Abstract
Objective:
Activation of the hedgehog pathway is an important signaling mechanism crucial in embryogenesis and has strong links to carcinogenesis. This study investigates the expression of the Sonic hedgehog pathway molecules in non-small cell lung tumors as it relates to clinical outcome of various non-small cell lung cancers.
Methods:
A tissue microarray with 81 samples from 42 patients with various non-small cell lung cancer histologies was examined without the aid of laser microdissection. All samples were stained with antibodies directed against Sonic hedgehog, Ptch-1, Smoothened, and Gli-1.
Results:
Most of the tumor samples showed negative to weak expression of the pathway proteins (Sonic hedgehog, 38% negative to 20% weak; Ptch-1, 100% negative; Smoothened, 69% negative to 7% weak; Gli-1, 57% negative to 5% weak) compared with higher expression in normal lung epithelial cells.
Conclusion:
The same pathway expression did not correlate with clinical outcome. While our results do not provide any indication that the pathway molecules are correlated to overall patient survival possibly due to the limited sample size, our study shows minimum overexpression of Sonic hedgehog pathway in non-small cell lung cancer and this did not correlate clinically with patient outcome.
Keywords: Gli-1, hedgehog pathway, lung cancer, prognosis, Sonic hedgehog
Introduction
Lung cancer is currently the leading cause of cancer-related deaths in men and women and the second most commonly diagnosed cancer in the United States [Jemal et al. 2010]. There are an estimated 222,520 new lung cancer cases each year and 157,300 deaths anticipated in 2010, constituting the most fatal cancer with a considerably higher number of deaths than other prevalent forms of cancer including colon, breast, and brain cancers combined [Jemal et al. 2010]. This is partly due to the aggressive and diverse nature of lung cancer and lack of effective treatment modalities. Treatment modalities conventionally used include surgery, chemotherapy, and radiation; however, substantial improvement in disease outcome and survival has not been achieved in the past 40 years. Novel treatment regimens under development to improve clinical outcome of patients include biological therapies targeting pathways driving tumor growth and development.
The hedgehog (Hh) pathway regulates fundamental processes in vertebrate embryonic development such as stem cell maintenance, cell differentiation, tissue polarity, and cell proliferation. In addition, constitutive activation of the Hh pathway leading to tumorigenesis has been noted in various cancers including basal cell carcinomas and medulloblastoma [Rubin and de Sauvage, 2006]. Other cancers, including brain, gastrointestinal, lung, breast, and prostate cancers, also have been found to show activation of this pathway [Rubin and de Sauvage, 2006]. Increased paracrine Hh signaling from the tumor to the surrounding stroma, increased Hh ligand production activating tumor cells and gain or loss of function mutations in the Hh pathways are mechanisms responsible for Hh signaling in cancer promoting tumorigenesis. This pathway has also been shown to regulate proliferation of cancer stem cells and to increase tumor invasiveness [Gupta et al. 2010]. Furthermore, the proteins involved in the pathway are also involved in the regulation of stem cell production and activation during tissue repair after injury [Waschek et al. 2006; Ingham and McMahon, 2001]. Aberrant activation and deregulation of the components of this pathway that regulates cell proliferation and differentiation has been implicated in numerous human malignancies including lung cancer [Watkins et al. 2003]. Thus, targeted inhibition of Hh signaling may be effective in the treatment and prevention of many types of cancers including lung cancer.
