Abstract
Study Design
Integrate theoretical and experimental approaches for annulus fibrosus (AF) functional tissue engineering.
Objective
Apply a hyperelastic constitutive model to characterize the evolution of engineered AF via scalar model parameters. Validate the model and predict the response of engineered constructs to physiologic loading scenarios.
Summary of Background Data
There is need for a tissue engineered replacement for degenerate AF. When evaluating engineered replacements for load-bearing tissues, it is necessary to evaluate mechanical function with respect to the native tissue, including nonlinearity and anisotropy.
Methods
Aligned nanofibrous poly-ε-caprolactone scaffolds with prescribed fiber angles were seeded with bovine AF cells and analyzed over 8 weeks using experimental (mechanical testing, biochemistry, histology) and theoretical methods (a hyperelastic fiber-reinforced constitutive model).
Results
The linear region modulus for φ = 0° constructs increased by ~25 MPa, and for φ = 90° by ~2 MPa from 1 day to 8 weeks in culture. Infiltration and proliferation of AF cells into the scaffold and abundant deposition of s-GAG and aligned collagen was observed. The constitutive model had excellent fits to experimental data to yield matrix and fiber parameters that increased with time in culture. Correlations were observed between biochemical measures and model parameters. The model was successfully validated and used to simulate time varying responses of engineered AF under shear and biaxial loading.
Conclusion
AF cells seeded on nanofibrous scaffolds elaborated an organized, anisotropic AF-like extracellular matrix, resulting in improved mechanical properties. A hyperelastic fiber-reinforced constitutive model characterized the functional evolution of engineered AF constructs, and was used to simulate physiologically relevant loading configurations. Model predictions demonstrated that fibers resist shear even when the shearing direction does not coincide with the fiber direction. Further, the model suggested that the native AF fiber architecture is uniquely designed to support shear stresses encountered under multiple loading configurations.
INTRODUCTION
The intervertebral disc confers stability, load transfer, motion, and energy dissipation to the spine. The annulus fibrosus (AF), a multi-lamellar fiber-reinforced collagenous soft tissue, is a key contributor to disc mechanical function due to its complex hierarchical structure and composition. Each AF layer possesses a densely packed, aligned population of collagen fiber bundles with alternating orientation in adjacent lamellae by approximately ± 30° with respect to the transverse axis of the spine1, 2. The oriented collagen fibers are embedded in nonfibrillar material comprised largely of hydrated proteoglycans. The AF organization and composition provide for complex mechanical behaviors that are nonlinear, anisotropic (direction dependent) and viscoelastic (rate dependent); these behaviors are key to disc function. Disc degeneration cascades from an unknown origin, in which the soft, hydrated nucleus pulposus progressivey becomes stiffer and more fibrous. Concomitant with this transformation in the nucleus pulposus, structural organization and biochemical composition of the AF are compromised, coincident with mechanical degradation3 . The sequelae of these alterations include tears and fissures of the AF, along with disc height loss, herniation, low back pain, and spinal stenosis. No treatment is available to restore the degenerated AF or nucleus pulposus. Current surgical treatments, such as discectomy, fusion, and total disc arthroplasty may alleviate pain, but fail to restore the function to the disc and may lack long term efficacy. There is a recognized need for an engineered replacement tissue for degenerate AF to both assuage lower back pain and restore disc function4.
Recent advances in AF tissue engineering have demonstrated the phenotypic stability of intervertebral disc cells in vitro and their ability to generate disc-like tissue in 3-D culture5–9. In some studies, scaffolding materials have been used to instruct organized extracellular matrix (ECM) deposition by a resident cell population7, 10, 11. Mizuno, et. al., constructed an anatomically shaped composite disc seeded with AF and nucleus pulposus cells and demonstrated increases in compressive properties of the construct following subcutaneous implantation in the mouse10. Despite the observed growth, constructs did not reach native disc mechanical properties. Further, although the gross morphology of the disc was replicated in the composite constructs, the fiber alignment and multilamellar organization of the native AF were not achieved. Shao and Hunter developed a scaffold consisting of unidirectionally aligned chitosan/alginate fibers and observed aggregation of AF cells along the organized fibers7. However, deposition of oriented ECM and construct mechanical properties were not investigated. Nerurkar et al. have recently utilized aligned electrospun nanofibrous scaffolds to recapitulate AF micro-architecture, with marked increases in ECM deposition and mechanical properties by AF cells, demonstrating promise for this technique in AF tissue engineering11.
It has become increasingly recognized that when evaluating load-bearing tissue engineered constructs such as the AF, it is not sufficient to simply address histological and biochemical outcomes12. It is necessary to evaluate mechanical function of the engineered construct with respect to the native tissue mechanics. Furthermore, complex mechanical behaviors require consideration, including nonlinearity and anisotropy; thus a linear measure of mechanical function such as modulus may not sufficiently assess the construct function with respect to native tissue benchmarks. Hyperelastic constitutive modeling, which has a long history in the study of the native AF 13–19 and other fiber-reinforced tissues, can provide insight into construct material behavior along the entire loading regime. Additionally, the terms of a structurally-based strain energy model have physical significance that can be particularly advantageous in understanding and redirecting the evolution of engineered tissue structure-function relationships.
Structural constitutive models have recently been utilized to study acellular electrospun mesh mechanical behavior20, 21. Studies like these are useful to determine application-specific design criteria for tissue engineering scaffold fabrication; however, the models were not used to evaluate cell-mediated changes in engineered tissue mechanics. The time-dependent functional growth of AF cell-seeded aligned electrospun nanofibrous scaffolds was quantified by Nerurkar et al. using a linear homogenization model that had been previously applied to native AF22. While this approach was limited by a model formulation that did not account for the material nonlinearity, it yielded quantitative measures of AF cell-mediated changes in construct mechanics. Together these studies provide strong evidence for the power of utilizing hyperelastic constitutive modeling to assess and optimize AF functional tissue engineering.
