Abstract
Objective: To assess the effects of Light Formulation, an oil-in-water emulsion, and Rich Formulation, a water-in-oil emulsion, for the treatment of xerosis. Design: Two double-blind, vehicle-controlled trials (both formulations); a double-blind, randomized regression study (Rich Formulation); and a single-blind tolerability study (Light Formulation). The two formulations were applied twice daily for two weeks, for five days in the regression study, and twice daily for two weeks in the tolerability study. Setting: Studies were conducted during winter in Hamburg, Germany. Participants: A total of 169 subjects were enrolled and 154 completed the studies. The majority were between 50 and 80 years of age, women, all with very dry skin. One withdrew because of an incompatibility reaction that reoccurred with the subject's own body lotion after sun exposure. Measurements: Skin hydration and skin barrier function with both formulations over two weeks, long-term moisturization effect after discontinuation of Rich Formulation, and symptom improvement and skin tolerability with Light Formulation. Results: Vehicle-controlled studies of Light and Rich Formulations demonstrated significantly improved hydration at Weeks 1 and 2 versus the untreated site and vehicles, and significantly reduced transepidermal water loss versus untreated site and basic vehicle. Both products significantly decreased visible dryness and tactile roughness. In the regression study, Rich Formulation maintained significant moisturization six days after treatment discontinuation. Light Formulation reduced symptoms of itching, burning, tightness, tingling, and feeling of dryness. Conclusion: These formulations represent a new approach for the treatment of xerosis by addressing multiple key deficiencies in skin hydration.
Xerosis is a common condition experienced by millions of people either chronically or acutely in response to changes in the environment, skin care regimen, age, or illness. The majority of people will experience xerosis or “dry skin” at some stage in their lives.1 Exogenous factors that can contribute to xerosis include living in cooler climates, particularly during winter months, where both cold, dry air outdoors and indoor heating cause blood to be drawn away from the dermis, or in dry, hot climates, where constant heat and air conditioning evaporate water from the skin. Excessive bathing can also dry the skin.2 Many cleansers contain surfactants that extract and emulsify skin surface lipids and the intracellular lipids between the corneocytes of the epidermis, damaging the skin barrier.3
Endogenous factors also contribute to xerosis. The prevalence of xerosis increases with age; this is thought to be caused by changes in the keratinization process and lipid content of the stratum corneum. While already common, xerosis is a condition that will continue to become more prevalent with the growing aging population. In addition, many chronic illnesses and medications can exacerbate xerosis.1
Three key deficiencies in the skin have been shown to contribute to xerosis. These include a deficiency in moisture-binding substances collectively known as the natural moisturizing factor (NMF)4,5; deficiencies in the skin barrier lipids, ceramides6–8; and more recently, a deficiency of the skin's own moisture network in the viable epidermis, mediated by the newly discovered aquaporin water channels.9–11
NMF components are found exclusively in the stratum corneum and are located in high concentrations within the corneocytes. The NMF consists primarily of amino acids (~40%) and their derivatives, including pyrrolidone carboxylic acid (PCA, ~12%), lactate (~12%), urea (~7%), and inorganic salts (~18%).5,12 These hygroscopic moisturizing factors attract and bind atmospheric water as well as internal water supplied from the dermis, allowing the corneocyte to remain hydrated despite the drying effects of the environment.4 Filaggrin, a histidine-rich keratin binding protein synthesized in the keratohyalin granules, was first hypothesized as a source of NMF several decades ago.13 Recent reports of filaggrin loss-of-function mutations as a causative factor in atopic xerosis and various dry skin conditions have brought wider appreciation of the importance of NMF. As the large granular cells progress through the stages of corneocyte maturation, filaggrin aids in corneocyte compaction, and is proteolyzed to produce a mixture of hygroscopic decomposition products (i.e., NMF) found within the flattened, outer layer corneocytes.9 Levels of the various NMF components, including PCA, urocanic acid, and amino acids, have been shown to be reduced in the skin of patients with atopic dermatitis, with the levels of reduction depending on filaggrin genotype and disease severity.14 Furthermore, deficiencies in stratum corneum levels of urea have also been associated with atopic disease, psoriasis, and xerosis in the elderly.15–18
Ceramides are the main intercellular lipids in the horny layer of skin, accounting for 40 to 50 percent of total lipids.19,20 There are nine ceramide subclasses in the stratum corneum, each one a combination of a fatty acid and a sphingoid base.19 Ceramides play an important role in maintaining skin barrier function. Most skin disorders exhibiting dry skin conditions, such as atopic dermatitis, have been shown to have reduced or altered ceramide profiles in the stratum corneum resulting in decreased barrier function.6,19,21 These changes in ceramide levels and profiles result in altered lipid packing exhibited by less ordered lipid structures, which has been identified as a cause of increased stratum corneum permeability.19,21 A few studies have reported that lipid levels in the stratum corneum are reduced with aging and that these levels are also subject to seasonal variation, being reduced in the winter months.6,22 Changes in ceramide composition have also been reported in seasonal and age-related xerosis.22
A more recent discovery of key importance in hydration of the skin is the identification of epidermal aquaporins.23 Aquaporins (AQPs) are a family of water channels found in both plants and animals, functioning as constitutively activated pores that allow water to follow the osmotic gradient generated by energy-dependent ion transporters.24 The discovery of these ubiquitous water channels finally explained the rapid movement of water between cells and led to their discoverer, Peter Agre, being awarded the Nobel Prize in Chemistry in 2003.
To date, at least 13 AQPs have been identified in mammals, each with its own characteristic distribution.9 AQP3, localized in the epidermis, is the most prevalent water channel in skin25; it is located in the cell membranes of keratinocytes in the viable epidermis.26,27 AQP3 belongs to the group of aquaglyceroporins, and as such it transports water and glycerol molecules as well as small solutes such as urea.9,25,28 AQP3 levels have been shown to be reduced in aged skin, dry skin, and diseased skin.9,29,30
Recently, glyceryl glucoside—a glycerol derivative with enhanced humectant properties—has been investigated for its ability to enhance the skin's own moisture network.31 The investigators propose that incorporating glyceryl glucoside into a moisturizing product may increase skin hydration from within the epidermis.
To effectively treat xerosis, each of the principal factors that are central to modulating and maintaining skin hydration should be addressed. With this in mind, a multifunctional therapeutic moisturizer was developed that addresses many of the known causes of xerosis to improve skin hydration in the following ways: by enhancing water supply to the stratum corneum with glyceryl glucoside, by augmenting water binding with various components of NMF, and by improving the barrier status with ceramides.
Two formulations with these ingredients have been developed. They include an oil-in-water formulation (Light Formulation) and a water-in-oil formulation (Rich Formulation). The clinical studies described here were designed to assess the effect of these moisturizers for the treatment of xerosis, using objective and subjective measures.
