Abstract
A major risk factor for cancer is increasing age, which suggests that syngeneic tumor implants in old mice would grow more rapidly. However, various reports have suggested that old mice are not as permissive to implanted tumor cells as young mice. In order to determine and characterize the age-related response to B16 melanoma, we implanted 5×105 tumor cells into 8, 16, 24, and 32-month-old male C57BL/6 (B6) and C57BL/6×BALB/c F1 (CB6 F1) mice subcutaneously in the inguinal and axillary spaces, or intradermally in the lateral flank. Results showed decreased tumor volume with increasing age, which varied according to mouse genetic background and the implanted site. The B6 strain showed robust tumor growth at 8 months of age at the inguinal implantation site, with an average tumor volume of 1341.25 mm3. The 16, 24, and 32-month age groups showed a decrease in tumor growth with tumor volumes of 563.69, 481.02, and 264.55 mm3, respectively (p≤0.001). The axillary implantation site was less permissive in 8-month-old B6 mice with an average tumor volume of 761.52 mm3. The 24- and 32-month age groups showed a similar decrease in tumor growth with tumor volumes of 440 and 178.19 mm3, respectively (p≤0.01). The CB6F1 strain was not as tumor permissive at 8 months of age as B6 mice with average tumor volumes of 446.96 and 426.91 mm3 for the inguinal and axillary sites, respectively. There was a decrease in tumor growth at 24 months of age at both inguinal and axillary sites with an average tumor volume of 271.02 and 249.12 mm3, respectively (p≤0.05). The strain dependence was not apparent in 8-month-old mice injected intradermally with B16 melanoma cells, with average tumor volumes of 736.82 and 842.85 mm3 for B6 and CB6 F1, respectively. However, a strain difference was seen in 32-month-old B6 mice with an average decrease in tumor volume of 250.83 mm3 (p≤0.01). In contrast, tumor growth significantly decreased earlier in CB6 F1 mice with average tumor volumes of 417.62 and 216.34 mm3 in the 16- and 24-month age groups, respectively (p≤0.005). Histologically, implanted tumors in young mice exhibited characteristics of aggressive, rapidly growing tumor cells including high vascularity, mitosis, and invasiveness compared to tumors in old mice. We contend that the decrease in B16 melanoma tumor growth seen with increasing age in B6 and CB6 F1 mice represents a biological process, which we are calling age-dependent cancer resistance (ADCR). Our data provide a detailed description of conditions necessary to use the model to investigate the mechanisms of ADCR and determine its biological and clinical relevance.
Keywords: aging, cancer, cancer resistance, B16 melanoma
It is well established that increasing age is a major risk factor for cancer (1). Therefore, the expectation is that older mice would be more susceptible to syngeneic tumor implants. Syngeneic cancer is cancer which is generated by the implantation of a tumor cell line into a recipient mouse with the same genetic background as that of the tumor cells. This type of mouse cancer model system has been used extensively in cancer research for a wide variety of investigations. There are a number of cell lines available representing specific organs and tissues that have consistently been used for many years including B16 melanoma. This line is very popular because it can easily be implanted in the skin and monitored visually without any special imaging equipment. The successful use of this cell line has traditionally been carried out in young mice ranging from 3 to 4 weeks of age to 3 to 4 months of age. However, it has periodically been reported over the last several decades that old mice show increased variability in response to implanted tumor cells. For example, B16 melanoma cells into old syngeneic C57BL/6 mice were shown by Ershler et al. (2) to have poor tumor growth compared to a very robust growth in young C57BL/6 mice.
These paradoxical observations have not been specifically characterized nor has any mechanism been particularly associated with them, but delayed tumor growth could potentially interfere with test results if an older age cohort is included as part of the research design, especially if experimental drugs or other compounds are being tested. For mice, middle age is generally represented as 15–18 months of age, which is equivalent to middle age (50–60 years) in people. Old age in mice is generally represented as 24–28 months of age, which is equivalent to old age (70–80 years) in people, and 32-month-old mice are representative of advanced age (90–100 plus years) in people. In this report, we describe the age-dependent delay in tumor progression of the B16F0 melanoma cell line when implanted into 8-, 16-, 24-, and 32-month age groups of respective syngeneic mice.
Materials and methods
Cell line
B16 melanoma F0 cells, a non-metastatic cell line obtained from the American Type Culture Collection (Manassas, VA), were grown and maintained in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS) with 1% pen/strep in a humidified atmosphere and 5% CO2 in air. B16 melanoma cells are syngeneic to C57BL/6 mice.