The objective of this study was to integrate theoretical and experimental approaches for functional tissue engineering of the AF. Aligned nanofibrous scaffolds were seeded with AF cells, and assayed for functional growth using experimental methods (e.g., mechanical testing, biochemistry, histology). A hyperelastic fiber-reinforced constitutive model formulation was used to characterize time-dependent evolution of the engineered AF. The constitutive model accounted for nonlinearity and anisotropy and provided scalar parameters associated with physical tissue function (e.g., fiber and matrix properties). These parameter values evolved with culture duration, elucidating physical mechanisms of functional growth. The model was validated and then used to predict time-dependent changes in the simulated response of engineered AF constructs to physiologic loading scenarios including biaxial and shear loading.
MATERIALS AND METHODS
Scaffold Fabrication
Aligned nanofibrous scaffolds of poly-ε-caprolactone (PCL, Sigma Chemicals, St. Louis, MO) were electrospun as described previously11, 23. Fibers were collected for 5 – 6 hours on a grounded shaft rotating at 7,500 rpm, yielding an aligned nanofibrous sheet approximately 250 µm thick. To create samples with discrete fiber orientations of 0°, 30°, and 90°, rectangular samples (5 mm × 30 mm) were excised from the nanofibrous sheet with the long axis of the sample rotated by an angle, φ, with respect to the prevailing fiber direction11.
AF Cell Isolation and Maturation of Cell-Laden Constructs
AF cells were isolated from the caudal discs of skeletally mature bovine donors (Moyer Packing Co., Souderton, PA) within 12 hours of slaughter as described previously11. Scaffolds with three fiber orientations (φ = 0°, 30°, 90°) were sterilized, rehydrated, and coated with 20 µg/mL fibronectin (Invitrogen, Carlsbad, CA). Second passage AF cells were seeded onto scaffolds at a density of 1 × 106 per construct, and scaffolds were cultured in 6 well plates, in 3 mL of a chemically defined growth medium comprised of Dulbecco’s modified eagle’s medium (Invitrogen), 0.1µM dexamethasone (Sigma), 40 µg/mL L-Proline (Sigma), 100 µg/mL Sodium Pyruvate (Sigma), 1% Insulin, Transferrin, Selenium/Premix (BD Biosciences, San Jose, CA), and 1% penicillin, streptomycin and fungizone supplemented with 10 ng/mL Transforming Growth Factor β-3 (R&D Systems, Minneapolis, MN)24.
Mechanical Testing and Biochemistry
After 1 day, 4 and 8 weeks in free swelling culture, intact cell-laden scaffolds from each orientation (φ = 0°, 30°, 90°) were collected for uniaxial tensile testing followed by biochemical analyses (n = 4 – 5 per φ per time point). Prior to testing, sample thickness was determined at three points along the length of each sample using a custom system25. Width and gage length were measured by digital calipers. Samples were lightly airbrushed with black enamel paint to generate texture for strain analysis (below). Testing in uniaxial tension was performed using an Instron 5542 (Canton, MA). All samples were placed into customized serrated grips and maintained in a phosphate buffered saline bath during the testing protocol described previously11. Briefly, following a 5 minute preload and 15 cycles of preconditioning, a quasi-static constant elongation test was performed until failure at a rate of 0.1% strain/sec. This protocol has been commonly used for testing native AF in uniaxial tension, and is sufficiently slow to eliminate viscoelastic effects.14, 26, 27 During the test, strain control was performed via crosshead displacement and images of the sample midsubstance were collected per 1.0% strain. Images were analyzed by texture correlation to determine the local surface deformations, and two-dimensional components of Lagrangian strain (E) were calculated (Vic-2D, Correlated Solutions Inc., Columbia, SC). Modulus was calculated by fitting a linear regression to the linear region of the resulting stress-strain plot for each sample.
Biochemical assays were performed to determine sulfated glycosaminoglycan (s-GAG), total collagen, and DNA content as described previously24. After tensile testing, samples were digested for 16 hours in papain (Sigma) at 60°C. Digested samples were analyzed for s-GAG content using the 1,9-dimethylmethylene blue dye-binding assay (Sigma), for orthohydroxyproline (OHP) content (after acid hydrolysis) by reaction with chloramine T (Sigma) and dimethylaminobenzaldehyde (Sigma) and for DNA content using the PicoGreen dsDNA Quantification kit (Molecular Probes, Eugene, OR). OHP was converted to collagen content for a 1:10 ratio of OHP:collagen24, 28, 29.
Histology and Actin Staining
Samples for each angle (n = 2 per φ per time point) were fixed overnight in 4% paraformaldehyde at 4°C, embedded in OCT freezing medium (Sakura Finetek USA Inc., Torrance, CA) and flash frozen in liquid nitrogen. Embedded samples were cryosectioned longitudinally to 8 µm thickness perpendicular to the fiber plane and stained for collagen with Picrosirius Red, for cellular nuclei with Haematoxylin and Eosin (H&E), and for s-GAG with Alcian Blue (Sigma). Additional 8 week samples for φ = 30° were sectioned in the fiber plane, and stained with Picrosirius Red to visualize collagen orientation (n = 2). Collagen orientation was measured by quantitative polarized light microscopy as described previously30. Cytoskeletal actin was visualized at 3 days23. Briefly, filamentous actin was labeled with Alexafluor 488 conjugated phalloidin (Invitrogen). Cell nuclei were counterstained with DAPI (Invitrogen). The time point of 3 days was selected for optimal visibility, as at earlier time points the cells are sparsely seeded, while at later time points, confluence and abundant ECM deposition obscured cell borders. Samples were imaged at 20× on a Nikon T30 inverted fluorescent microscope equipped with a CCD camera and the NIS Elements software package (Nikon Instruments, Melville, NY).