Methods
Formulations. Two clinical moisturizers were tested: 1) Light Formulation: a light, oil-in-water emulsion (oil droplets suspended in a continuous aqueous phase) containing 5% urea (Eucerin Smoothing Repair, Beiersdorf Inc., Wilton, Connecticut); and 2) Rich Formulation: a rich, water-in-oil emulsion (aqueous droplets suspended in a continuous oil phase) containing 10% urea (Eucerin Professional Repair, Beiersdorf Inc., Wilton, Connecticut).
Both products contain glyceryl glucoside; the NMF components urea, lactate, a variety of amino acids, PCA, inorganic salt, and several sugars; and ceramide 3 (Table 1).
TABLE 1.
Ingredients of test products and vehicles as registered by the International Nomenclature of Cosmetic Ingredients
| LIGHT FORMULATION AND VEHICLES | RICH FORMULATION AND VEHICLES | |
|---|---|---|
| Emulsion type | Oil-in-water | Water-in-oil |
| Vehicle ingredients | Water, cetearyl alcohol, cyclomethicone, butyrospermum parkii butter, caprylic/capric triglyceride, methylpropanediol, octyldodecanol, dicaprylyl ether, tapioca starch, glyceryl stearate SE, hydrogenated coco-glycerides, ceramide 3, dimethiconol, chondrus crispus, sodium cetearyl sulfate, 1,2-hexanediol, phenoxyethanol | Water, isopropyl stearate, dicaprylyl ether, butyrospermum parkii butter, nylon-12, polyglyceryl-4 diisostearate/polyhydroxy-stearate/sebacate, cetearyl alcohol, ceramide 3, 1,2-hexanediol, phenoxyethanol, potassium sorbate |
| Vehicle plus, additional ingredients | 5% urea, sodium lactate, lactic acid | 10% urea, sodium lactate, lactic acid |
| Test formulations, additional ingredients | Glycerin, glyceryl glucoside, carnitine, sodium PCA, arginine HCL, serine, alanine, histidine, citrulline, lysine, sodium chloride, glycogen, mannitol, sucrose, glutamic acid, threonine | Glycerin, glyceryl glucoside, carnitine, sodium PCA, arginine HCL, serine, alanine, histidine, citrulline, lysine, sodium chloride, glycogen, mannitol, sucrose, glutamic acid, threonine |
Clinical studies. Four studies are reported here. Studies 1 and 2 tested both the Light Formulation and Rich Formulation separately in vehicle-controlled studies to determine changes in skin hydration and skin barrier function over two weeks of regular use. Study 3 is a regression study performed with the Rich Formulation to determine the long-term moisturization effect after treatment discontinuation. Study 4 investigated symptom improvement and skin tolerability with the Light Formulation.
Studies 1 and 2: Vehicle-Controlled Studies
Test products. Light Formulation (Study 1) and Rich Formulation (Study 2) were each tested alongside a basic vehicle (vehicle), and this same vehicle enriched with urea and lactate (vehicle plus). The vehicles did not contain glyceryl glucoside or NMF components (beyond urea and lactate in the vehicle plus formulation). Both the vehicle and vehicle plus for the Light Formulation were oil-in-water formulations, with vehicle plus containing 5% urea and 5% lactate. The vehicles for the Rich Formulation comparisons were water-in-oil formulations, with vehicle plus containing 10% urea and 5% lactate.
Subjects. For Study 1, female subjects ages 50 to 80 years with Fitzpatrick phototype I, II, or III and demonstrating very dry skin were eligible for enrollment. For Study 2, female subjects between ages 50 and 70 years with very dry skin were enrolled.
Subjects were excluded if they had any acute skin disease, general illness, skin irregularities (such as scars, tattoos, or wounds), or were using any medication or topical treatments that could hinder or endanger the subjects or the performance of the study. Individuals with any known allergy or sensitivity to any cosmetics, ingredients of cosmetics, latex, plaster, or any other study materials were excluded.
The recommendations of the Declaration of Helsinki and the International Conference on Harmonisation guidelines for Good Clinical Practice were observed as applicable to a nondrug study. All subjects provided written, informed consent to participate in the trial.
Study design. Studies 1 and 2 were single-center, randomized trials. A block enrollment was used ensuring that all subjects started and completed their respective study on the same day. The Light Formulation trial was conducted in January and February 2011, while the Rich Formulation trial was conducted in April and May 2011. Both studies were carried out in Hamburg, Germany.
All subjects took part in a one-week preconditioning phase in which forearms were washed with a standard mild shower gel twice daily (lathering on moistened skin for 60 seconds before rinsing with tap water). The standard shower gel contained the following ingredients: water, sodium laureth sulfate, cocamidopropyl betaine, parfum, panthenol, polyquaternium-10, disodium cocoyl glutamate, PEG-40 hydrogenated castor oil, citric acid, sodium chloride, PEG-200 hydrogenated glyceryl palmate, benzophenon-4, tetrasodium iminodisuccinate, sodium benzoate, sodium salicylate, benzyl salicylate, linalool, limonene, amyl cinnamal, CI 74160, CI 61570, CI 4209, and CI 13015. This shower gel was selected to be neutral as it does not contain any moisturizers. Contact with other skin care products and with household cleaning products was also forbidden during this period.
Each study was double blind for the test products and open for the untreated site. Subjects applied both vehicle test products and either Light Formulation or Rich Formulation as applicable to marked test areas on their inner forearms according to a preassigned, blinded randomization scheme. A fourth area was left untreated and used as a control. Treatments were applied twice daily for two weeks.
Evaluations. All measurements were performed after subjects were allowed to acclimatize for at least 30 minutes under standard atmospheric conditions (21.5°C±1.0°C and 45%±5% relative humidity). Skin hydration levels were measured using a Corneometer CM 825 (Courage + Khazaka, Cologne, Germany) according to European Group for Efficacy Measurements on Cosmetics and Other Topical Products (EEMCO) guidelines.32 Ten measurements were taken per area and the median result reported as arbitrary units. Skin barrier function was assessed by measuring transepidermal water loss (TEWL) according to published guidelines.33 The Light Formulation study used a Tewameter 300 (Courage + Khazaka, Cologne, Germany) while the Rich Formulation study used a DermaLab TEWL Module (Cortex, Denmark). Five measurements were taken per area and the mean result reported in g/m2·h. Instrumental measurements taken at Week 1 and Week 2 were performed 10 to 20 hours after the last application of the test products. Clinical grading of visible dryness and tactile roughness was assessed by a single expert dermatologist according to a five-point scale (Table 2).34 Subjects were also observed for incompatibility reactions.
TABLE 2.
