Abstract
For decades, transepidermal water loss and corneometry have been accepted as measures of skin barrier function. However, these tests are not capable of informing clinicians of the biochemical constituents and biophysical status of the stratum corneum. Knowledge of how the stratum corneum reacts to topical agents is important, as it reveals significant detail regarding the composition and function of this vital skin layer. Furthermore, transepidermal water loss and corneometry serve only as surrogate markers of barrier function. A more precise method of assessing stratum corneum hydration and lipid levels is emerging; in vivo confocal Raman spectroscopy is able to detect and quantify specific biochemical constituents in skin. This information then allows for assessment of the actual physiological status of this vital layer of the skin. This pilot study sought to elucidate a biophysical rationale for the clinical improvement achieved by hyaluronic acid/ceramide barrier repair foam in prior studies as measured by in vivo confocal Raman spectroscopy. Study results include increased lipid and hydration levels in the stratum corneum to depths of 25µm and 40µm, respectively, at the 2-hour, 48-hour, and 7-day time points.
Background
Due to its role in skin disease and health maintenance, the stratum corneum (SC) is a key therapeutic target when treating skin disease. This multifunctional epidermal layer is responsible for critical barrier functions including permeability, antimicrobial, and immune response activities.1 These functions are dependent upon proper hydration and optimal lipid levels, which, in turn, are altered by dermatological disease.1 This creates a cycle of dysfunction in the SC as the innate physiological processes designed to repair the barrier are compromised due to biochemical deficiencies. The ability of humectants and lipids to restore skin barrier functions may provide a basis for improved clinical outcomes in skin diseases associated with barrier dysfunction. Until recently, the means to assess precisely the physiological impact of topical agents designed to improve skin barrier function were few.
Multiple studies have examined transepidermal water loss (TEWL) as a surrogate marker of SC health and to assess the impact of topical agents designed to improve SC function. However, TEWL does not directly measure lipid composition or the hydration state of the SC and its use as an effective measure of SC function is debated.2 Similar controversies are associated with corneometry studies, as results may be attributable to ambient changes in hydration of the skin, and are not necessarily due to the topical agent(s) being tested. More precise study methods to assess hydration state and lipid composition of the SC are desired and would provide clinicians with vital data regarding topical agents that directly improve the barrier function of the skin.
In contrast with corneometry and TEWL measurements, which are subject to environmental influences and other uncontrolled variations within test protocol, in vivo confocal Raman spectroscopy (IVCRS) measures the actual biochemical composition of the SC.2,3 In brief, Raman spectroscopy, as a whole, is a technique premised upon inelastic scattering of energy provided to a target from an external source. Greatly simplified, in IVCRS, some of the energy supplied by a beam of light from the spectrometer and focused upon a precise location in the skin is inelastically scattered in a characteristic way based upon the vibrational, rotational, and other low frequency transitions in molecules excited by the beam. By measuring this inelastic scattering with the sophisticated spectrometer, the biochemical composition of the target and the surrounding biochemical molecules may be determined. Therefore, IVCRS is able to provide information about fluctuations in the lipid and hydration levels of the SC subsequent to therapeutic intervention with great specificity including the localization of these measurements to precise depths within the skin. However, spatially resolved measurements are limited to the SC and the upper viable epidermis with a depth resolution of 3 to 5µm at concentrations ≥0.1%, on average.
Objective
The objective of this evaluation was to assess the changes in molecular composition of the SC as a result of topical hyaluronic acid/ceramide barrier repair foam (HA/CER foam, HylatopicPlus®, Onset Dermatologics, Cumberland, Rhode Island) application on intact and purposefully damaged skin. Specifically, levels of lipid composition and hydration were targeted for analysis, as these components are critical to the function of the SC to provide essential barrier functions.1 As the test article has already demonstrated clinical evidence of improvement in SC function, the aim of this study was to better understand the biophysiological rationale of the observed improvement.
Methods
This was an open-label, bilateral, split comparison study of intact and damaged skin. Study volunteers ranged in age from 25 to 42 years and were of equal gender distribution. Four skin sites were selected and demarcated on the volar aspect of both forearms. Per randomized schedule, two of the four sites were exposed to 1% sodium lauryl sulfate (SLS) in the form of a hydrated patch affixed to the skin for 24 hours to simulate a damaged skin barrier. Upon removal of the patch, the skin was allowed to air dry for 20 minutes to address the potential problem of excessive skin hydration associated with the SLS patch exposure (Figure 1). Afterward, HA/CER foam was applied to two randomized skin sites (one intact site and one SLS-exposed site) three times daily for the duration of the study. The remaining two skin sites (one intact site and one SLS-exposed site) were left untreated and served as controls.
Figure 1.