Constitutive Model
Hyperelastic materials are described by a scalar strain energy density function, W, that can describe material behaviors for finite and nonlinear deformations. Determining the optimal form of W to capture the complex behavior of biological materials is an active area of study14, 31–36. Modifications to hyperelastic constitutive models have been developed to account for the anisotropy of fiber-reinforced composites37 by prescribing special properties along axes of anisotropy, representing fiber populations. A hyperelastic fiber-reinforced constitutive model was formulated to model the tensile behavior of AF cell-seeded scaffolds. The strain energy density function (W) for cell-seeded constructs was decomposed into the sum of a matrix strain energy (Wm) and a fiber strain energy (Wf)37. This approach was used in place of a constrained mixture model in order to eliminate the need to calculate mass fractions. Determination of mass fractions of fibers and extrafibrillar material is not experimentally straightforward for engineered fibrocartilage tissues; an advantage of the theory outlined in Spencer (1972) is that these mass fractions are absorbed into the modulus-type parameters in the model (see below). Throughout the discussion of the model formulation, implementation, and results, we refer to cell-deposited extracellular material as 'ECM', while the term 'matrix' is reserved for nonfibrillar material such as s-GAGs, which we assume contribute only to isotropic material behavior. The model ‘fiber’ phase refers to the fibrous ECM, such as collagen, as well as polymeric nanofibers of the scaffold. Scaffold fibers and newly deposited collagen fibers were assumed to lie along the same direction; this assumption was verified by experimental results (see below).
The matrix phase was described as a Neo-Hookean compressible material38 according to:
| (1) |
where µ and are the two scalar material parameters that characterize the matrix mechanics. µ is related to the modulus of matrix, while υ is related to matrix compressibility (the proclivity of a material to change volume during a given deformation). The scalars I1 = trC and I3 = det(C) are invariants of the Right Cauchy Green Tensor C = FTF, where F is the deformation gradient tensor39. The fiber phase was described according to an exponential law commonly used for fibrous soft tissues14, 19, 38, 40:
| (2) |
where γ and ξ are the two scalar material parameters that characterize fiber mechanics. γ is related to the fiber modulus, while ξ represents the degree of stress-strain nonlinearity of the fiber material. The scalar I4 is an invariant defined as I4 = a · Ca, where a is a unit vector along the fiber direction37. To account for the inability of the fiber phase to resist compressive deformations, I4 was set to unity for I4 < 141. From (Eq. 1) and (Eq. 2), the full constitutive law can be written according to as follows:
| (3) |
The reorientation of fibers was accounted for by , where ao is the original fiber direction, which deforms to the new fiber direction a according to the deformation gradient F. Because T is a second order tensor, the tensoral equation (Eq. 3) is a system of nine equations for the nine components of Cauchy stress. In summary, the complete model contains four scalar material parameters: µ and ν to describe the matrix phase, and γ and ξ to represent the fiber phase.
In order to apply the model to experimental tensile test data for engineered AF constructs, we adhered to the following coordinate system throughout the study: the loading direction for all experiments was along the x1 direction, and 2D strains were measured in the fiber-containing plane formed by the x1 and x2 axes. φ is the angle formed between the fiber direction a and the loading axis (x1) (Fig. 1A). For uniaxial and biaxial loading in the x1–x2 plane, the nonzero components of Eq. 3 in component form are:
| (4a) |
| (4b) |
| (4c) |
where λi are stretch ratios along the directions xi for i=1,2,3. For uniaxial tension along the x1 direction, T22 = 0.
Figure 1.
Schematic representation (A) of coordinate system for uniaxial tension (T11) with fiber angle (φ) indicated by the unit vector a. Representative curves (B) for engineered AF (φ = 0°) demonstrate a nonlinear response in uniaxial tension. The nonlinearity and modulus increased from 1 day to 8 weeks of culture. The model (solid lines) successfully fit experimental tensile behavior (open circles).
Least squares curve fits of the model stress to full experimental stress-strain curves for φ = 90° samples were performed to yield values for the two matrix parameters, µ and ν, at 1 day, 4 and 8 weeks. For each sample, two-dimensional strains were measured during tensile tests, and extrapolated to three dimensional strains according to a Poisson’s ratio ν13, computed as part of the curve fit. These values were used to compute model stresses, which were fit simultaneously to the three normal components of the Cauchy stress: T11 was experimentally measured during the tensile test, while a traction free boundary condition was enforced for the transverse stresses (T22 = T33 = 0). This condition ensured uniqueness of the resulting parameter values. For uniaxial tension along φ = 90°, I4 = 1 and the fiber term in (Eq. 3) is identically zero, leaving the fiber parameters γ and ξ undetermined. Least squares curve fits of the model to full stress-strain curves for φ = 0° samples were performed to yield values for the two fiber parameters, γ and ξ, at each 1 day, 4 and 8 weeks. For each time point, the corresponding average values of µ and ν (obtained above) were employed. Out of plane strains for φ=0° were assumed based on transversely isotropic material symmetry (E33 = E22). As above, the fit was constrained by traction free boundary conditions. The constitutive law used in this study is a simple form used widely in biomechanics applications. For further details on model robustness and parameter sensitivity the reader is directed to Guerin and Elliott, 2007.14
Upon calculation of the four material parameters (µ, ν, γ, and ξ) from the φ=0° and 90° data sets at each 1 day, 4 weeks and 8 weeks, model validation was carried out by predicting the stress-strain behavior of φ=30° samples. Because ν13 varies with φ42, its value was determined by least squares curve fitting of the traction free boundary conditions for each parameter set and sample-specific 2-D deformations. The resulting 3-D strain data was used with time-point matched material parameter values to compute a model-predicted stress T11. Agreement of the model predicted stress with the corresponding experimental stress was assessed to indicate the suitability of the proposed model as a full quantitative description of engineered AF mechanics during maturation. The contribution of fiber and matrix phases to the uniaxial tensile response at φ = 30° was computed by additive decomposition of (Eq. 3) into fiber stress (Tf) and matrix stress (Tm). Fraction of total stress for the fibers was computed as . Growth related changes in the fiber contribution were determined by using the material parameters associated with 1 day and 8 weeks.