Clinical grading scores for visible dryness and tactile roughness evaluations
| SCORE | VISIBLE DRYNESS | TACTILE ROUGHNESS |
|---|---|---|
| 0 (absent) | None | Perfectly smooth and pliable |
| 1 (slight) | Slight scaling, slight roughness, and dull appearance | Slightly irregular and rough on tangential tactile evaluation |
| 2 (moderate) | Small scales in combination with a few larger scales, slight roughness, whitish appearance | Definitely irregular and rough and possibly slightly stiffened on vertical tactile evaluation |
| 3 (severe) | Small and larger scales uniformly distributed, definite roughness, possibly somewhat red, and possibly a few superficial cracks | Advanced irregularly and rough feeling associated with some stiffening |
| 4 (extreme) | Dominated by large scales, advanced roughness, redness, eczematous changes, and cracks | Gross irregularly and severe disturbance of skin markings and definite stiffening |
Statistical analysis. Statistical changes from baseline and between treatments for corneometry and TEWL were measured using the Shapiro-Wilk test with a significance level set at 0.05. Clinical grading results were compared using Wilcoxon's signed rank test.
Study 3: Regression Study
Subjects. Female, Caucasian subjects between 60 and 80 years of age with very dry skin were eligible for enrollment. Exclusion criteria were the same as per Studies 1 and 2, with the addition that any subjects who had experienced a severe reaction after exposure to sunlight were excluded. This study was conducted using a block enrollment and completed in January 2011 in Hamburg, Germany.
Study design. This was a single-center, randomized trial. The study was double blind with respect to the product applied and open for the untreated site. A one-week preconditioning phase was completed during which the application of skin care products was prohibited, as for the vehicle-controlled trials. Rich Formulation was applied twice daily to a test area on the inner forearm for five days, with the outer upper arm left untreated and used as a control. Treatment was discontinued after five days, with subjects returning two and six days later for further evaluations.
Evaluations. Skin hydration levels were measured using a Corneometer CM 825 (Courage + Khazaka, Cologne, Germany).32 Ten measurements were taken per area and the median result reported as arbitrary units. Corneometry was performed at baseline, after five days of treatment (measurement taken at least 8–20 hours after the last treatment), and after two and six days of regression. Clinical grading of visible skin condition and tactile roughness were completed at baseline and after five days of treatment using a 0 to 4 grading scale.
Study 4: Skin Tolerability Study
Study design. Male and female adult subjects with dry skin who use body care products on a regular basis were eligible for enrollment. Exclusion criteria were similar to other studies.
Study 4 was completed in January 2011 in Hamburg, Germany, using a block enrollment. The subjects, blinded to the product identity, applied Light Formulation to their arms and legs twice daily according to their normal use conditions for two weeks.
Evaluations. Subjects assessed their skin for symptoms of itching, burning, tightness, tingling, and feeling of dryness at baseline and after two weeks of treatment. A dermatologist evaluated the skin at baseline and study end for erythema, dryness, scaling, fissures, and other symptoms of dry skin. Clinical parameters were graded according to the following scale: 0=no result, 0.5=very slight, 1=slight, 2=moderate, 3=strong.
Results
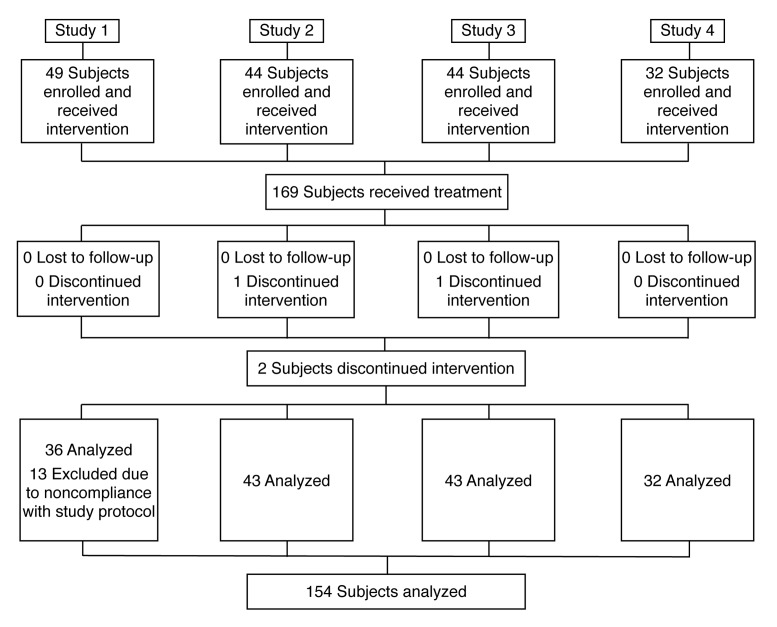
Study 1: Light Formulation comparison with vehicle and vehicle plus. Forty-nine subjects were enrolled in the study; 36 were included in the analyses, with 13 excluded due to noncompliance with the study protocol (Figure 1). The subjects' ages ranged from 51 to 76 years, with a mean age of 64.03 years.
Figure 1.
Flow diagram of subject progress through the trials
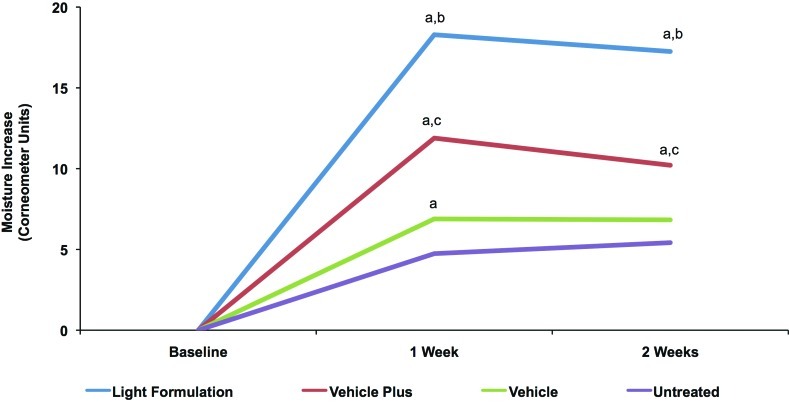
Corneometry readings indicated that all products significantly improved skin hydration after one week of regular twice-daily use compared with the untreated control site. The Light Formulation and vehicle plus also showed significant improvement in skin hydration after two weeks of use compared with the untreated site (Figure 2). Corneometry readings are reported in Table 3. Subjects demonstrated an average baseline corneometry reading of 27 units and there were no statistically relevant differences between the values of the different treatment areas at baseline. Comparison of test products showed that the Light Formulation significantly improved skin hydration at Week 1 and Week 2 compared with both vehicle and vehicle plus (Figure 2).
Figure 2.