Mouse lines
C57BL/6 (B6) and Balb/c×C57BL/6 F1 (CB6F1) mice were obtained from the NIA mouse colony (contracted by Charles River, Inc., Wilmington, MA) at ages of 8, 16, 24, or 32 months. Mice were housed at the animal research facility at the University of Washington and were acclimatized to the facility conditions while provided with free access to a standard rodent diet (PicoLab® Rodent Diet 20, Lab Diet, PMI, St Louis, MO, USA) and water. Animals were maintained throughout the study in a controlled environment and a 12 h light/dark cycle. All experiments were conducted in accordance with procedures and protocols approved by the Animal Care and Use Committee of the University of Washington.
Tumor assessment
Cells were implanted into recipient mice by injecting 5×105 B16F0 melanoma cells in 200 µl phosphate buffered saline subcutaneously in the inguinal and axillary spaces, or in 20 µl intradermally in the lateral flank. Mice were monitored daily for tumor incidence and growth. When palpable, tumors were measured every other day using digital calipers and measured in two dimensions. Mice were sacrificed 14 days after implantation, and tumors were weighed and measured for length (L), width (W), and depth (D) using a digital caliper as described (3). Tumor volume (V) was determined by using the formula V=L×W×D×3.14/6. Tumor tissue was collected into 10% neutral buffered formalin and processed for hematoxylin and eosin staining and histological evaluation.
Statistics
Results were expressed as mean±SEM. Statistical analyses of data were performed using Student's t-test, using fx for Microsoft Excel version 10.1 and the significance of the difference was defined and considered highly significant when p<0.01.
Results and discussion
Tumor volumes decrease with increasing age
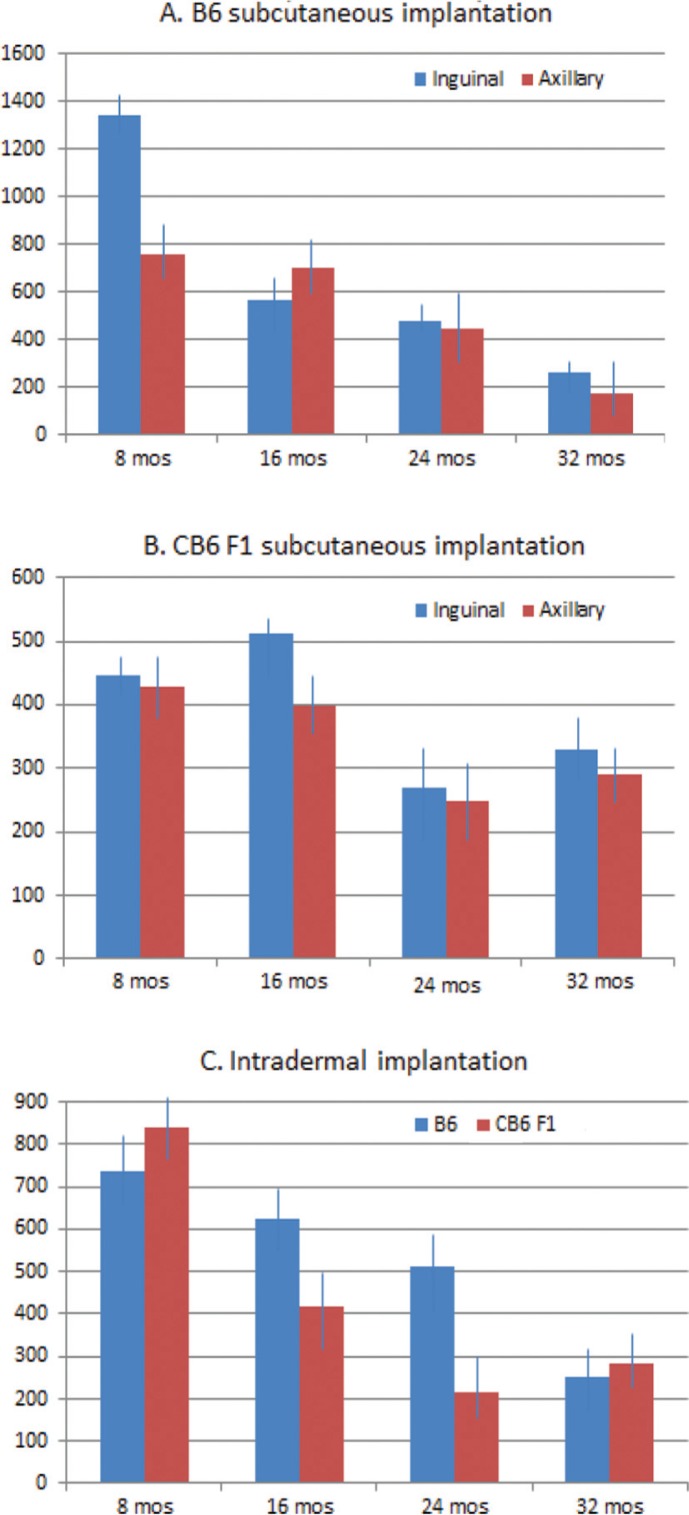
Our results showed a decrease of tumor volume with increasing age, which varied according to the genetic background and the implanted site, that is, subcutaneous axillary, subcutaneous inguinal or intradermal. The B6 strain showed an especially robust tumor growth at 8 months of age at the inguinal implantation site, with an average tumor volume of 1341.25 mm3 (Fig. 1A). The subsequent age groups at 16, 24, and 32 months showed a dramatic decrease in tumor growth with tumor volumes of 563.69, 481.02, and 264.55 mm3, respectively (p≤0.001). The axillary implantation site was less permissive in 8-month-old mice with an average tumor volume of 761.52 mm3. The subsequent age groups at 24 and 32 months showed a similar decrease in tumor growth as at the inguinal site with tumor volumes of 440 and 178.19 mm3, respectively (p≤0.01).
Fig. 1.