Model Simulations
Two simulations to compute stresses under physiological loading of engineered AF mechanics were performed for φ = 30°: 1) shear stresses associated with uniaxial and biaxial extension, analogous to AF loading during axial compression, flexion, and extension, and 2) circumferential and shear stresses associated with shear loading, analogous to torsion. First, because stress and strain tensors are not co-axial for anisotropic materials, normal deformations that preserve the rectangular shape of a body can generate shear stresses in addition to normal stresses. For φ = 30°, the shear stress T12 associated with uniaxial (λ1 >1) and equibiaxial (λ1 = λ2) extension were computed according to Eqn. 4c. For the uniaxial case, deformations along the loading axis were prescribed, and transverse strains were computed according to Poisson’s ratios either experimentally measured (time point matched ν12), or as described above (ν13). For the simulation of equibiaxial extension, plane strain was assumed, with equal extension along the x1 and x2 axes. Second, the model was used to simulate the shear response of engineered AF constructs and how the response evolved with time in culture. Shear was simulated as an isochoric deformation due to a force on the face perpendicular to x2 in the direction x1. The amount of shear (κ) was prescribed, and the resulting nonzero components of T were computed according to:
| (6a) |
| (6b) |
| (6c) |
Statistics
Significance (p≤0.05) was evaluated by one way ANOVA with a Bonferroni post hoc test. Goodness of model fits and predictions are reported in R2 and Bland Altman limits of agreement (bias ± standard dev), reported in MPa43. Correlations between model parameters and biochemical measures were determined by linear regression. Correlations were considered significant for p≤0.05.
RESULTS
Experimental
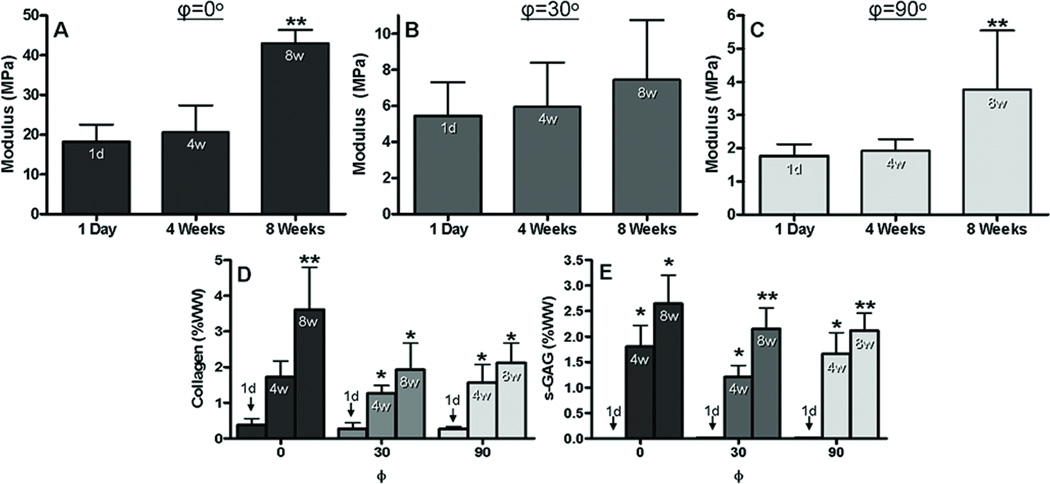
In this study, scaffolds with prescribed fiber angles (φ = 0°, 30°, 90°) were seeded with AF cells and cultured over 8 weeks. For φ = 0° and φ = 30°, constructs behaved nonlinearly in uniaxial tension and underwent finite elastic deformations (Fig. 1). New tissue growth was evident from a time-dependent improvement in mechanical behavior and biochemical composition for all fiber orientations (Fig. 2). From 1 day to 8 weeks, the linear region modulus for φ = 0° increased by ~25 MPa (138%, p<0.05, Fig. 2A), and for φ = 90° by ~2 MPa (118%, p<0.05, Fig. 2C). The modulus increase for φ = 30° was not significant (Fig. 2B). Collagen and s-GAG increased by 4 weeks, and continued to increase up to 8 weeks in culture (Fig. 2D,E). Average DNA content over all φ increased by nearly 5-fold, from 0.49 ± 0.14 µg at 1 day to 1.93 ± 0.74 µg by 8 weeks (p<0.05).
Figure 2.
Experimentally measured tensile moduli for φ = 0° (A), φ = 30° (B), and φ = 90° (C). Collagen (D) and s-GAG (E) increased with time for all φ. WW = wet weight. 1d = 1 day, 4w = 4 weeks, and 8w = 8 weeks. * = p<0.05 compared to day 1, ** = p<0.05 compared to day 1 and 4 weeks. n = 4 –5 per group.
Histologically, infiltration and proliferation of AF cells into the scaffold was observed by 8 weeks, with abundant and relatively uniform deposition of collagen and s-GAG (Fig. 3A–C). However, AF cell infiltration was restricted to the outer two-thirds of the scaffold thickness, with a dense border of cells at the periphery (Fig. 3B). At 4 weeks, infiltration into the scaffold was limited, and collagen and s-GAG, although readily detected, were less abundant (not shown). These findings agreed with bulk biochemical findings (Fig. 2D,E). AF cells were highly aligned with the underlying scaffold fiber direction (Fig. 3D). Cytoskeletal actin was organized into many prominent stress fibers, also oriented parallel to scaffold fibers. These observations are supported by previous work, in which the maintenance of cellular alignment was observed up to 4 weeks after seeding.23 Samples sectioned in-plane with the scaffold fibers revealed newly formed collagen, also organized along the prevailing fiber direction (arrow, Fig. 3E, F); however, a thin, randomly organized capsule of collagen around the construct perimeter was observed. The fiber angle of newly formed collagen in the center of the scaffold, measured by quantitative polarized light microscopy at 8 weeks, was 31.1° for φ = 30° samples.
Figure 3.
Histological sections of a φ = 90° sample at 8 weeks, stained with Picrosirius Red (A), H&E (B), and Alcian Blue (C). Actin (green) stained AF cells with counter-stained nuclei (blue) aligned with nanofibers, viewed in the plane of the scaffold at 3 days (D). Picrosirius Red stained fiber-plane sections for φ = 30° (E, higher magnification in F) revealed oriented collagen in the scaffold mid-substance (white arrow), and a thin disorganized capsule at the periphery (black asterisk). (F) Scale bar = 100 µm (A–C and F), 50 µm (D) and 200 µm (E).