Sodium lauryl sulfate patch-exposed skin sites allowed to air dry
Biophysical measurements, via Confocal Raman Spectrometer Model 3510 SCA, were obtained at all four skin sites per the following schedule:
Baseline, prior to SLS exposure
T0 = 24 hours post-SLS exposure (24 hours + 20 minutes post SLS exposure for sites randomized to receive SLS patch)
T2 = 2 hours post initial HA/CER foam application
T48hrs = 48 hours post initial HA/CER foam application
T7days = 7 days post initial HA/CER foam application.
Measures
Prior to placement of the spectrometer, treated skin sites were wiped gently with dry cotton to ensure the readings obtained were attributable to the constituents of the SC and not residue of the test article. Water measurements were obtained to a depth of 40µm, with a stepsize of 4µm to include the SC and the first layers of living cells. The average depth of the SC on the volar forearm is 15µm. Lipid and natural moisturizing factor (NMF) measurements were obtained to a depth of 25µm with a stepsize of 4µm. Lipid assessments were ceramide based with ceramide-3 as the model spectrum.
Molecular concentration profiles were determined from the Raman spectra using software (SkinTools 2.0) to conduct SC analyses per established practices.3 In these analyses, a set of in vitro spectra representing the major skin constituents was fitted to the measured skin spectra. The resulting fit coefficients represent the relative proportions of the targeted skin constituents. Each concentration profile, per time point, is reported as an average of 10 readings to account for the heterogeneity of the SC. Comparison was done over all 10 repeats of four volunteers, resulting in N=40. The concentration profiles, per site, were compared per time point to T0. Statistical analyses were conducted using a two-sided, paired Student’s t-test with a 95% confidence interval.
Results
Water content in the epidermis of treated skin was significantly higher than that of untreated skin for both intact and disrupted SC sites. This effect increased with duration of use for the test article. As water content at the surface (10µm) decreased, water content in the deeper layers (20–40µm) increased. This pattern closely mirrors the physiological hydration gradient of the SC and may reflect a change in SC composition subsequent to the replacement of physiological lipids to the outermost layer. Conversely, NMF content was not significantly changed over the same period except for the variations noted due to SLS application. This may be attributed to alterations in SC content due to over hydration created by the SLS patch as well as variances in biophysical compositions secondary to lipid deposition as detected with hydration assessments. Additionally, it has been reported that disruption of the SC may alter lipid levels within the SC due to exocytosis of lipids in the lamellar granules of the corneocytes secondary to calcium gradient changes imposed by SC perturbation.4 The current study did not measure or account for changes to the calcium gradient; thus, a positive or negative relationship to the study outcomes cannot be established.
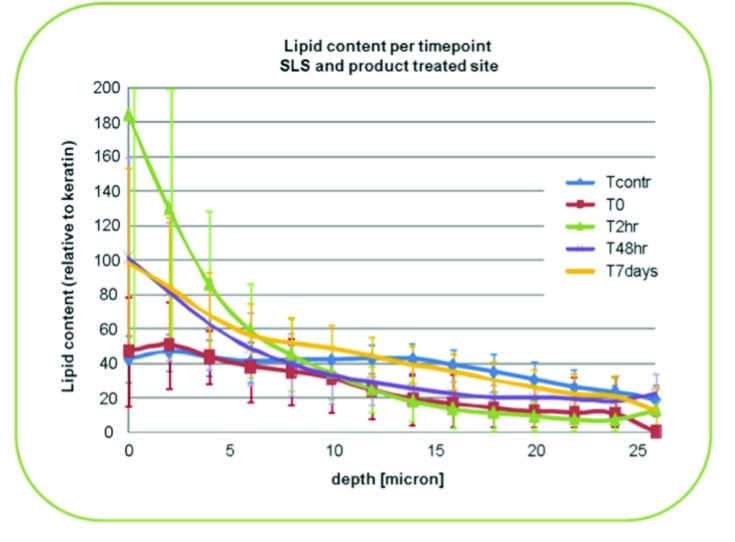
Lipid composition assessments revealed an increase in ceramide-3 spectrum content of the outer layer of the SC of treated skin for all time points. As depicted in Figure 2, lipids in the top 10µm were consistently improved as compared with baseline measures throughout the study period. The greatest increases were noted at the 2-hour and 7-day time points. The greatest increase in lipids in the deeper layers (25µm) of the SC was reported at the 48-hour time point. Due to IVCRS limitations, it is not known if these increases are attributed to the HC/CER foam ceramides that were absorbed into the measured SC depths or perhaps due to newly incorporated ceramides. Although an increase in lipids is associated with improved barrier function, such differentiation of lipid source has not been established via IVCRS methods.
Figure 2.