B16 melanoma tumor volumes decrease with increasing age in an implantation site and strain-dependent manner. (A) Cohorts of C57BL/6 (B6) mice, with 12 animals in each age group, were implanted with 5×105 cells in the subcutaneous axillary and inguinal areas and terminated 14 days later. Average tumor volume was higher at the inguinal site compared to the axillary site in 8-month-old mice (p≤0.05), and was significantly lower at 32 months at both sites (p≤0.001). (B) The experimental procedures and cohort numbers were the same for C57BL/6×BALB/c F1 (CB6 F1) mice but with lower average tumor volumes than B6 mice at 8 months of age at both implantation sites. An age-related decrease occurred only from 16 to 24 months of age at both sites (p≤0.05). (C) Intradermal melanoma implants, with the same cell number and eight mice in each age group for both mouse strains, resulted in average tumor volumes that were similar for both strains at 8 months of age, but with a significant decrease in average tumor volume at 16 months and again at 24 months for CB6 F1 mice (p≤0.005). Average tumor volumes did not decrease until 32 months for B6 mice (p≤0.01).
The CB6F1 strain was much less tumor permissive at 8 months of age with average tumor volumes of 446.96 and 426.91 mm3 for the inguinal and axillary sites, respectively (Fig. 1B). There was a moderate decrease in tumor growth at 24 months of age at both inguinal and axillary sites with an average tumor volume of 271.02 and 249.12 mm3, respectively (p≤0.05). Interestingly, there was no additional decrease in tumor growth from 24 to 32 months at either implantation site.
Phenotypic variation between the two genotypes at 8 months of age could certainly be explained by minor genotypic differences responsible for immune responsiveness. These differences disappeared with increasing age. The robust tumor growth in 8-month-old C57BL/6 mice at the inguinal site might be due to increased vascularization, which is no longer present in the older age groups.
The background strain appeared to influence subcutaneously implanted tumor growth only at a young age where tumor volumes were twice as large in 8-month-old B6 mice compared to CB6 F1 mice of the same age. Interestingly, this strain dependence was not apparent in young mice injected intradermally with B16 melanoma cells, with average tumor volumes of 736.82 and 842.85 mm3 for B6 and CB6 F1, respectively (Fig. 1C). However, a striking strain difference was seen in tumor growth in subsequent age groups with tumor volumes not significantly decreasing in B6 mice until the 32-month age group at 250.83 mm3 (p≤0.01). In contrast, tumor growth drastically decreased in CB6 F1 mice at the 16- and 24-month age groups with tumor volumes of 417.62 and 216.34 mm3, respectively (p≤0.005).
