Abstract
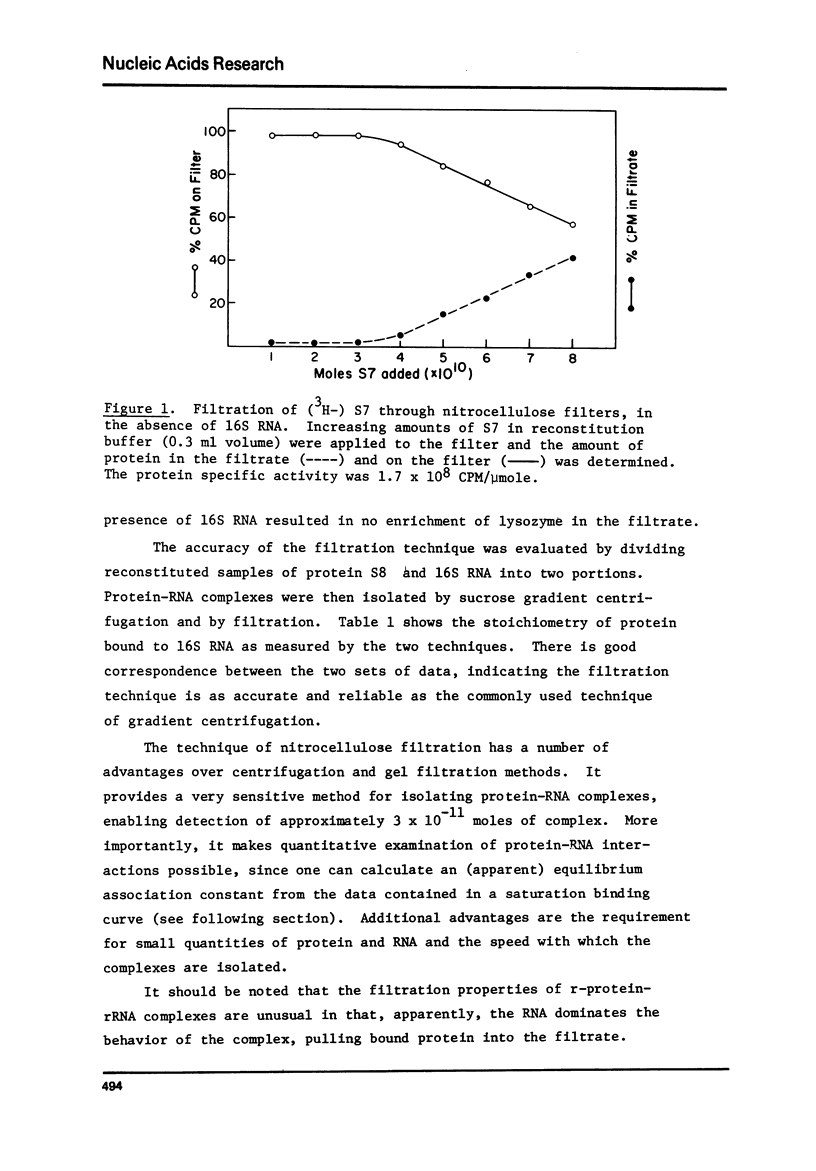
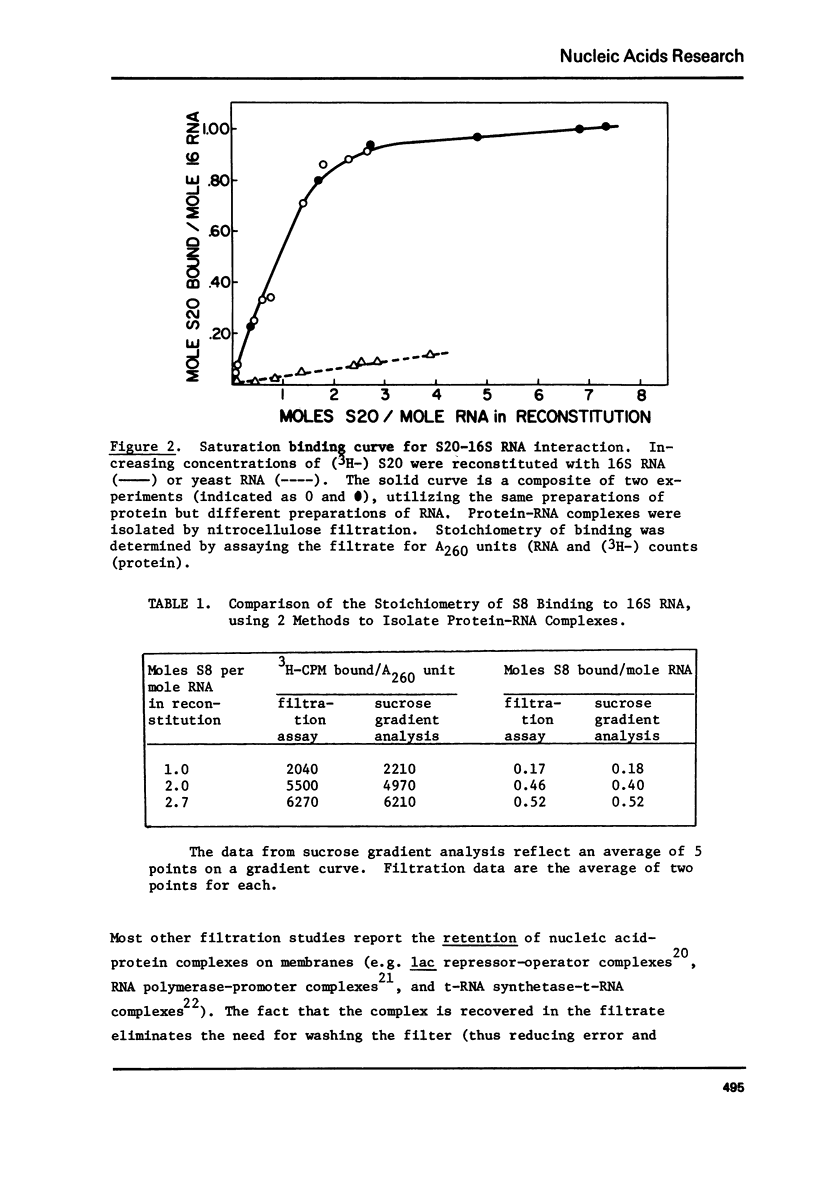
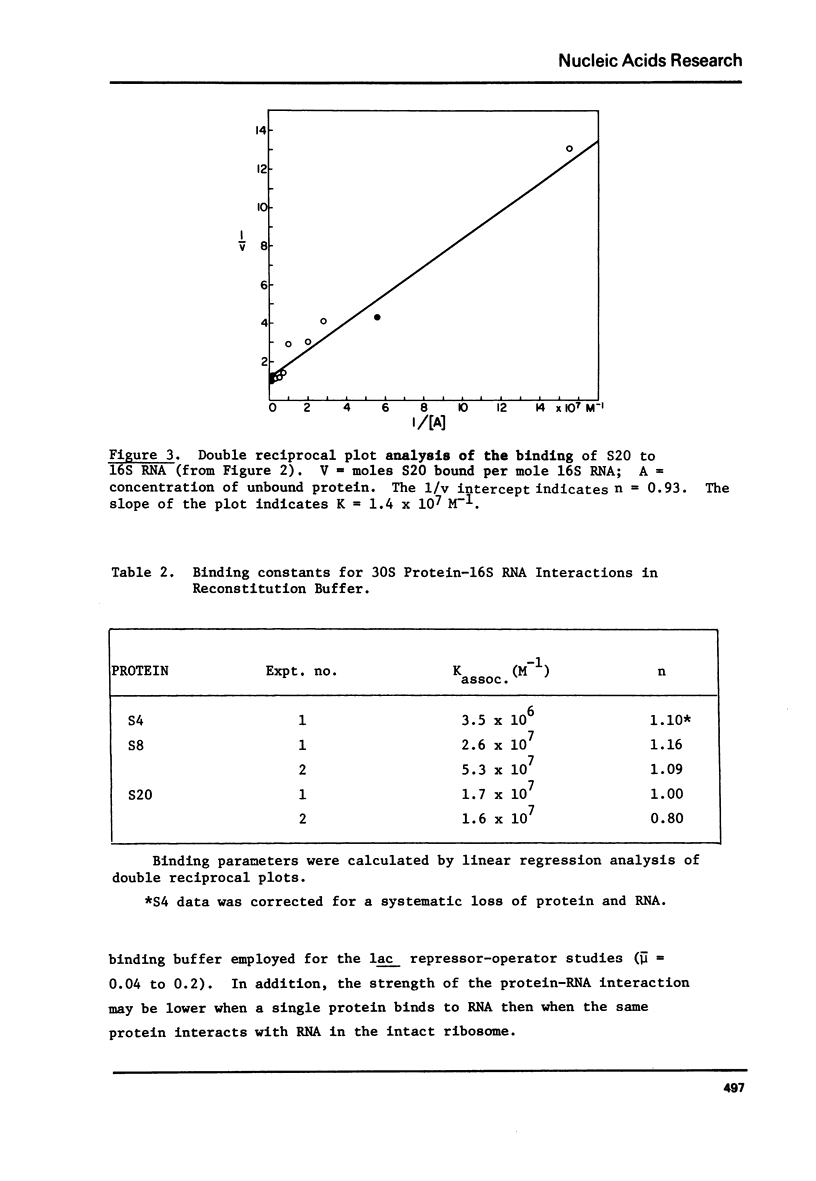
E. coli ribosomal proteins are retained by nitrocellulose filters. In contrast, 16S RNA passes through nitrocellulose filters. We have found that specific protein-RNA complexes involving single proteins also pass through nitrocellulose filters. Thus, by utilizing radioactively labeled r-proteins, nitrocellulose filtration can be used to study directly and sensitively the stoichiometry of r-protein-RNA association. The filtration process maintains near equilibrium conditions, making it applicable to weak as well as strong protein-RNA associations. We have used nitrocellulose filtration to obtain saturation binding curves for the association of proteins S4, S7, S8 and S20 with 16S RNA. In each case, the stoichiometry of binding was one mole of protein or less per mole of RNA. The stoichiometry of protein S8 binding to 16S RNA measured by filtration is comparable to that observed by sucrose gradient centrifugation. Association constants for the binding of proteins S4, S8 and S20 to 16S RNA have been determined by analysis of the saturation binding curves and were found to range from .3-6 X 10(7)M-1.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craven G. R., Voynow P., Hardy S. J., Kurland C. G. The ribosomal proteins of Escherichia coli. II. Chemical and physical characterization of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2906–2915. doi: 10.1021/bi00835a032. [DOI] [PubMed] [Google Scholar]