Lipid content changes in sodium lauryl sulfate and product-treated skin sites
Conclusion
The stratum corneum is a therapeutic target for many topical dermatological products. In particular, skin barrier repair agents seek to restore the lipid and water balance of the SC as a means of improving skin barrier function. Methods to establish the ability of these agents to replace physiological lipids and hydration to the SC, such as TEWL and corneometry, are imprecise. Using confocal Raman spectroscopy, this study evaluated precise levels of physiological lipids and water within the SC following application of HA/CER foam.
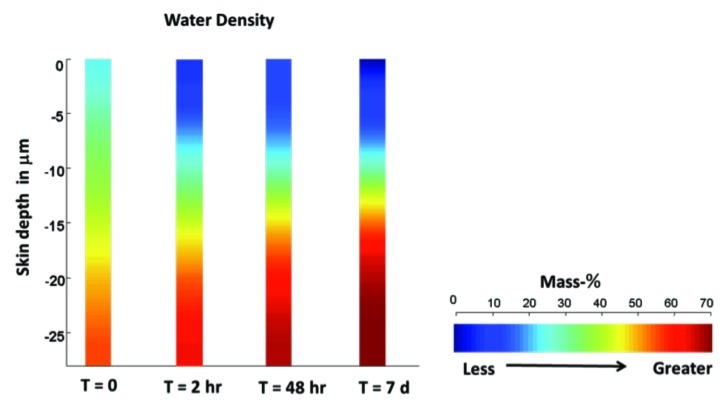
As early as two hours postapplication, skin treated with HA/CER foam showed an increase in water and lipid content compared with untreated skin. Improvements were noted in both HA/CER treatment groups (intact skin and SLS perturbed skin); however, lipid and water increases were greater in the SLS perturbed skin as compared with intact skin. These improvements continued through to the 48-hour and 7-day time points (Figure 3).
Figure 3.
Changes in water density of topical hyaluronic acid/ceramide barrier repair foam-treated skin
Collectively, these results suggest that the test article (HA/CER foam) improves barrier function through the restoration of lipid and hydration profiles associated with intact skin. Elevations in lipid and water concentrations improved with continued use of HA/CER foam. These data support the hypothesis that the study article supports restoration of essential skin barrier components that are vital to SC function. This finding is congruent with prior studies that resulted in reduced TEWL and improved atopic dermatitis symptoms with the use of HA/CER foam.5,6 It is unknown if similar results would be attained with other barrier products, namely other moisturizers, humectants, emollients and occlusives.
Improved barrier function is known to improve outcomes when treating dermatitis and has also been associated with improved immune defense, including stabilization of antimicrobial peptides in atopic dermatitis patients.1,7 Additional research to assess these effects in atopic skin, and to compare the effects of differing products and/or formulations, is needed to better understand the therapeutic effects of barrier restoration in atopic diseased skin.
References
- 1.Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4(9):22–42. [PMC free article] [PubMed] [Google Scholar]
- 2.Chilcott RP, Dalton CH, Emmanuel AJ, Allen CE, Bradley ST. Transepidermal water loss does not correlate with skin barrier function in vitro. J Invest Dermatol. 2002;118(5):871–875. doi: 10.1046/j.1523-1747.2002.01760.x. doi: 10.1046/j.1523-1747.2002.01760.x. [DOI] [PubMed] [Google Scholar]
- 3.Caspers PJ, Lucassen GW, Carter EA, Bruining HA, Puppels GJ. In vivo confocal Raman microspectroscopy of the skin: noninvasive determination of molecular concentration profiles. J Invest Dermatol. 2001;116(3):434–442. doi: 10.1046/j.1523-1747.2001.01258.x. doi: 10.1046/j.1523-1747.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- 4.Elias P, Ahn S, Brown B, Crumrine D, Feingold KR. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs. passive mechanisms. J Invest Dermatol. 2002;119(6):1269–1274. doi: 10.1046/j.1523-1747.2002.19622.x. [DOI] [PubMed] [Google Scholar]
- 5. Trumbore M, Gurge R. A hyaluronic acid-ceramide based prescription emollient foam provides significant improvement of skin barrier function and skin hydration. Poster presented at Fall Clinical Dermatology Meeting. October 2010; Las Vegas, Nevada. [Google Scholar]
- 6.Frankel A, Sohn A, Patel RV. Lebwohl, M. Bilateral comparison study of pimecrolimus cream 1% and a ceramide-hyaluronic acid emollient foam in the treatment of patients with atopic dermatitis. J Drugs Dermatol. 2011;10(6):666–672. [PubMed] [Google Scholar]
- 7.Park KY, Kim DH, Jeong MS, Li K, Seo SJ. Changes of antimicrobial peptides and transepidermal water loss after topical application of tacrolimus and ceramide-dominant emollient in patients with atopic dermatitis. J Korean Med Sci. 2010;25(5):766–771. doi: 10.3346/jkms.2010.25.5.766. doi: 10.3346/jkms.2010.25.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]