Change in skin hydration with Light Formulation compared with vehicles. Corneometry measurements after twice-daily treatment with Light Formulation, vehicle (basic vehicle), and vehicle plus (basic vehicle containing 5% lactate and 5% urea) as well as an untreated control area. Values shown are mean differences between the respective Week 1 and Week 2 recordings and baseline levels. Significance was set at P<0.05.
a=significantly higher than untreated control; b=significantly higher than vehicle and vehicle plus; c=significantly higher than vehicle
TABLE 3.
Corneometry and TEWL values for Study 1 and Study 2
| CORNEOMETRY(MEAN) | TEWL (MEAN) | ||||||
|---|---|---|---|---|---|---|---|
| BASELINE | WEEK 1 | WEEK 2 | BASELINE | WEEK 1 | WEEK 2 | ||
| Study 1 | Control | 26.8 | 31.6 | 32.2 | 7.0 | 6.7 | 6.4 |
| Vehicle | 26.5 | 33.4 | 33.3 | 7.3 | 6.8 | 7.0 | |
| Vehicle plus | 26.7 | 38.6 | 36.9 | 6.8 | 6.0 | 5.6 | |
| Light Formulation | 27.9 | 46.2 | 45.2 | 6.7 | 5.4 | 5.3 | |
| Study 2 | Control | 21.6 | 24.0 | 23.2 | 7.4 | 6.0 | 5.2 |
| Vehicle | 21.2 | 30.7 | 29.8 | 7.3 | 6.4 | 5.8 | |
| Vehicle plus | 21.3 | 34.8 | 31.7 | 7.7 | 5.3 | 4.1 | |
| Rich Formulation | 21.2 | 43.2 | 42.9 | 7.7 | 5.1 | 4.0 | |
Corneometry measurements are reported as arbitrary units. TEWL measurements are reported in g/m2·h.
TEWL=transepidermal water loss
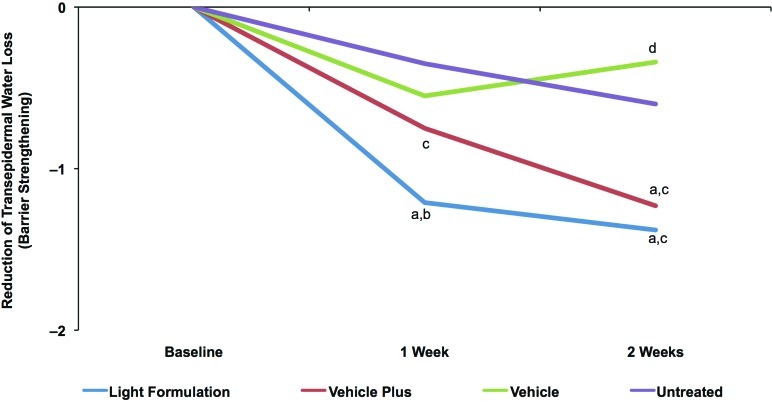
The average pretreatment TEWL value was 6.95g/m2 ·h, indicative of dehydrated skin (defined as 5–9 g/m2 ·h; see Table 3). TEWL was significantly decreased after treatment with Light Formulation at Weeks 1 and 2 compared with the untreated control, indicating an improvement in skin barrier function with twice-daily treatment (Figure 3). In addition, the Light Formulation significantly decreased TEWL compared with all other products at Week 1 and compared with vehicle at Week 2. The vehicle plus significantly reduced TEWL compared with the untreated area at Week 2 and showed significant improvement compared with vehicle at both Weeks 1 and 2. The vehicle-treated area showed a small but significant increase in TEWL relative to the untreated control at Week 2, indicating a worsening of skin barrier function.
Figure 3.
Change in skin barrier function with Light Formulation compared with vehicles as measured by TEWL. TEWL measures of areas after twice-daily treatment with Light Formulation, vehicle, and vehicle plus as well as an untreated control area. Values are mean differences between the respective Week 1 and Week 2 recordings and baseline levels. Significance was set at P<0.05. a=significantly lower than untreated control (improvement); b=significantly lower than vehicle and vehicle plus; c=significantly lower than vehicle; d=significantly higher than untreated control (worsening) (P<0.05)
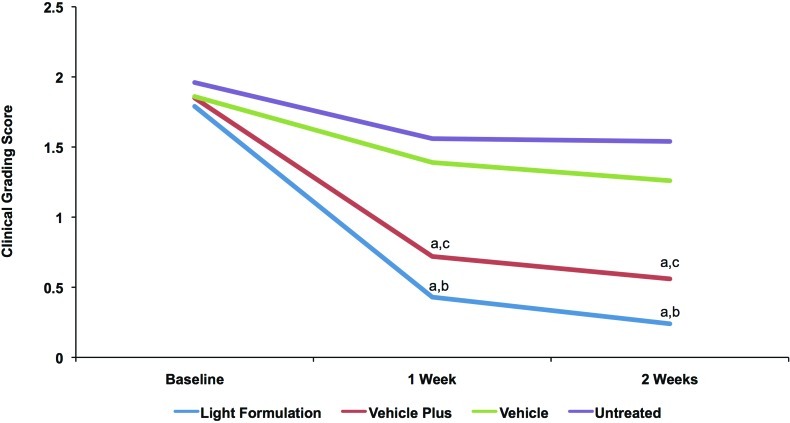
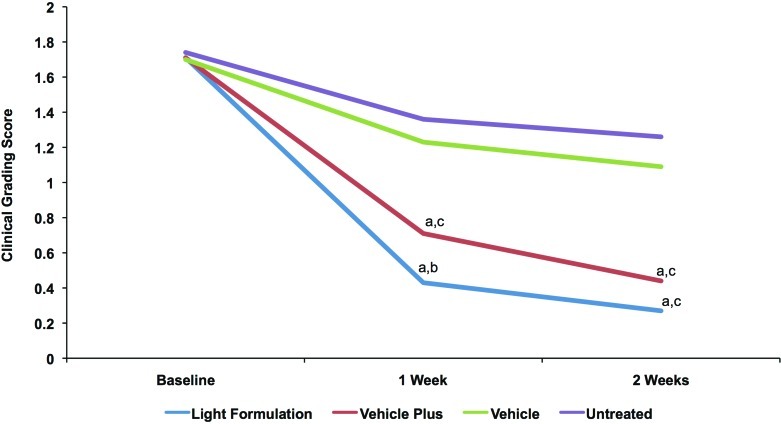
Clinical grading results showed that both Light Formulation and vehicle plus significantly decreased visible skin dryness and tactile skin roughness at Week 1 and Week 2 compared with vehicle and the untreated site (Figures 4a and 4b). The Light Formulation was also superior to vehicle plus for visible dryness at Weeks 1 and 2 and tactile roughness at Week 1. The average dryness score at baseline was 1.86. Light Formulation significantly reduced this score by 1.36 at Week 1 and 1.56 at Week 2. Vehicle plus reduced the visible dryness score by 1.13 at Week 1 and 1.29 at Week 2. The average tactile roughness score was 1.72 at baseline. Light Formulation significantly reduced this score by 1.29 at Week 1 and 1.44 at Week 2. Vehicle plus reduced the visible dryness score by 1.00 at Week 1 and 1.27 at Week 2. No incompatibility reactions were observed and the product was well tolerated.