Implanted melanoma tumors in young and old mice are morphologically and histologically distinct
Intradermally implanted melanoma tumors in 32-month-old CB6 F1 mice were more shallow with an average width of 7.7 mm and an average depth of 4.8 mm compared to intradermally implanted melanoma tumors in 8-month-old mice, with average widths of 11.69 mm and average depths of 12.7 mm. This observation is of clinical interest because it has been reported that vertical growth of melanomas is correlated with an increase in aggressive and metastatic behavior (4).
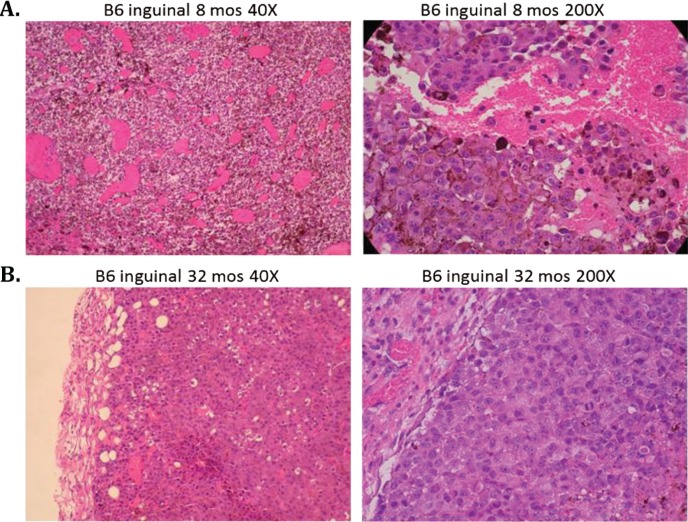
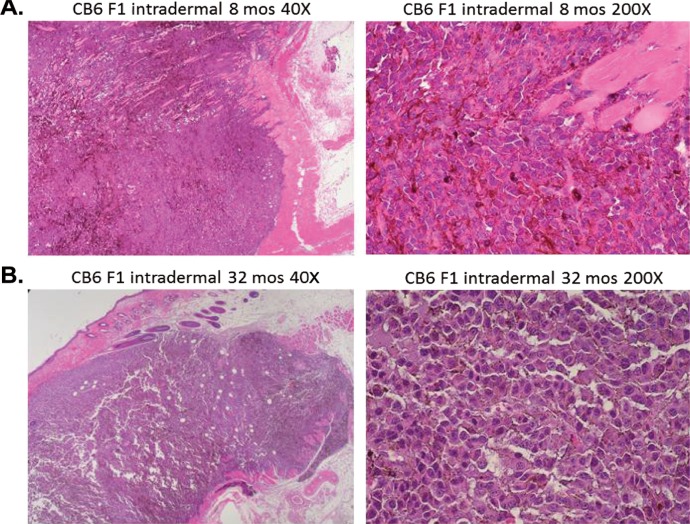
Histologically, intradermal as well as subcutaneous implanted tumors in young mice exhibited characteristics of aggressive, rapidly growing tumor cells including high vascularity, mitosis, and invasiveness. Tumors in old mice showed a much slower growth pattern with markedly decreased vascularity and invasiveness consistent with clinical morphology described above. The contrast was most striking in 8-month-old B6 mice implanted subcutaneously in the inguinal site compared to 24-month-old B6 mice implanted at the same site and in the same manner. Fig. 2A shows the typical high vascularity in 8-month-old B6 mice, which is markedly decreased in 32-month-old B6 mice (Fig. 2B). The same histological pattern is also seen in CB6 F1 mice implanted intradermally at 8 and 32 months of age (Fig. 3A,B). In addition, the 8-month-old mice allowed a more vertical tumor growth pattern (Fig. 3A; 40×) compared to a more horizontal growth pattern in 32-month-old mice (Fig. 3B; 40×).
Fig. 2.
Subcutaneous B16 melanoma tumors in C57BL/6 mice are histologically more aggressive in young animals compared to old animals as determined from hematoxylin and eosin stained slides. (A) The main histological features in 8-month-old mice were high neovascularization, high mitotic activity, and lack of any surrounding confining borders (40× and 200× power). (B) The main histological features in 32-month-old mice were presence of a fibrous capsule, few mitotic figures, and low vascularity (40× and 200× power).
Fig. 3.