Theoretical/Model Results
The constitutive model had an excellent fit to φ = 90° samples at all time points to yield matrix constants µ and ν (Fig. 4A,B, and Table 1), with an average R2 = 0.98 (n = 15). Both matrix constants increased with time, however not significantly, illustrating extra-fibrillar ECM deposition by AF cells. The constitutive model also had an excellent fit to φ = 0° samples (Fig. 1) to yield fiber parameters γ and ξ (Fig. 4C,D) with R2 = 0.98 (n = 13). γ increased significantly by 8 weeks (p < 0.05). ξ increased slightly by 4 weeks, however the change was not significant. Significant correlation was observed between the biochemical measures of collagen and the model fiber parameter γ (R = 0.81, Fig 5A) and between s-GAG and the model matrix parameter ν (R = 0.53, Fig 5B). No correlations were found between matrix parameters and collagen content or between fiber parameters and s-GAG content (p > 0.05).
Figure 4.
The model fit to uniaxial tensile responses for φ = 90° produced matrix parameters µ (A) and ν (B). The average values of µ and ν were used to fit φ = 0° at each corresponding time-point to yield fiber material parameters γ (C) and ξ (D). ** = p<0.05 compared to 1 day and 4 weeks. n = 4 – 5 per φ per time point.
Table 1.
Model fit and prediction measures. ν13 was computed as part of the curve fit. R2 and Bland Altman limits of agreement (BA) are reported as the mean difference ± average standard deviation of the mean difference. For φ = 0° and φ = 90°, R2 and BA represent the model fit, while for φ = 30°, they represent model prediction.
| ν13 |
R2 |
BA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| φ | 1 Day | 4 Weeks | 8 Weeks | 1 Day | 4 Weeks | 8 Weeks | 1 Day | 4 Weeks | 8 Weeks |
| 0° | 1.10 ± 0.62 | 0.72 ± 0.29 | 0.69 ± 0.19 | 0.97 ± 0.03 | 0.98 ± 0.02 | 0.99 ± 0.01 | −0.01± 0.05 | 0.01 ± 0.05 | 0.03 ± 0.10 |
| 30° | < 0.01 | < 0.01 | 1.09 ± 0.78 | 0.98 ± 0.03 | 0.99 ± 0.01 | 0.98 ± 0.03 | 0.13 ± 0.16 | 0.02 ± 0.05 | 0.35 ± 0.2 |
| 90° | 0.07 ± 0.04 | 0.20 ± 0.29 | 0.34 ± 0.34 | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.98 ± 0.01 | 0.00 ± 0.02 | 0.00 ± 0.01 | 0.00 ± 0.01 |
Figure 5.
Significant correlations were observed between the fiber parameter γ and the collagen content (A) and between the matrix parameter ν and the s-GAG content (B).
To validate the model, the measured strains for tensile tests of each φ = 30° sample were input into the model and a prediction of the resulting stress was computed using the four constant values determined above from φ = 0° and φ = 90°. These predicted stress-strain curves had excellent agreement with their corresponding experimental curves (Fig. 6, Table 1), with R2 = 0.98 (n = 13). Using the average values of parameters µ, ν, γ and ξ for 8 week AF constructs, the uniaxial tensile behavior for φ = 30° was decomposed into contributions from the fiber and matrix phases (Fig. 7). Fibers provided the predominant stress contribution, with fiber stress increasing with strain. Comparison at 1 day and 8 weeks revealed an upward shift in the stress contribution of the fiber phase (inset, Fig. 7).
Figure 6.
For 4 weeks, the model predicted stress-strain behavior at φ = 30° (solid lines) agreed well with the experimentally measured stress (circles). Predicted and experimental curves for each sample are paired by color.
Figure 7.
Uniaxial stress-strain response for φ = 30° at 8 weeks (Total), decomposed into matrix and fiber stresses. Inset: The fraction of total stress contributed by the fibers was computed at 1 day and 8 weeks.
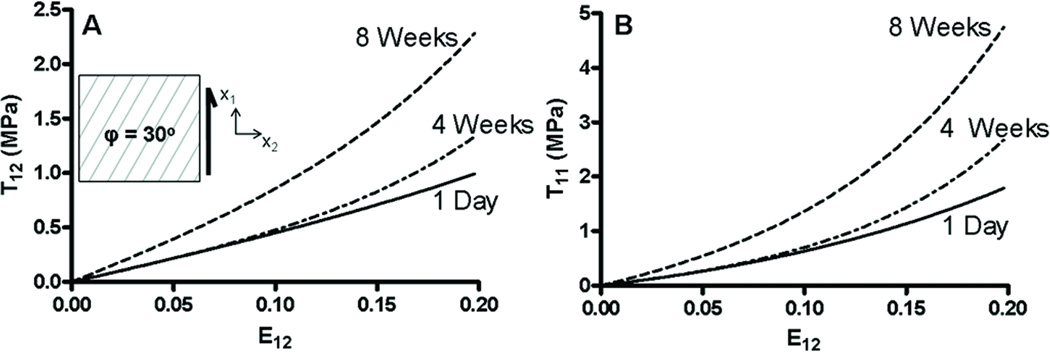
Because of the anisotropy of fiber-reinforced materials, normal deformations may generate shear stresses when the deformed configuration is constrained to a rectangular form (as in uniaxial and biaxial tensile testing). The computed magnitude of shear stress (T12) resulting from uniaxial extension (E11 = 0.10) varied nonlinearly with fiber angle for 1 day and 8 weeks (Fig 8A). Interestingly, shear stress peaked at approximately φ = 30°. Shear stress reduced to zero near φ = 60°, implying that, at E11 = 0.1, fibers were predominantly in compression; however, at much larger strains, fiber reorientation will eventually reestablish tensile (nonzero) fiber stresses. While the magnitude of shear stress increased from 1 day to 8 weeks, the dependence of shear stress on fiber angle was similar. Computing the full stress-strain curve for φ = 30° revealed a time-dependent increase in slope and nonlinearity of the shear stress that accompanied uniaxial extension (Fig. 8B). Similarly, equibiaxial extension in-plane with the fibers can generate shear stress when the fibers lie oblique to the loading axes. For φ = 30°, equibiaxial extension induced a larger and more nonlinear shear stress response than uniaxial tension (Fig. 8B). Simulating 1 day and 8 week responses illustrated that time-dependent effects were even more pronounced in biaxial tension.
Figure 8.
The dependence of shear stress T12 in uniaxial tension (computed at E11 = 0.10) on φ (A). Shear stress T12 associated with uniaxial (UA) and equibiaxial (EB) deformations (B). 1 day = solid lines, 8 weeks = dashed lines.