First discovered in Drosophila, the hedgehog has three distinct homologues in mammals: Sonic hedgehog (Shh), Indian hedgehog (Ihh) and Desert hedgehog (Dhh). The pathway is activated with the binding of the Hedgehog proteins to a transmembrane receptor Patched (Ptch-1), which in the absence of a Hh ligand normally serves as a suppressor of another transmembrane protein, a G-protein coupled receptor Smoothened (Smo). The derepression of Smoothened causes a signal cascade that leads to translocation of active form of the transcription factor Gli-1 to the nucleus where transcription of hedgehog responsive genes Ptch-1 and Gli-1 takes place [Watkins et al. 2003; Olsen et al. 2004; Morton and Lewis, 2007]. Smo and other downstream pathway components require cilia to transit in order to activate Gli transcription factors, and the zinc-finger transcription factor cubitus interruptus (Ci). This signaling cascade causes the translocation of Gli and the zinc finger to eventually make their way to the nucleus, which further activates in target gene [Liao et al. 2009]. The function of the zinc-finger transcription factor Ci can be inferred from the roles of the three Gli proteins, each distinguished by their transcriptional activity; while the oncoprotein Gli-1 serves as a regulator of the Hh pathway targets, Gli-2 and Gli-3 possess both transcriptional activation and repression properties [Watkins and Peacock, 2004].
Overexpression of the proteins Gli-1 and Ptch-1 has been linked with lung [Watkins et al. 2003], prostate [Datta and Datta, 2006], and ovarian cancers [Liao et al. 2009]. Extensive studies have been conducted in one of the main Hh proteins, Shh, since it is the predominant signaling ligand in the brain, lung, and limb development [Watkins and Peacock, 2004]. It also plays an essential role in mediating epithelial–mesenchymal interactions in lung development [Watkins et al. 2003]. Hence, a mutation or deregulation in the Shh could cause a serious lung defect by hindering bronchial morphogenesis. Fujita and colleagues have found that overexpression of Shh in lung epithelial cells has a mitogenic effect on lung mesenchymal cells [Fujita et al. 1997].
In this study, we investigated the expression of the Hh signaling pathway proteins in 81 tumor samples from 42 lung cancer patients and determined the clinical correlation between Hh pathway expression and clinical outcome including survival. By investigating the expression of the signaling molecules, Shh, Ptch-1, Smo, and Gli-1, involved in the Hh pathway, we hoped to evaluate the role of these molecules as a prognostic indicator in patients with non-small cell lung cancer (NSCLC) and potentially identify new therapeutic targets for lung cancer management.
Patients and methods
Tissue samples and patients
All patients signed the approved informed consent documents obtained from Vanderbilt Institutional Review Board and appropriate protocol consent form prior to the study. All samples were treated with the highest degree of confidentiality according to the ethical and legal standards. In this study, samples from 42 patients (Table 1) (29 women, 13 men; mean age, 64 years; range 12–87 years) with biopsy-proven NSCLC were collected and tissue cores were placed on tissue microarrays. A total of 7% of the patients were African-American while 93% were White. Out of the 42 patients, 21 were nonsmokers, 15 were current smokers, 5 were exsmokers, and 1 of the patient’s smoking history was unknown. The histological breakdown was the following: adenocarcinoma (30), squamous cell carcinoma (4), adenosquamous carcinoma (2), large cell carcinoma (1), carcinoid (1) and NSCLC not otherwise specified (NOS, 8). Patients underwent treatment and were followed at Vanderbilt University Medical Center between 1998 and 2011.
Table 1.
Patient characteristics.