Figures 4a and 4b.
Clinical grading scores of visible dryness (A) and tactile roughness (B) with Light Formulation, vehicle plus, and vehicle. Grading was performed by a dermatologist according to the descriptions in Table 2. A reduction in score indicates an improvement. Significance was set at P< 0.05. a=significantly lower than untreated control (improvement); b=significantly lower than vehicle and vehicle plus; c=significantly lower than vehicle
Study 2: Rich Formulation comparison with vehicle and vehicle plus. Forty-four subjects were enrolled and 43 completed the study, although TEWL data were not recorded for one subject on the final reading (Figure 1). Subjects' ages ranged from 50.2 to 70.7 years, with a mean of 59.9 years.
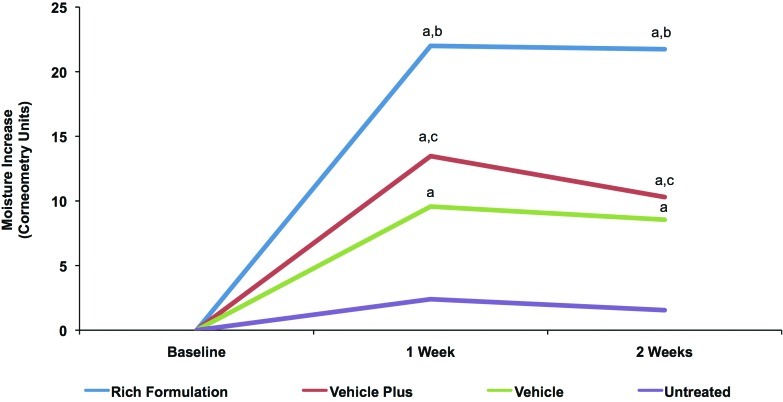
Corneometry and TEWL readings for baseline and Weeks 1 and 2 are reported in Table 3. Corneometry results showed that all products significantly improved skin hydration at Weeks 1 and 2 compared with the untreated site (Figure 5). Subjects demonstrated an average baseline corneometry reading of 21.3 units and there were no statistically relevant differences between the values of the different treatment areas. The Rich Formulation increased hydration of the skin significantly more than all other treatments. Vehicle plus significantly improved hydration compared with vehicle alone (Figure 5).
Figure 5.
Change in skin hydration with Rich Formulation compared with vehicles. Corneometry measurements after twice-daily treatment with Rich Formulation, vehicle, and vehicle plus as well as an untreated control area. Values shown are mean differences between the respective Week 1 and Week 2 recordings and baseline levels. Significance was set at P<0.05. a=significantly higher than untreated control; b=significantly higher than vehicle plus and vehicle; c=significantly higher than vehicle
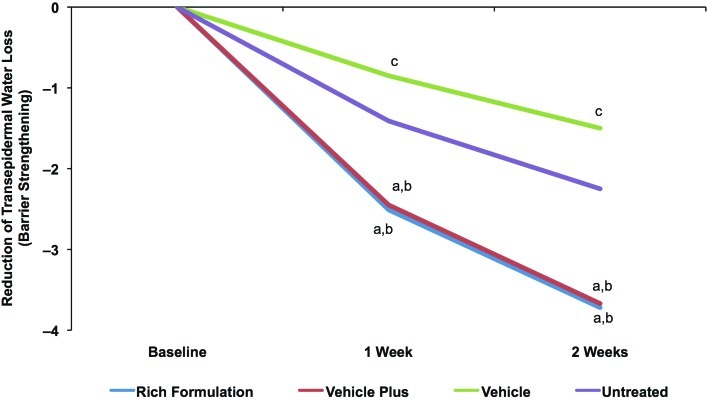
The average pretreatment TEWL was 7.51g/m2 ·h, as expected for dry skin. TEWL was significantly decreased after treatment with Rich Formulation and vehicle plus at Weeks 1 and 2 compared with the untreated site, indicating an improvement in skin barrier function (Figure 6). Vehicle alone showed significantly higher TEWL at Week 1 and Week 2 compared with the untreated site, indicating a relative worsening of skin barrier function. Rich Formulation and vehicle plus significantly improved skin barrier function between Weeks 1 and 2.
Figure 6.
Change in skin barrier function with Rich Formulation compared with vehicles as measured by TEWL. TEWL measures after twice-daily treatment with Rich Formulation, vehicle, and vehicle plus as well as an untreated control area after 1 week and 2 weeks of twicedaily treatment. Values shown are mean differences between the respective Week 1 and Week 2 recordings and baseline levels. Significance was set at P<0.05. a=significantly lower than untreated control (improvement); b=significantly lower than vehicle; c=significantly higher than untreated control (worsening)
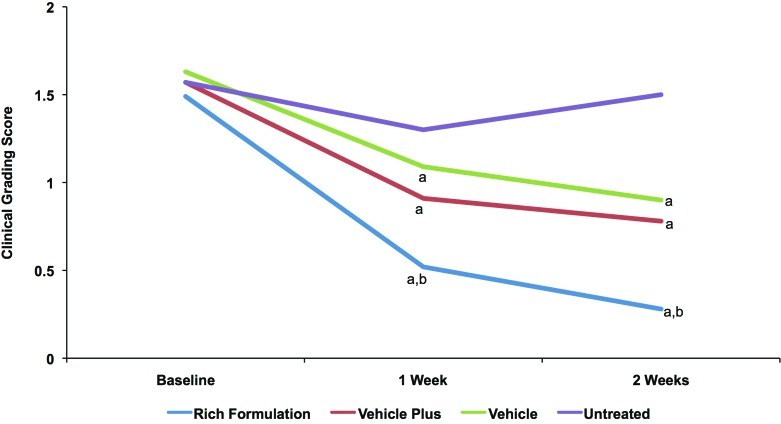
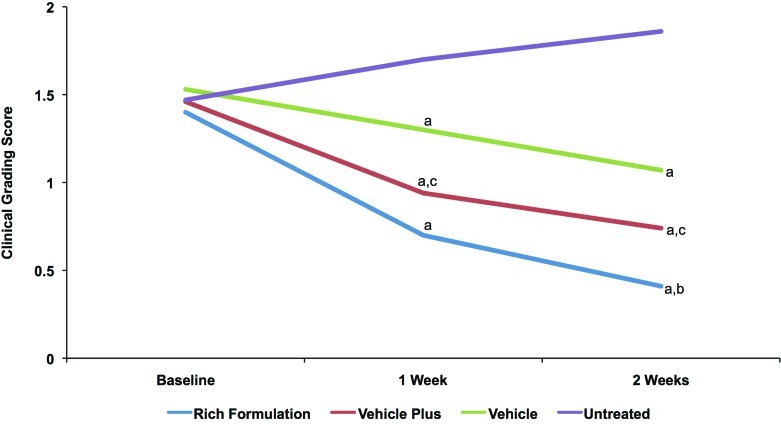
Clinical grading results showed that all test products significantly improved visible dryness and tactile roughness at Weeks 1 and 2 and that the improvements at Week 2 were significantly greater than at Week 1, showing progressive improvement with treatment (Figure 7). The average dryness score at baseline was 1.56. Rich Formulation significantly improved dryness compared with other products at Weeks 1 and 2. Rich Formulation significantly reduced the dryness score by 0.97 at Week 1 and 1.20 at Week 2, while vehicle plus reduced the visible dryness score by 0.65 at Week 1 and 0.80 at Week 2 (Figure 7a). The average roughness score at baseline was 1.47. Rich Formulation and vehicle plus significantly improved roughness compared with vehicle at Week 1, and Rich Formulation was superior to both vehicle and vehicle plus at Week 2 (Figure 7b). Rich Formulation significantly reduced the tactile roughness score by 0.70 at Week 1 and 0.97 at Week 2, while vehicle plus reduced the tactile roughness score by 0.52 at Week 1 and 0.70 at Week 2 (Figure 7b).