Intradermal B16 melanoma tumors in young mice show features of aggressive, rapidly growing cells compared to tumors in old mice. (A) Aggressive behavior in 8-month-old CB6 F1 mice is seen as vertical growth and invasive spreading with irregular nodular contour and irregular infiltrating cells within the epidermal and dermal layers (40× and 200× power). (B) Intradermal tumors in 32-month-old CB6 F1 mice showed a radial growth pattern with superficial spreading of tumor cells in a well demarcated nodular contour (40× and 200× power).
B16 melanoma is a prototype model for age-dependent cancer resistance
We contend that the decrease in B16 melanoma tumor growth seen with increasing age in B6 and CB6 F1 mice represents a biological process, which we are calling age-dependent cancer resistance (ADCR). Although we have not yet determined the mechanisms involved, we suspect that stromal cells from aged mice are somehow unable to respond to the type of pro-tumor signaling that readily triggers stromal cells from young mice into a tumor supporting mode. In this regard, it is tantalizing to speculate that the ADCR process may be responsible for limiting the increased incidence of cancer with increasing age, that is, the increase in cancer would be much greater without ADCR such that a much higher percentage of the population would be afflicted with the disease in some form or another. We know ADCR is not unique to the B16 melanoma line as other lines have been reported to grow more slowly in old mice (5–7) and our lab has unpublished data showing that the 4T1 mouse breast cancer cell line and PyMT breast cancer primary cells do not grow as well in old syngeneic mice as in young syngeneic mice (manuscripts in preparation). There are several recent reports of cell lines that have been described to proliferate equally well in young and old syngeneic recipient mice (8,9). This could very well be dependent on the type of mutations in the tumor cells that affect cytokine signaling and cellular cross talk that might attenuate ADCR. However, our data confirm the delay of B16 melanoma tumor growth with increasing age in mice using age groups from 8 to 32 months, and provide a detailed description of conditions necessary to use the model to investigate the mechanisms of ADCR and determine its biological and clinical relevance.
Acknowledgements
This study was supported by the Nathan Shock Center for Aging at the University of Washington.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Dix D, Cohen P, Flannery J. On the role of aging in cancer incidence. J Theor Biol. 1980;83(1):163–73. doi: 10.1016/0022-5193(80)90377-x. [DOI] [PubMed] [Google Scholar]
- 2.Ershler WB, Stewart JA, Hacker MP, Moore AL, Tindle BH. B16 murine melanoma and aging: slower growth and longer survival in old mice. J Natl Cancer Inst. 1984;72(1):161–4. doi: 10.1093/jnci/72.1.161. [DOI] [PubMed] [Google Scholar]
- 3.Goh J, Enns L, Fatemie S, Hopkins H, Morton J, Pettan-Brewer C, et al. Mitochondrial targeted catalase suppresses invasive breast cancer in mice. BMC Cancer. 2011;11:191. doi: 10.1186/1471-2407-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy GF, Mihm M. The skin. In: Kumar V, Abbas A, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia: Elsevier Saunders; 2005. pp. 1227–71. [Google Scholar]
- 5.Gravekamp C, Sypniewska R, Gauntt S, Tarango M, Price P, Reddick R. Behavior of metastatic and nonmetastatic breast tumors in old mice. Exp Biol Med (Maywood) 2004;229(7):665–75. doi: 10.1177/153537020422900711. [DOI] [PubMed] [Google Scholar]
- 6.Pili R, Guo Y, Chang J, Nakanishi H, Martin GR, Passaniti A. Altered angiogenesis underlying age-dependent changes in tumor growth. J Natl Cancer Inst. 1994;86(17):1303–14. doi: 10.1093/jnci/86.17.1303. [DOI] [PubMed] [Google Scholar]
- 7.Kaptzan T, Skutelsky E, Itzhaki O, Sinai J, Huszar M, Siegal A, et al. Efficacy of anti-angiogenic treatment of tumors in old versus young mice. Mech Ageing Dev. 2006;127(4):398–409. doi: 10.1016/j.mad.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Reed MJ, Karres N, Eyman D, Cruz A, Brekken RA, Plymate S. The effects of aging on tumor growth and angiogenesis are tumor-cell dependent. Int J Cancer. 2007;120(4):753–60. doi: 10.1002/ijc.22351. [DOI] [PubMed] [Google Scholar]
- 9.Norian LA, Allen PM. No intrinsic deficiencies in CD8+ T cell-mediated antitumor immunity with aging. J Immunol. 2004;173(2):835–44. doi: 10.4049/jimmunol.173.2.835. [DOI] [PubMed] [Google Scholar]