| Number of patients: 42 | |
|---|---|
| Male | 13 |
| Female | 29 |
| Mean age (years) | 64 |
| Age range (years) | 12–87 |
| Social history | |
| Current smoker | 15 |
| Exsmoker | 5 |
| Nonsmoker | 21 |
| Unknown | 1 |
| Histology | |
| Adenocarcinoma | 30 |
| Squamous cell carcinoma | 4 |
| Not otherwise specified | 8 |
| Pathological stage | |
| Stage I | 22 |
| Stage II | 4 |
| Stage III | 12 |
| Stage IV | 1 |
| Treatment | |
| Surgery | 38 |
| Radiation therapy | 13 |
| Chemotherapy | 18 |
| Surgery and chemotherapy | 9 |
| Surgery and radiation therapy | 5 |
| Chemotherapy and radiation therapy | 2 |
| Surgery and chemotherapy and radiation therapy | 6 |
| Patient response to treatment | |
| Progressive disease | 17 |
| Complete response | 16 |
| Stable disease | 6 |
| Partial response | 1 |
| N/A | 2 |
| Survival | |
| Dead | 25 |
Immunohistochemistry
Deparaffinized and rehydrated 5 µm section slides of tissue samples on tissue microarrays were prepared and placed in heated Target Retrieval Solution, High pH (Dako, Carpinteria, CA). Endogenous peroxidase was neutralized with 0.03% hydrogen peroxide followed by a casein-based protein block (Dako, Carpinteria, CA) to minimize nonspecific staining. The sections were incubated with Gli-1 (sc-20687, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:25 and incubated overnight at 4°C. The sections were incubated with Ptch-1 (sc-9016, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:25 and incubated for 60 minutes at room temperature. The sections were incubated with Smo (sc-13943, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:25 and incubated for 60 minutes at room temperature. The sections were incubated with Shh (sc-9024, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:25 and incubated for 60 minutes at room temperature. Human lung carcinoma samples served as positive controls. The Dako Envision+ HRP/DAB System (Dako) was used to produce localized, visible staining. The slides were lightly counterstained with Mayer’s hematoxylin, dehydrated and coverslipped.
Scoring of results
Scoring methods proposed by Sinicrope and colleagues were utilized in the evaluation of immunoreactivity for both intensity of staining and the percentage positive of stained tumor cells [Sinicrope et al. 1995]. The percentage of positive cells that revealed stronger staining intensity in proportion to the adjacent epithelial cells were scored as follows: 0 (0–4% tumor cells stained), 1 (5–25% tumor cells stained), 2 (26–50% tumor cells stained), 3 (51–75% tumor cells stained), and 4 (>75% tumor cells stained). In order to determine the staining intensity, these categories were further subclassified as follows: 0, no expression; 1, extremely weak; 2, weak; 3, moderate; 4, strong expression. Evaluation of the percentage expression of the pathway molecules was repeated multiple times without the knowledge of patients’ clinical data to avoid observer bias.
Statistical analysis
Overall survival (OS; defined as the interval between the date of first treatment and the date of death from any cause) was estimated by the Kaplan–Meier method. Multivariable-adjusted Cox proportional hazards regression models were used to examine the association of Gli-1, Ptch-1, Smo, and Shh expression, separately with patient survival. Pathologic stage, histology, surgery (yes/no), and age at diagnosis were used as covariates. Kaplan–Meier curves of biomarker expression levels were constructed in the top 25% and bottom 25% expressers to show whether the downregulation or overregulation of the biomarker has an effect on patient survival.
Results
Expression of Hh pathway proteins in normal lung epithelial cells
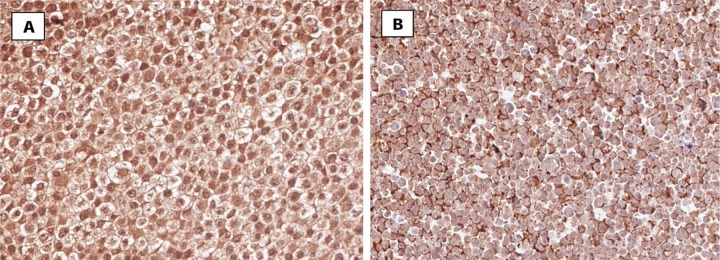
The tissue microarrays which contained the core biopsies of NSCLC patients were stained immunohistochemically to analyze the expression pattern of Hh signaling molecules in these tumor samples. Antibodies directed against the Hh signaling molecules Shh, Gli-1, Ptch-1, and Smo were utilized. The percentage expression patterns of these molecules in lung tumor samples were correlated to the smoking history, histopathological diagnosis, pathological stage, patient response to treatment, distant metastasis, and overall survival. Moderate to high expression of Smo and Ptch-1 were found in normal lung epithelial cells as seen in Figure 1. With respect to the expression levels of Smo, 9 out of 78 samples (11%) displayed extremely weak expression while 19 revealed weak (24%) expression. Moderate expression was found in 25 samples (32%), and 25 presented strong expression (32%). Overall, high levels of Smo were seen in the normal lung epithelial cells. In terms of the prevalence of Ptch-1 in normal lung epithelial cells, expression levels were not nearly as high relative to those for Smo. Out of 70 samples, 19 displayed no expression (27%) of Ptch-1 molecules while 16 displayed extremely weak expression (23%). Weak expression was seen in 13 samples (19%), and 19 samples (27%) exhibited moderate levels of Ptch-1. Only 3 samples (4%) displayed strong expression of Ptch-1.