Finally, an isochoric shear deformation was simulated for 1 day, 4 and 8 week AF constructs, with shearing along the x1 direction for φ = 30° (inset, Fig. 9A). A considerable increase in the stress response of AF constructs was observed for both normal and shear stresses (Fig. 9A, B). Shear and normal stresses under shearing were similar in magnitude to uniaxial tensile tests along the fiber direction.
Figure 9.
Simulation of shear deformation, shown schematically (A, inset) produced both shear (A) and normal (B) stress responses.
DISCUSSION
This study integrated theoretical and experimental approaches for AF tissue engineering utilizing aligned electrospun nanofibrous scaffolds seeded with AF cells. We implemented a hyperelastic fiber-reinforced constitutive model to characterize the evolution of engineered AF constructs with time in culture. The constitutive model was successfully fit to stress-strain responses at each culture time point with nanofibrous scaffold orientations of φ = 0° and φ = 90°, and then was validated by successfully predicting the stress-strain response of φ = 30° constructs at each time point.
A key advantage of this approach was that the nonlinear stress-strain behavior of engineered AF constructs was fully described by the model (Fig. 1B). This is preferable to simply measuring linear modulus11, 23, 44, as under physiological conditions the AF operates throughout the nonlinear regime of both the ‘toe region’ (or ‘neutral zone’) and ‘linear region’. Another advantage is that the model material parameters have physical interpretations, representing mechanics of the extrafibrillar matrix (µ and ν) and fibrous ECM (γ and ξ). For instance, the observation that the linear region moduli increased over 8 weeks (Fig. 2) simply implies general construct growth. However, the increase in γ with time suggests that AF cells deposited oriented collagen that contributed to construct mechanical function. This is supported by the correlation of the fiber parameter γ with the experimentally measured collagen content (Fig. 5). Furthermore, although significance was not achieved, an increase in fiber property ξ with time may suggest that the degree of nonlinearity increased with culture duration. This may represent a shift in load-bearing from the scaffold to the newly deposited collagen fibers, as PCL remains linearly elastic over the culture duration, while collagen increased with time in culture, and confers nonlinearity to most biological tissues40, 45. The matrix parameters µ and ν also increased with time in culture (although not significantly), indicating that AF cells deposited extrafibrillar ECM such as s-GAG, and that this newly deposited matrix also contributed to mechanical function. This is supported by the positive correlation between the model matrix parameter, ν, and experimentally measured s-GAG. In uniaxial tension at φ = 30°, the total stress response was largely dictated by the fiber phase (Fig 7) and the relative fiber contribution increased with construct growth (Fig 7 inset), implying that AF cells elaborated fibrous ECM that improved the tensile load bearing capacity of constructs with an anatomic fiber orientation.
In addition to physical interpretation of model parameters as above, another advantage of the validated constitutive model is the ability to investigate the effect of construct growth for loading configurations encountered under physiologic conditions that cannot be readily measured. Specifically, we considered 1) multi-axial stresses encountered in uniaxial and biaxial extension, and 2) shear loading. Uniaxial tensile testing of φ ≠ 0° or 90° applies loads oblique to the fiber direction. This generates shear stresses in anisotropic materials because the grips are constrained to one-dimensional motion along the loading axis, despite a tendency of the material to undergo rotations. In other words, uniaxial loading oblique to the fibers generates reaction stresses in the transverse directions of the crosshead, opposed by shear stresses in the material. Because these shear stresses increase with culture duration, it is likely that newly deposited ECM enhances fiber-matrix continuity, and thereby encourages fiber reorientation under load. This improved load transfer shields matrix and interfibrillar materials from large strains at low to moderate stresses by allowing the fibrillar components to bear more stress, even when the loading direction does not coincide with the prevailing fiber direction (as is common in the native AF in vivo). This shielding effect is a critical requirement in preventing failure of fiber-reinforced soft tissues under physiologic loading, as the formation of tears and fissures in the AF has been attributed, to some extent, to delaminations and debonding between the matrix and fibers46, 47. The magnitude of shear stress varied with fiber angle, and, interestingly, was highest near the anatomic fiber angle φ = 30° (Fig. 8A). This result suggests that AF fibers may be optimally oriented to resist large circumferential strains of the disc, while also resisting other complex multi-axial deformations, including those occurring during torsion. In the engineered construct, this phenomenon was present in constructs at 1 Day, and was accentuated by ECM deposition with time in culture (Fig. 8A, B). These changes may be indicative of improved load transfer between fibers and matrix in these developing constructs.
Although uniaxial tension is a standard test to evaluate material properties, it does not represent AF physiologic loading: freely contracting boundaries do not occur in vivo for the constrained AF. All AF loading, including disc compression and bending, generate AF biaxial loads. Much larger magnitudes of shear stress were observed under equibiaxial extension (Fig. 8B). Like the uniaxial case, however, the shear stress for a given equibiaxial deformation increased from 1 day to 8 weeks of growth. This suggests that ECM deposition improved the performance of engineered AF under biaxial deformations. Finally, shear loading of the AF was modeled, representing torsion of the spine. We simulated the effect of a torsional motion of the spine on engineered AF as a shear stress applied 30° from the fiber direction (Fig. 9A, inset). The deformation produced nonzero shear and normal components of stress. Interestingly, the normal stresses T11 and T22 (not shown) were of similar magnitude to the shear stress T12, although slightly larger, demonstrating that fibers resist shear deformations, even when the shearing direction does not coincide with the prevailing fiber direction. This result is in contrast to the notion that shear behavior of fiber-reinforced tissues is driven primarily by the matrix48. As with uniaxial and biaxial loading, time-dependent growth of constructs revealed an improvement in the shear response of engineered AF with time in culture. These simulations not only provide insight into the physical mechanisms of functional growth of cell-laden constructs, but also indicate that AF cells deposit ECM on aligned elctrospun scaffolds that improve function over a wide range of mechanical environments that are of direct relevance to the intervertebral disc in vivo.