- Garrett R. A., Rak K. H., Daya L., Stöffler G. Ribosomal proteins. XXIX. Specific protein binding sites on 16S rRNA of Escherichia coli. Mol Gen Genet. 1972;114(2):112–124. doi: 10.1007/BF00332782. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Held W. A., Ballou B., Mizushima S., Nomura M. Assembly mapping of 30 S ribosomal proteins from Escherichia coli. Further studies. J Biol Chem. 1974 May 25;249(10):3103–3111. [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Hochkeppel H. K., Spicer E., Craven G. R. A method of preparing Escherichia coli 16 S RNA possessing previously unobserved 30 S ribosomal protein binding sites. J Mol Biol. 1976 Feb 25;101(2):155–170. doi: 10.1016/0022-2836(76)90369-7. [DOI] [PubMed] [Google Scholar]
- Kurland C. G. The requirements for specific sRNA binding by ribosomes. J Mol Biol. 1966 Jun;18(1):90–108. doi: 10.1016/s0022-2836(66)80079-7. [DOI] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S., Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970 Jun 27;226(5252):1214–1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- Moore G., Crichton R. R. Reductive methylation: a method for preparing functionally active radioactive ribosomes. FEBS Lett. 1973 Nov 15;37(1):74–78. doi: 10.1016/0014-5793(73)80429-6. [DOI] [PubMed] [Google Scholar]
- Paulus H. A rapid and sensitive method for measuring the binding of radioactive ligands to proteins. Anal Biochem. 1969 Oct 15;32(1):91–100. doi: 10.1016/0003-2697(69)90107-9. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Schaup H. W., Green M., Kurland C. G. Molecular interactions of ribosomal components. II. Site-specific complex formation between 30S proteins and ribosomal RNA. Mol Gen Genet. 1971;112(1):1–8. doi: 10.1007/BF00266926. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of Escherichia coli ribosomes. I. Partial fractionation of the functionally active ribosomal proteins and reconstitution of artificial subribosomal particles. J Mol Biol. 1968 Jun 28;34(3):575–593. doi: 10.1016/0022-2836(68)90182-4. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of Escherichia coli ribosomes. VI. Mechanism of assembly of 30 s ribosomes studied in vitro. J Mol Biol. 1969 Mar 28;40(3):391–413. doi: 10.1016/0022-2836(69)90161-2. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Garrett R., Ehresmann C., Stiegler P., Fellner P. An investigation of the 16-S RNA binding sites of ribosomal proteins S4, S8, S15, and S20 FROM Escherichia coli. Eur J Biochem. 1975 Feb 3;51(1):165–180. doi: 10.1111/j.1432-1033.1975.tb03917.x. [DOI] [PubMed] [Google Scholar]
- Voynow P., Kurland C. G. Stoichiometry of the 30S ribosomal proteins of Escherichia coli. Biochemistry. 1971 Feb 2;10(3):517–524. doi: 10.1021/bi00779a026. [DOI] [PubMed] [Google Scholar]
- Yarus M., Berg P. On the properties and utility of a membrane filter assay in the study of isoleucyl-tRNA synthetase. Anal Biochem. 1970 Jun;35(2):450–465. doi: 10.1016/0003-2697(70)90207-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Mackie G. A., Muto A., Garrett R. A., Ungewickell E., Ehresmann C., Stiegler P., Ebel J. P., Fellner P. Location and characteristics of ribosomal protein binding sites in the 16S RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):279–302. doi: 10.1093/nar/2.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Muto A., Fellner P., Ehresmann C., Branlant C. Location of ribosomal protein binding sites on 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1972 May;69(5):1282–1286. doi: 10.1073/pnas.69.5.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]