Figure 1.
Immunohistochemical staining for Patched and Smoothened in normal lung epithelial cells. Parts (A) and (B) show representative images of positive expression of Patched (A) and Smoothened (B) in normal lung epithelial cells.
Expression of Hh pathway proteins in lung tumors
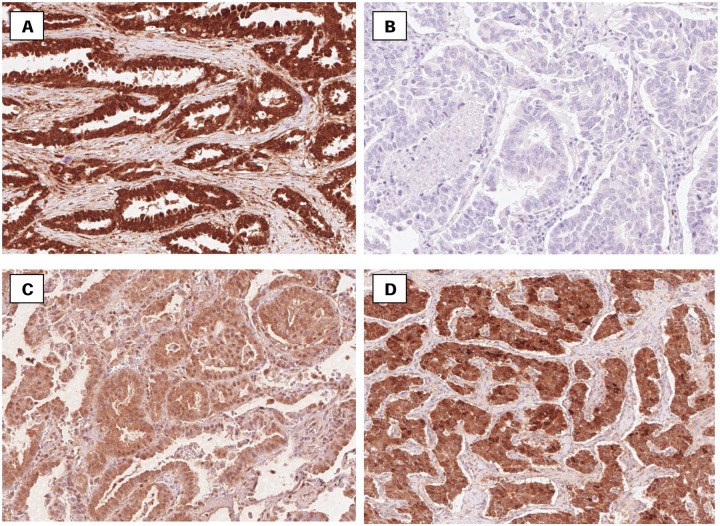
Ptch-1 was negative in every tumor that was stained, as indicated in Table 2 and can be seen in Figure 2. Gli-1 was negative in 46 out of 81 lung sections (57%), 29 out of 81 displayed extremely weak expression (36%), 4 out of 81 revealed weak expression (5%) whereas only 2 out of 81 had moderate expression (2%). Lung tumors also revealed mostly negative to weak expression of Smo. There was moderate expression in only 4 out of 81 samples (5%), whereas 56 out of 81 lung tumors (69%) presented negative expression of Smo. There was extremely weak expression in 15 out of 81 samples (19%) and weak expression in 6 out of 81 samples (7%). Shh was weakly and moderately expressed in most tumor sections. In 17 out of 81 samples (20%) and 15 out of 81 samples (19%), weak and moderate expression was exhibited, respectively. In 31 samples (38%), Shh remained completely negative.
Table 2.
Percentage expression of Sonic hedgehog pathway molecules in tissue microarrays.