Figures 7a and 7b.
Clinical grading scores of visible dryness (A) and tactile roughness (B) with Rich Formulation, vehicle plus, and vehicle. Grading was performed by a dermatologist according to the descriptions in Table 2. A reduction in score indicates an improvement. Significance was set at P<0.05. a=significantly lower than untreated control (improvement); b=significantly lower than vehicle and vehicle plus; c=significantly lower than vehicle
One subject did not complete the study due to an incompatibility reaction showing erythema, some papules, and strong pruritus with both the Rich Formulation and vehicle plus. These reactions occurred after sun exposure following the 13th application of test products. Symptoms resolved after discontinuation, but reoccurred with the subject's own body lotion after sun exposure.
Study 3: Regression study. Forty-four female subjects were enrolled in this study with one subject discontinuing after five days due to illness (Figure 1). Ages ranged from 60.2 to 80.8 years, with a mean of 66.5 years.
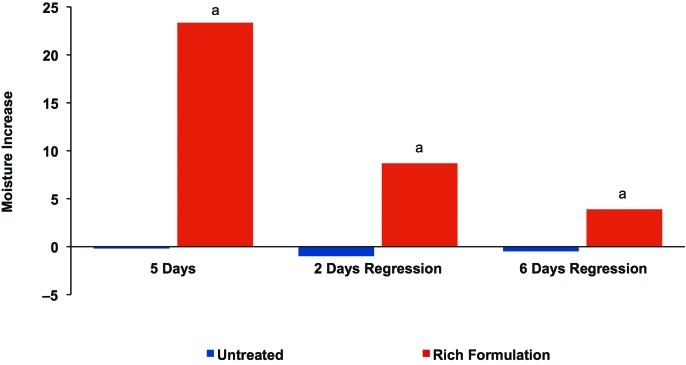
Corneometry results showed that the Rich Formulation significantly improved skin hydration after five days of twice-daily treatment compared with the untreated site (Figure 8). In addition, skin hydration remained significantly higher than the untreated site at two and six days after treatment discontinuation, demonstrating that water content was maintained in the skin for at least six days. The average pretreatment corneometry value was 23.3 units, indicating that the skin hydration level almost doubled after five days of treatment with Rich Formulation, while the untreated area remained relatively constant over the study period. No incompatibility reactions were observed or reported in connection with application of the Rich Formulation.
Figure 8.
Maintenance of skin hydration with Rich Formulation in regression study. Change in skin hydration as measured by corneometry at baseline, after 5 days of twice-daily use of Rich Formulation, and 2 and 6 days after treatment discontinuation. Significance was set at P<0.05. a=significantly higher values than untreated control areas
Study 4: Skin tolerability study. Thirty-two subjects (81% female) with dry skin, aged between 28 and 73 years with a mean age of 55.9 years, were enrolled (Figure 1). This group included patients with diabetes (47%) and patients with atopic dermatitis (31%). The remaining patients were otherwise healthy. To assess skin tolerability after normal use, subjects applied Light Formulation to their arms and legs twice daily for two weeks. Symptoms of dry skin were recorded by both the subjects and a dermatologist.
Light Formulation alleviated the frequency of symptoms associated with dry skin after two weeks of treatment. Before treatment, 36 dermatological discomforts were noted by subjects, while only three were noted after treatment (Table 4). These were three subjects still noting a “feeling of dryness,” while symptoms of itching, burning, tightness, tingling, and the majority of cases of dryness had resolved. Dermatologists noted 59 cases of symptoms associated with dry skin before treatment, predominantly dryness and scaling. After two weeks of treatment, only seven cases of symptoms were still unresolved (Table 4). The product was weighed before and after application to determine how much Light Formulation subjects had used. The average amount used by study subjects was 164g, although this amount ranged from 49 to 259g. The median amount used by subjects was 181g over the two-week period. The Light Formulation was well tolerated and there were no incompatibility results.
TABLE 4.
Frequency of symptoms of xerosis as assessed by subjects and dermatologists (N=36 subjects)
| FREQUENCY | ||
|---|---|---|
| DAY 1 | DAY 15 | |
| Subjects' assessment of symptoms | ||
| Itching | 5 | 0 |
| Burning | 1 | 0 |
| Tightness | 7 | 0 |
| Tingling | 4 | 0 |
| Feeling of dryness | 18 | 3 |
| Other | 1 | 0 |
| Total | 36 | 3 |
| Dermatologists' assessment of symptoms | ||
| Erythema | 2 | 1 |
| Dryness | 30 | 4 |
| Scaling | 23 | 2 |
| Fissures | 0 | 0 |
| Papules | 3 | 0 |
| Pustules | 0 | 0 |
| Edema | 0 | 0 |
| Weeping | 0 | 0 |
| Other | 1 | 0 |
| Total | 59 | 7 |
Discussion
Xerosis can be caused by deficiencies in NMF, ceramides, and/or aquaporins. These deficiencies may be a consequence of aging, environmental factors, genetic abnormalities, or illness. The therapeutic moisturizers, Light Formulation and Rich Formulation, have been specifically developed to address the effects of these deficiencies in the skin, providing the skin with the necessary components to improve skin hydration, enhance the barrier function, and ameliorate xerosis. The corneometry results from the double-blind, vehicle-controlled trials demonstrated that both of these products significantly improved hydration of the skin compared with both basic vehicle and vehicle plus, which is supplemented with lactate and urea. In addition, both products significantly improved skin barrier function compared with vehicles, as shown by the reduction in TEWL. Both Light Formulation and Rich Formulation improved visible dryness and tactile roughness, as assessed by the investigator. The studies indicate that these therapeutic moisturizers improve skin hydration and barrier function as assessed by objective measures, as well as improving subjectively assessed symptoms of xerosis.