Anisotropic increases in modulus indicate that AF cells preferentially deposit ECM under the directional guidance of the aligned nanofibrous scaffold. AF cells aligned along the scaffold fibers, with numerous actin stress fibers observed parallel to the fiber direction (Fig. 3). During embryonic AF development and for various cell types in vitro, cellular orientation and actin patterning play an important role in the directional deposition of structurally organized ECM49–53. Similarly in this study, patterned ECM elaboration was evidenced by quantitative polarized light microscopy of sections in the scaffold fiber-plane, where the collagen fiber direction at 8 weeks closely mirrored the scaffold fiber direction. These results demonstrate that aligned electrospun scaffolds direct organized formation of new collagenous tissue by AF cells, and verify the model assumption that scaffold and collagen fiber orientations are defined by a single direction.
Of the three fiber orientations (φ =0°, 30°, 90°), the largest increase in modulus was observed along the fiber direction (φ = 0°), where over 8 weeks the modulus increased by approximately 25 MPa to 43 MPa. Despite this large increase, the fiber direction linear modulus remains below that of a single lamella of the AF (80 – 100 MPa)54, 55. This result is in contrast to a previous study11, in which no increase was observed for φ = 0°. The difference is likely due to modifications to the testing protocol, including the use of serrated grips and optical surface strains to reduce the effects of grip slippage. Additionally, scaffold thickness was reduced from 1 mm to 250 µm to enhance the relative infiltration of AF cells into the scaffold.
In this study, we provided a framework for the rigorous functional evaluation of engineered AF, with specific application to the mechanics of a single lamella. Although tissue engineering treatments for degenerative disc disease remain beyond the realm of clinical practice, the current work may contribute to the development of annular patches for the treatment of disc herniation. Moreover, development of multilamellar fibrous constructs, when coupled with an engineered nucleus pulposus material, may eventually lead to fully biologic intervertebral disc replacements 56.
In conclusion, this study integrated theoretical and experimental approaches for AF functional tissue engineering. We implemented a hyperelastic fiber-reinforced constitutive model to characterize the functional time-dependent evolution of engineered AF constructs. The constitutive model parameters elucidated AF fiber and matrix functional growth. The model was validated and then applied to simulate complex physiologically relevant loading configurations including shear strains in tensile loading, biaxial loading, and shear loading. Finally, utilizing standard assays, we demonstrated that AF cells seeded on electrospun nanofibrous scaffolds elaborate aligned, organized disc-like ECM, correlating with measurable changes in mechanical behavior.
Acknowledgements
This work was funded by NIH grant EB0242. The authors gratefully acknowledge An M. Nguyen and Pamela Tsing for their help with data collection.
REFERENCES
- 1.Hickey DS, Hukins DW. X-ray diffraction studies of the arrangement of collagenous fibres in human fetal intervertebral disc. J Anat. 1980;131(Pt 1):81–90. [PMC free article] [PubMed] [Google Scholar]
- 2.Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23(1):75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- 3.Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc annulus fibrosus. Spine. 1990;15(5):402–410. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Richardson SM, et al. Intervertebral disc biology, degeneration and novel tissue engineering and regenerative medicine therapies. Histol Histopathol. 2007;22(9):1033–1041. doi: 10.14670/HH-22.1033. [DOI] [PubMed] [Google Scholar]
- 5.Gruber HE, et al. Three-dimensional culture of human disc cells within agarose or a collagen sponge: assessment of proteoglycan production. Biomaterials. 2006;27(3):371–376. doi: 10.1016/j.biomaterials.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Chou AI, et al. The effect of serial monolayer passaging on the collagen expression profile of outer and inner anulus fibrosus cells. Spine. 2006;31(17):1875–1881. doi: 10.1097/01.brs.0000229222.98051.9a. [DOI] [PubMed] [Google Scholar]
- 7.Shao X, Hunter CJ. Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J Biomed Mater Res A. 2007;82(3):701–710. doi: 10.1002/jbm.a.31030. [DOI] [PubMed] [Google Scholar]
- 8.Wan Y, et al. Novel Biodegradable Poly (1,8-octanediol malate) for Annulus Fibrosus Regeneration. Macromol Biosci. 2007 doi: 10.1002/mabi.200700053. [DOI] [PubMed] [Google Scholar]
- 9.Chang G, et al. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine J. 2007 doi: 10.1007/s00586-007-0364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuno H, et al. Biomechanical and biochemical characterization of composite tissue-engineered intervertebral discs. Biomaterials. 2006;27(3):362–370. doi: 10.1016/j.biomaterials.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 11.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25(8):1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 12.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122(6):570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 13.Wu HC, Yao RF. Mechanical behavior of the human annulus fibrosus. Journal of Biomechanics. 1976;9:1–7. doi: 10.1016/0021-9290(76)90132-9. [DOI] [PubMed] [Google Scholar]
- 14.Guerin HL, Elliott DM. Quantifying the contributions of structure to annulus fibrosus mechanical function using a nonlinear, anisotropic, hyperelastic model. J Orthop Res. 2007;25(4):508–516. doi: 10.1002/jor.20324. [DOI] [PubMed] [Google Scholar]
- 15.Wagner DR, Lotz JC. Theoretical model and experimental results for the nonlinear elastic behavior of human annulus fibrosus. J Orthop Res. 2004;22(4):901–909. doi: 10.1016/j.orthres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Klisch SM, Lotz JC. Application of a fiber-reinforced continuum theory to multiple deformations of the annulus fibrosus. Journal of Biomechanics. 1999;32:1027–1036. doi: 10.1016/s0021-9290(99)00108-6. [DOI] [PubMed] [Google Scholar]
- 17.Sun DD, Leong KW. A nonlinear hyperelastic mixture theory model for anisotropy, transport, and swelling of annulus fibrosus. Ann Biomed Eng. 2004;32(1):92–102. doi: 10.1023/b:abme.0000007794.87408.1e. [DOI] [PubMed] [Google Scholar]
- 18.Caner FC, et al. Hyperelastic Anisotropic Microplane Constitutive Model for Annulus Fibrosus. J Biomech Eng. 2007;129(5):632. doi: 10.1115/1.2768378. [DOI] [PubMed] [Google Scholar]