| Patient | Shh | Gli-1 | Patched | Smoothened |
|---|---|---|---|---|
| 1 | 7.91 | 0.74 | 0.02 | 19.35 |
| 2 | 59.37 | 6.98 | 0.17 | 0.19 |
| 3 | 53.13 | 19.48 | 0.33 | 0.13 |
| 4 | 63.45 | 41.94 | 0.02 | 0.15 |
| 5 | 42.73 | 11.20 | 0.26 | 0.44 |
| 6 | 15.08 | 3.05 | 0.02 | 0.17 |
| 7 | 38.04 | 7.57 | 0.47 | 0.73 |
| 8 | 53.81 | 14.74 | 0.08 | 0.09 |
| 9 | 29.21 | 5.59 | 0.00 | 0.02 |
| 10 | 20.84 | 4.73 | 1.29 | 1.33 |
| 11 | 29.90 | 13.28 | 0.11 | 2.17 |
| 12 | 22.77 | 9.94 | 0.03 | 0.82 |
| 13 | 48.46 | 23.31 | 0.10 | 0.30 |
| 14 | 41.53 | 5.81 | 0.04 | 0.05 |
| 15 | 19.61 | 6.97 | 0.06 | 0.13 |
| 16 | 14.47 | 7.35 | 0.01 | 0.25 |
| 17 | 52.83 | 5.72 | 0.01 | 0.07 |
| 18 | 34.90 | 5.31 | 0.05 | 0.17 |
| 19 | 42.67 | 22.74 | 0.01 | 0.08 |
| 20 | 58.63 | 34.18 | 0.00 | 0.03 |
| 21 | 36.62 | 5.78 | 0.02 | 0.09 |
| 22 | 20.08 | 5.83 | 0.03 | 0.66 |
| 23 | 40.69 | 12.88 | 0.04 | 0.06 |
| 24 | 4.91 | 3.31 | 1.47 | 13.30 |
| 25 | 2.59 | 2.90 | 0.06 | 22.51 |
| 26 | 1.00 | 2.75 | 0.07 | 60.13 |
| 27 | 17.34 | 1.69 | 0.03 | 19.81 |
| 28 | 1.02 | 1.60 | 0.01 | 4.97 |
| 29 | 0.79 | 2.49 | 0.09 | 36.62 |
| 30 | 14.24 | 0.76 | 0.10 | 5.18 |
| 31 | 2.45 | 1.24 | 0.13 | 21.56 |
| 32 | 5.36 | 9.18 | 0.07 | 13.58 |
| 33 | 0.17 | 0.18 | 0.36 | 23.72 |
| 34 | 20.76 | 0.77 | 0.11 | 5.07 |
| 35 | 0.19 | 0.50 | 0.07 | 29.60 |
| 36 | 0.81 | 2.54 | 0.01 | 0.11 |
| 37 | 0.01 | 0.06 | 0.01 | 2.97 |
| 38 | 0.03 | 0.24 | 0.27 | 45.62 |
| 39 | 7.48 | 0.52 | 0.11 | 1.51 |
| 40 | 3.41 | 0.73 | 0.03 | 11.66 |
| 41 | 0.30 | 5.69 | 0.01 | 8.33 |
| 42 | 0.04 | 0.27 | 0.02 | 12.88 |
Figure 2.
Representative immunostaining of Shh (A), Patched (B), Smo (C), and Gli-1 (D) in samples from non-small cell lung tumors. The expression of Shh, Ptch-1, Smo, and Gli-1 was found in the epithelial and stromal components of cancers.
Impact of Hh pathway molecules expression on survival
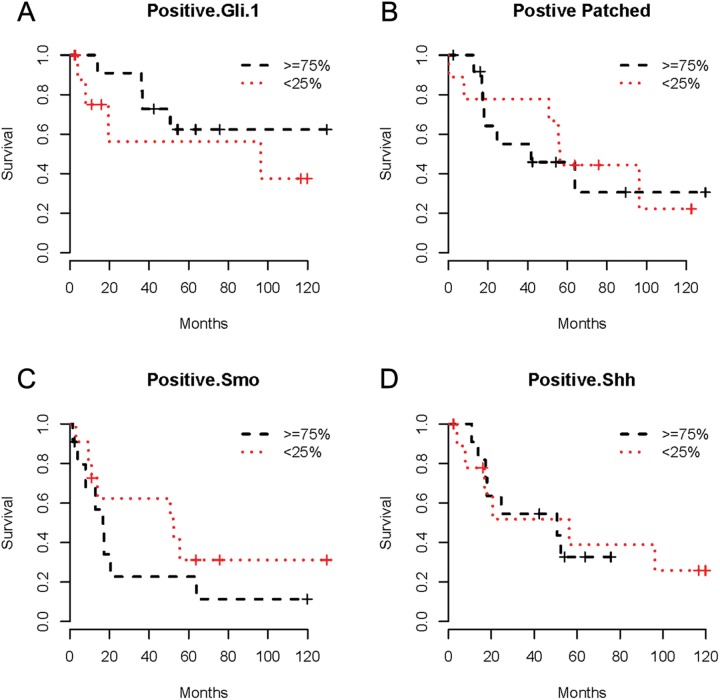
Statistical analysis revealed no correlation between the expression of Hh pathway proteins and histopathology, stage, smoking status, or overall survival. Survival analysis curves are shown in Figure 3. At the end of our analysis, mortality reached 53%.