The regression study showed that after only five days of treatment, there was a significant improvement in skin hydration that almost doubled the water content in the skin, and that at two and six days after cessation of product use, hydration remained significantly improved relative to the untreated site. In fact, on Day 6 of the regression phase, hydration was still approximately 20 percent of the highest level achieved before discontinuation of treatment. This clearly indicates that hydration is maintained even when the product is not used daily.
Xerosis can cause many un-comfortable symptoms, such as skin itching and tightness. Chronic or severe xerosis can also progress to cause scaling and fissures, increasing the risk for inflammation and infections.21 In Study 4, Light Formulation was shown to dramatically reduce the frequency of such symptoms after two weeks of treatment. In addition, both products were shown to significantly reduce signs of visible dryness and tactile roughness compared with both the basic vehicle and vehicle plus formulations.
Both products were well tolerated. Contact dermatitis is common in older adults who have used multiple treatments for xerosis and can be caused by one or more ingredients in applied products.35 No cases of contact dermatitis were seen in these studies. A total of 169 subjects were treated with either the Light or Rich Formulations during these four studies. In all of the studies, only one patient experienced an incompatibility reaction. This reaction did not appear until after the 13th application of the product and sun exposure. However, the subject also experienced a similar reaction with the vehicle formulation and their own body lotion after sun exposure. As no follow-up patch testing was conducted, the cause of these reactions is unknown.
All subjects in these studies exhibited xerosis. Many cases of xerosis may have been age related due to the fact that approximately 73 percent of subjects enrolled were age 60 years or over. Additionally, some subjects were diagnosed with atopic xerosis, while others had both diabetes and atopic dermatitis. While the study populations were not large enough for any statistical analyses to be performed on these subgroups, it is important to note that both the Light Formulation and the Rich Formulation were effective and well tolerated for the treatment of various types of dry skin.
It should be noted that these clinical studies were rigorously controlled. These were all double-blind, randomized, controlled studies. All studies were single-center block enrollments in which all subjects started and finished the trial on the same day in the same location, effectively controlling for changing environmental factors. All studies were conducted in Hamburg, Germany, during the winter, which is typically a cold environment that can exacerbate seasonal dry skin. In addition, all studies used a standard neutral cleanser for the preconditioning phase. This is in contrast to many trials in which subjects are allowed to use their own cleansers prior to the study commencement.
A limitation of these studies is their relatively small size, with each study enrolling between 32 and 49 subjects. While identically designed, vehicle-controlled studies were performed for both products, a head-to-head comparison between the Light Formulation and Rich Formulation was not carried out, and the vehicle-controlled TEWL results cannot be directly compared due to differences in the instruments used. The regression study and tolerability study were each only performed for one product. Consequently, conclusions cannot be made as to the capability of the Light Formulation in maintaining skin hydration after treatment discontinuation, or the change in symptoms of dry skin with Rich Formulation, beyond what was demonstrated in the vehicle-controlled analysis.
Light Formulation and Rich Formulation have similar formulations. Light Formulation is an oil-in-water formulation containing 5% urea as well as NMF, ceramide 3, and glyceryl glucoside. This product is suited for the majority of patients with dry skin. Rich Formulation is a water-in-oil formulation containing 10% urea in addition to NMF components, ceramide 3, and glyceryl glucoside. Because of the external oil phase in this formulation, it is more protective and substantive on the skin, shielding against TEWL that can be pronounced in patients with very dry or severely dry skin.
The Light Formulation and Rich Formulation described and tested here were designed to combat key deficiencies that have been identified in xerosis: insufficient water supply to the stratum corneum, deficiency in water-binding capacity, and barrier perturbation. Glyceryl glucoside, a novel humectant, enhances water supply to the stratum corneum; various components of the NMF attract and hold water in the skin's upper layers; and ceramide 3 augments the barrier lipid composition to strengthen the skin barrier function.
Treatment with glyceryl glucoside in an emulsion formulation was shown to significantly decrease TEWL in preliminary clinical trials.31 Here, these formulations containing glyceryl glucoside in addition to NMF and ceramide 3 were not only observed to significantly decrease TEWL (indicating enhanced barrier function), but also significantly improved skin hydration with daily use, which was maintained after product discontinuation. These product formulations assist the self-repair processes of the skin barrier and represent a multidimensional, enhanced treatment for xerosis.
Therefore, the authors would maintain that a good moisturizer does not just supply the skin with ingredients to replace those of which it is deficient, but creates a physiologically compatible environment that allows the skin to repair itself. These products contain glyceryl glucoside to promote water delivery to the stratum corneum, ceramide 3 to strengthen the lipid barrier, and numerous components of the NMF, such as urea and others, to moisturize and help promote barrier repair. These unique and novel formulations, constructed to hydrate skin by multiple mechanisms, appear to promote the self-repair of the barrier and represent a new approach for the treatment of xerosis.
Footnotes
DISCLOSURE:Dr. Weber is an employee of Beiersdorf Inc., Wilton, Connecticut. Ms. Kausch and Drs. Rippke, Schoelermann, and Filbry are employees of Beiersdorf AG, Hamburg, Germany. These studies were conducted and funded by Beiersdorf AG, Hamburg, Germany.