- 19.Eberline R, Holzapfel GA, Schulze-Bauer CA. An anisotropic constitutive model for annulus tissue and enhanced finite element analyses of intact lumbar disc bodies. Computer Methods in Biomechanics and Biomedical Engineering. 2001;4:209–230. [Google Scholar]
- 20.De Vita R, et al. A Constitutive Law for Poly(butylene terephthalate) Nanofiber Mats. Journal of Applied Polymer Science. 2006;102:5280–5283. [Google Scholar]
- 21.Courtney T, et al. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27(19):3631–3638. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Yin L, Elliott DM. A homogenization model of the annulus fibrosus. J Biomech. 2005;38(8):1674–1684. doi: 10.1016/j.jbiomech.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Li WJ, et al. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40(8):1686–1693. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14(2):179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Soslowsky LJ, et al. Geometric and mechanical properties of the coracoacromial ligament and their relationship to rotator cuff disease. Clin Orthop Relat Res. 1994;(304):10–17. [PubMed] [Google Scholar]
- 26.Elliott DM, Setton LA. Anisotropic and inhomogeneous tensile behavior of the human anulus fibrosus: experimental measurement and material model predictions. J Biomech Eng. 2001;123:256–263. doi: 10.1115/1.1374202. [DOI] [PubMed] [Google Scholar]
- 27.Guerin HA, Elliott DM. Degeneration affects the fiber reorientation of human annulus fibrosus under tensile load. J Biomech. 2006;39(8):1410–1418. doi: 10.1016/j.jbiomech.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Vunjak-Novakovic G, et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17(1):130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 29.Hollander AP, et al. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96(6):2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gimbel JA, et al. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Humphrey JD, Yin FCP. New constitutive formulation for characterizing the mechanical behavior of soft tissues. Biophysical Journal. 1987;52:563–570. doi: 10.1016/S0006-3495(87)83245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bischoff JE. Continuous versus discrete (invariant) representations of fibrous structure for modeling nonlinear anisotropic soft tissue behavior. International Journal of Non-Linear Mechanics. 2006;41(2):167–179. [Google Scholar]
- 33.Gasser TC, Ogden RW, Holzapfel GA. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J R Soc Interface. 2006;3(6):15–35. doi: 10.1098/rsif.2005.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klisch SM. A bimodular theory for finite deformations: Comparison of orthotropic second-order and exponential stress constitutive equations for articular cartilage. Biomech Model Mechanobiol. 2006;5(2–3):90–101. doi: 10.1007/s10237-006-0027-0. [DOI] [PubMed] [Google Scholar]
- 35.Limbert G, Middleton J. A constitutive model of the posterior cruciate ligament. Med Eng Phys. 2006;28(2):99–113. doi: 10.1016/j.medengphy.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Wagner DR, Reiser KM, Lotz JC. Glycation increases human annulus fibrosus stiffness in both experimental measurements and theoretical predictions. J Biomech. 2006;39(6):1021–1029. doi: 10.1016/j.jbiomech.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Spencer AJM. Deformations of Fibre-reinforced Materials. London: Oxford University Press; 1972. p. 128. [Google Scholar]
- 38.Holzapfel GA. Nonlinear Solid Mechanics: A Continuum Approach for Engineering. 2000. p. 455. [Google Scholar]
- 39.Ogden RW. Non-Linear Elastic Deformations. 1997. [Google Scholar]
- 40.Fung YC. Biomechanics: Mechanical Properties of Livign Tissues. 2nd Ed. 1982. p. 568. [Google Scholar]
- 41.Ateshian GA. Anisotropy of fibrous tissues in relation to the distribution of tensed and buckled fibers. J Biomech Eng. 2007;129(2):240–249. doi: 10.1115/1.2486179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nerurkar N, et al. Engineering of fiber-reinforced tissue with anisotropic biodegradable nanofibrous scaffolds. Transactions of IEEE Engineering in Medicine and Biology Society. 2006 doi: 10.1109/IEMBS.2006.259395. [DOI] [PubMed] [Google Scholar]
- 43.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–310. [PubMed] [Google Scholar]
- 44.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28(11):1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanir YA. structural theory for the homogeneous biaxial stress-strain relationships in flat collagenous tissues. J Biomech. 1979;12(6):423–436. doi: 10.1016/0021-9290(79)90027-7. [DOI] [PubMed] [Google Scholar]
- 46.Pezowicz CA, et al. Mechanisms of anular failure resulting from excessive intradiscal pressure: a microstructural-micromechanical investigation. Spine. 2006;31(25):2891–2903. doi: 10.1097/01.brs.0000248412.82700.8b. [DOI] [PubMed] [Google Scholar]
- 47.Iatridis JC, ap Gwynn I. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J Biomech. 2004;37(8):1165–1175. doi: 10.1016/j.jbiomech.2003.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss JA, Gardiner JC, Bonifasi-Lista C. Ligament material behavior is nonlinear, viscoelastic and rate-independent under shear loading. J Biomech. 2002;35(7):943–950. doi: 10.1016/s0021-9290(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 49.Hayes AJ, Benjamin M, Ralphs JR. Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly. Dev Dyn. 1999;215(3):179–189. doi: 10.1002/(SICI)1097-0177(199907)215:3<179::AID-AJA1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 50.Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20(2):107–121. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 51.Canty EG, et al. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J Biol Chem. 2006;281(50):38592–38598. doi: 10.1074/jbc.M607581200. [DOI] [PubMed] [Google Scholar]
- 52.Wang JH, et al. Cell orientation determines the alignment of cell-produced collagenous matrix. J Biomech. 2003;36(1):97–102. doi: 10.1016/s0021-9290(02)00233-6. [DOI] [PubMed] [Google Scholar]
- 53.Lee CH, et al. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26(11):1261–1270. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 54.Skaggs DL, et al. Regional variation in tensile properties and biochemical composition of the human lumbar anulus fibrosus. Spine. 1994;19(12):1310–1319. doi: 10.1097/00007632-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Holzapfel GA, et al. Single lamellar mechanics of the human lumbar anulus fibrosus. Biomech Model Mechanobiol. 2005;3(3):125–140. doi: 10.1007/s10237-004-0053-8. [DOI] [PubMed] [Google Scholar]
- 56.Nerurkar NL, et al. Multiscale tissue engineering of the intervertebral disc; Transactions of the 54th Annual Meeting of the Orthopaedic Research Society; 2008. [Google Scholar]