Figure 3.
Kaplan–Meier survival curves for Gli-1 (A), Ptch (B), Smo (C), and Shh (D) protein expression in non-small cell lung cancer patients.
Discussion
The purpose of the present study was to investigate the expression level of Hh pathway proteins in patients with NSCLC and investigate the correlation between Hh pathway proteins and clinical outcome of NSCLC patients. Overall, there was weak expression of the Hh pathway proteins in the lung cancer tissue microarrays and the expression did not correlate with clinical parameters including patient survival.
Previous studies have shown an important role of the Hh signaling pathway in the development of a variety of solid tumors and have revealed its impact on the outcome of most incurable aggressive malignancies [Watkins et al. 2003; Liao et al. 2009; Watkins and Peacock, 2004; Datta and Datta, 2006; Brunner et al. 2010; Schneider et al. 2011; Vestergaard et al. 2006]. The Hh signaling pathway plays a crucial role in tumorigenesis when reactivated in adult tissues through sporadic mutations and other mechanisms. Mutations and triggered deregulations of the pathway could contribute to the onset of tumorigenesis or accelerate tumor growth [Rubin and de Sauvage, 2006].
Interestingly, a recent study by Watkins and colleagues found expression of Shh in NSCLC lines and decreased levels of Ptch, but not Gli-1 [Watkins et al. 2003]. The authors suggested that one explanation for the differential staining pattern may be that some NSCLC may retain the Shh signaling pathway initially noted in primitive lung endodermal cells that signal to adjacent mesenchymal cells in early development. Lauth and colleagues expanded on the importance of the stromal component for Hh signaling noting that Gli-1 signal was associated with stromal cells and suggested that these cells respond to the tumor cell-secreted Shh and not primarily to the tumor cells [Lauth et al. 2010]. The utilization of a laser microdissection method would have allowed us to observe the expression levels of the stroma and tumor sections separately, and perhaps assisted in revealing a more accurate expression level of Gli-1. The lack of its use could, in part, explain the low expression of Gli-1 in our study. In fact, Yauch and colleagues have observed ligand-dependent Hh activation of the Hh pathway is primarily observed in the stromal microenvironment [Yauch et al. 2008] While established tissue arrays are a useful technique for immunohistochemical analysis, the laser microdissection would have helped us separate the stromal and tissue sections to better analyze the expression levels of the proteins involved in the pathway. Since the expression in the pathway arises from the stromal component while our study focuses on the expression levels within lung tumors, our expression levels would certainly be low to account for the fact that the stromal component is not taken into account. The above-mentioned study also found increased levels of Shh in cancer cell lines with KRAS gene mutation in comparison to normal KRAS function.
Moreover, it is noted that approximately 10% of female nonsmokers had an epidermal growth factor receptor (EGFR) mutation [Dacic et al. 2010]. However, data suggests that there is emerging evidence of cooperation between EGFR and hedgehog pathway. Eberl and colleagues have found that Hh/Gli cooperation with EGRF signaling promotes transformation of and cancer cell progression in vitro [Eberl et al. 2012]. In addition, a study conducted by Raz and colleagues examined the correlation between immunohistochemical staining, RDRF and KRAS mutations, and OS [Raz et al. 2012]. They found a 6.8% EGRF mutation and no correlation between the pathway proteins and overall survival. Moreover, Mimeult and Batra also found that the Hh/Gli cascade implicates the cooperation of other oncogenic products including mutated KRAS and EGFR [Mimeult and Batra, 2010].