References
- 1.White-Chu EF, Reddy M. Dry skin in the elderly: complexities of a common problem. Clin Dermatol. 2011;29(1):37–42. doi: 10.1016/j.clindermatol.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Pons-Guiraud A. Dry skin in dermatology: a complex physiopathology. J Eur Acad Dermatol Venereol. 2007;21(2 suppl):1–4. doi: 10.1111/j.1468-3083.2007.02379.x. [DOI] [PubMed] [Google Scholar]
- 3.Draelos ZD. Clinical situations conducive to proactive skin health and anti-aging improvement. J Investig Dermatol Symp Proc. 2008;13(1):25–27. doi: 10.1038/jidsymp.2008.9. [DOI] [PubMed] [Google Scholar]
- 4.Rawlings AV, Scott IR, Harding CR, Bowser PA. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103(5):731–741. doi: 10.1111/1523-1747.ep12398620. [DOI] [PubMed] [Google Scholar]
- 5.Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17(1 suppl):43–48. doi: 10.1111/j.1396-0296.2004.04s1005.x. [DOI] [PubMed] [Google Scholar]
- 6.Jungersted JM, Hellgren LI, Jemec GB, Agner T. Lipids and skin barrier function—a clinical perspective. Contact Dermatitis. 2008;58(5):255–262. doi: 10.1111/j.1600-0536.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 7.Elias PM, Feingold KR. Lipids and the epidermal water barrier: metabolism, regulation, and pathophysiology. Semin Dermatol. 1992;11(2):176–182. [PubMed] [Google Scholar]
- 8.Grubauer G, Feingold KR, Harris RM, Elias PM. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30(1):89–96. [PubMed] [Google Scholar]
- 9.Bonté F. Skin moisturization mechanisms: new data. Ann Pharm Fr. 2011;69(3):135–141. doi: 10.1016/j.pharma.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Verdier-Sévrain S, Bonté F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6(2):75–82. doi: 10.1111/j.1473-2165.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 11.Draelos ZD. New channels for old cosmeceuticals: aquaporin modulation. J Cosmet Dermatol. 2008;7(2):83. doi: 10.1111/j.1473-2165.2008.00367.x. [DOI] [PubMed] [Google Scholar]
- 12.Clar EJ, Fourtanier A. L'acide pyrrolidone carboxylique (PCA) et la peau. Intl J Cosmet Sci. 1981;3(3):101–113. doi: 10.1111/j.1467-2494.1981.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 13.Scott IR, Harding CR, Barrett JG. Histidine-rich proteins of the keratohyalin granule: source of the free amino acids, urocanic acid and pyrrolidone carboxylic acid in the stratum corneum. Biochim Biophys Acta. 1982;719(1):110–117. doi: 10.1016/0304-4165(82)90314-2. [DOI] [PubMed] [Google Scholar]
- 14.Kezic S, O'Regan GM, Yau N, et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy. 2011;66(7):934–940. doi: 10.1111/j.1398-9995.2010.02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller KH, Pflugshaupt C. [Urea in dermatology I] Hautarzt. 1989;40(9 suppl):1–12. [PubMed] [Google Scholar]
- 16.Müller KH, Pflugshaupt C. [Urea in dermatology II] Hautarzt. 1989;40(9 suppl):13–19. [PubMed] [Google Scholar]
- 17.Proksch E. Urea in dermatology. Dtsch Med Wochenschr. 1994;119(33):1126–1130. doi: 10.1055/s-2008-1058813. [DOI] [PubMed] [Google Scholar]
- 18.Wellner K, Wohlrab W. Quantitative evaluation of urea in stratum corneum of human skin. Arch Dermatol Res. 1993;285(4):239–240. doi: 10.1007/BF00372018. [DOI] [PubMed] [Google Scholar]
- 19.Choi MJ, Maibach HI. Role of ceramides in barrier function of healthy and diseased skin. Am J Clin Dermatol. 2005;6(4):215–223. doi: 10.2165/00128071-200506040-00002. [DOI] [PubMed] [Google Scholar]
- 20.Mizutani Y, Mitsutake S, Tsuji K, et al. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 2009;91(6):784–790. doi: 10.1016/j.biochi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Lebwohl M, Herrmann LG. Impaired skin barrier function in dermatologic disease and repair with moisturization. Cutis. 2005;76(6 suppl):7–12. [PubMed] [Google Scholar]
- 22.Rogers J, Harding C, Mayo A, Banks J, et al. Stratum corneum lipids: the effect of aging and the seasons. Arch Dermatol Res. 1996;288(12):765–770. doi: 10.1007/BF02505294. [DOI] [PubMed] [Google Scholar]
- 23.Hara-Chikuma M, Verkman AS. Roles of aquaporin-3 in the epidermis. J Invest Dermatol. 2008;128(9):2145–2151. doi: 10.1038/jid.2008.70. [DOI] [PubMed] [Google Scholar]
- 24.Chrispeels MJ, Agre P. Aquaporins: water channel proteins of plant and animal cells. Trends Biochem Sci. 1994;19(10):421–425. doi: 10.1016/0968-0004(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 25.Boury-Jamot M, Daraspe J, Bonté F, et al. Skin aquaporins: function in hydration, wound healing, and skin epidermis homeostasis. Handb Exp Pharmacol. 2009;(190):205–217. doi: 10.1007/978-3-540-79885-9_10. [DOI] [PubMed] [Google Scholar]
- 26.Frigeri A, Gropper MA, Umenishi F, et al. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci. 1995;108(pt 9):2993–3002. doi: 10.1242/jcs.108.9.2993. [DOI] [PubMed] [Google Scholar]
- 27.Sougrat R, Morand M, Gondran C, et al. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J Invest Dermatol. 2002;118(4):678–685. doi: 10.1046/j.1523-1747.2002.01710.x. [DOI] [PubMed] [Google Scholar]
- 28.Boury-Jamot M, Sougrat R, Tailhardat M, et al. Expression and function of aquaporins in human skin: Is aquaporin-3 just a glycerol transporter? Biochim Biophys Acta. 2006;1758(8):1034–1042. doi: 10.1016/j.bbamem.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Bonté F, Noblesse E, Juan M, Verbavatz JM, et al. A study of the importance of aquaporins for human skin. Chin J Dermatol. 2009;42(5):327–329. [Google Scholar]
- 30.Cao C, Wan S, Jiang Q, et al. All-trans retinoic acid attenuates ultraviolet radiation-induced down-regulation of aquaporin-3 and water permeability in human keratinocytes. J Cell Physiol. 2008;215(2):506–516. doi: 10.1002/jcp.21336. [DOI] [PubMed] [Google Scholar]
- 31. Schrader A, Siefken W, Kueper T, et al. Glyceryl glucoside increases aquaporin 3 expression in human skin keratinocytes. Poster presented at: 30th Anniversary Fall Clinical Dermatology Conference; October 27-30, 2011: Las Vegas, Nevada.
- 32.Berardesca E. European Group for Efficacy Measurements on Cosmetics and Other Topical Products. EEMCO guidance for the assessment of stratum corneum hydration: electrical methods. Skin Res Technol. 1997;3(2):126–132. doi: 10.1111/j.1600-0846.1997.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 33.Rogiers V EEMCO Group. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Skin Physiol. 2001;14(2):117–128. doi: 10.1159/000056341. [DOI] [PubMed] [Google Scholar]
- 34.Serup J. EEMCO guidance for the assessment of dry skin (xerosis) and ichthyosis: clinical scoring systems. Skin Res Technol. 1995;1(3):109–114. doi: 10.1111/j.1600-0846.1995.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 35.Jankiçeviç J, Vesiç S, Vukiçeviç J et al. Contact sensitivity in patients with venous leg ulcers in Serbia: comparison with contact dermatitis patients and relationship to ulcer duration. Contact Dermatitis. 2008;58(1):32–36. doi: 10.1111/j.1600-0536.2007.01253.x. [DOI] [PubMed] [Google Scholar]