A recent study by Liao and colleagues revealed a significant correlation between considerably elevated levels of Gli-1 and Ptch-1 protein expression with poor overall survival of ovarian cancer patients [Liao et al. 2009]. Patients with cancers that expressed high Gli-1 expression had an inferior survival (37.3 ± 8.7 months) than patients with cancers having lower Gli-1 expression (128.2 ± 14 months). In the same way, patients with elevated levels of Ptch-1 also had decreased survival (38.7 ± 7.4 months) compared with those with lower Ptch-1 expression (130.3 +/- 14.3 mo). Gli-1 expression is also related to disease-free survival. Their data further revealed that Gli-1, not Ptch-1, was an independent prognostic factor adjusted by tumor grade, stage, histologic type and patients’ age. Furthermore, studies have also confirmed the role for Hh signaling in advanced prostate cancer through the examination of the autocrine signaling by tumor cells that is crucial for proliferation, viability, and invasive behavior of the tumor. Datta and Datta discovered that blocking the Hh signaling results in tumor shrinkage and remission in preclinical tumor xenograft models, which is significant in terms of targeted therapy for prostate cancer [Datta and Datta, 2006]. In a study examining the Hh signaling in airway epithelial progenitors and in small cell lung cancer, Watkins and colleagues demonstrated that small cell lung cancer cells display a persistent activation of the Hh signaling pathway and a discernible reduction in their ability to signal to adjacent cells [Watkins et al. 2003]. Data also demonstrated that lung cancer cells recapitulate different aspects of Shh signaling seen in lung development and repair mechanism. In addition, Fujita and colleagues examined human lung squamous cell carcinoma cells and found positive expression of Shh in the squamous carcinoma, while noting its disappearance in normal lung tissues of the same patient [Fujita et al. 1997]. Shh in general seems to disappear in the adult human lung epithelial cells during the aging process, but it seems to become prevalent in the lung squamous carcinoma cells.
In summary, the present study confirmed elevated Shh expression in NSCLC but its expression level or the low expression of the additional Hh pathway proteins did not correlate with prognostic factors in NSCLC including patient survival. Our analysis is limited by the small sample size and inherent limitations of its retrospective nature. However, this study could instigate larger population-based studies or analyses of larger registry data to study whether Shh pathway activation is predictive of prognosis of lung cancer patients.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Malvi Savani, Division of Radiation Oncology, Department of Medicine, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA.
Yan Guo, Division of Radiation Oncology, Department of Medicine, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA.
David P. Carbone, Division of Radiation Oncology, Department of Medicine, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA
Ildiko Csiki, Division of Radiation Oncology, Vanderbilt University Medical Center, 22nd at Pierce Avenue, B1034, Nashville, TN 37232-5671, USA.
References
- Brunner M., Thurnher D., Pammer J., Heiduschka G., Petzelbauer P., Schmid C., et al. (2010) Expression of hedgehog signaling molecules in Merkel cell carcinoma. Head Neck 32: 333–340 [DOI] [PubMed] [Google Scholar]
- Dacic S., Shuai Y., Yousem S., Ohori P., Nikiforova M. (2010) Clinicopathological predictors of EGFR/KRAS mutational status in primary lung adenocarcinomas. Mod Pathol 23: 159–168 [DOI] [PubMed] [Google Scholar]
- Datta S., Datta M.W. (2006) Sonic Hedgehog signaling in advanced prostate cancer. Cell Mol Life Sci 63: 435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl M., Klingler S., Mangelberger D., Loipetzberger A., Damhofer H., Zoidl K., et al. (2012) Hedgehog-EGRF cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cells. EMBO Mol Med 4: 218–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E., Khoroku Y., Urase K., Tsukahara T., Momoi M., Kumagai H., et al. (1997) Involvement of Sonic hedgehog in the cell growth of LK-2 cells, human lung squamous carcinoma cells. Biochem Biophys Res Commun 238: 658–664 [DOI] [PubMed] [Google Scholar]
- Gupta S., Takebe N., Lorusso P. (2010) Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol 2: 237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham P.W., McMahon A.P. (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15: 3059–3087 [DOI] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Xu J., Ward E. (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300 [DOI] [PubMed] [Google Scholar]
- Lauth M., Bergström A., Shimokawa T., Tostar U., Jin Q., Fendrich V., et al. (2010) DYRK1B-dependent autocrine-to-paracrine shift of Hedgehog signaling by mutant RAS. Nat Struct Mol Biol 17: 718–725 [DOI] [PubMed] [Google Scholar]
- Liao X., Siu M., Au C., Wong E., Chan H., Ip P., et al. (2009) Aberrant activation of hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion and differentiation. Carcinogenesis 30: 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeult M., Batra S. (2010) Frequent deregulation in the hedgehog signaling network and cross-talks with the epidermal growth factor receptor pathway involved in cancer progression and targeted therapies. Pharmacol Rev 62: 497–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J.P., Lewis B.C. (2007) Shh signaling and pancreatic cancer: implications for therapy? Cell Cycle 6: 1553–1557 [DOI] [PubMed] [Google Scholar]
- Olsen C.L., Hsu P.P., Glienke J., Rubanyi G.M., Brooks A.R. (2004) Hedgehog-interacting protein is highly expressed in endothelial cells but down-regulated during angiogenesis and in several human tumors. BMC Cancer 4: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz G., Allen K.E., Kingsley C., Cherni I., Arora S., Watanabe A., et al. (2012) Hedgehog signaling pathway molecules and ALDH1A1 expression in early-stage non-small cell lung cancer. Lung Cancer 76: 191–196 [DOI] [PubMed] [Google Scholar]
- Rubin L.L., de Sauvage F.J. (2006) Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov 5: 1026–1033 [DOI] [PubMed] [Google Scholar]
- Schneider S., Thurnher D., Kloimstein P., Leitner V., Petzelbauer P., Pammer J., et al. (2011) Expression of the Sonic hedgehog pathway in squamous cell carcinoma of the skin and the mucosa of the head and neck. Head Neck 33: 244–250 [DOI] [PubMed] [Google Scholar]
- Sinicrope F.A., Ruan S.B., Cleary K.R., Stephens L.C., Lee J.J., Levin B. (1995) bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res 55: 237–241 [PubMed] [Google Scholar]
- Vestergaard J., Pedersen M.W., Pedersen N., Ensinger C., Tümer Z., Tommerup N., et al. (2006) Hedgehog signaling in small-cell lung cancer: frequent in vivo but a rare event in vitro. Lung Cancer 52: 281–290 [DOI] [PubMed] [Google Scholar]
- Waschek J.A., Cicco-Bloom E., Nicot A., Lelievre V. (2006) Hedgehog signaling: new targets for GPCRs coupled to cAMP and protein kinase A. Ann N Y Acad Sci 1070: 120–128 [DOI] [PubMed] [Google Scholar]
- Watkins D.N., Berman D.M., Burkholder S.G., Wang B., Beachy P.A., Baylin S.B., et al. (2003) Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 422: 313–317 [DOI] [PubMed] [Google Scholar]
- Watkins D.N., Peacock C.D. (2004) Hedgehog signalling in foregut malignancy. Biochem Pharmacol 68: 1055–1060 [DOI] [PubMed] [Google Scholar]
- Yauch R.L., Gould S.E., Scales S.J., Tang T., Tian H., Ahn C.P., et al. (2008) A paracrine requirement for hedgehog signalling in cancer. Nature 455: 406–410 [DOI] [PubMed] [Google Scholar]