Congestive heart failure (CHF) is the leading cause of hospitalization in those older than 65 years in the United States. More than 5 million Americans have CHF, with an annual incidence approaching 10 per 1000 among the United States population older than 65. The disease is costly and deadly. More than 5% of the national health care budget dollars are spent on CHF.1 The 5-year mortality associated with CHF still remains high at 50%, despite recent advances in treatment.2 CHF results in approximately 300,000 deaths each year.
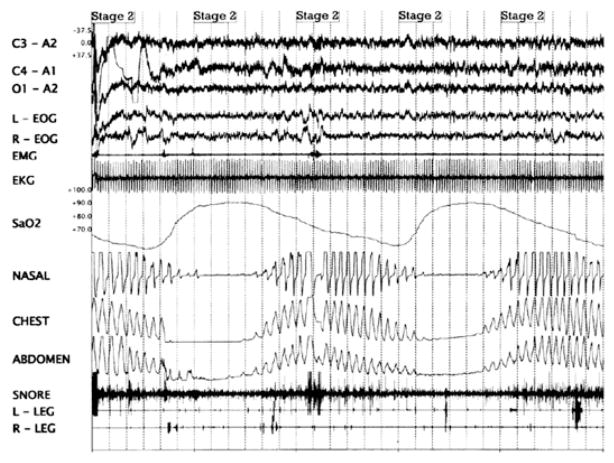
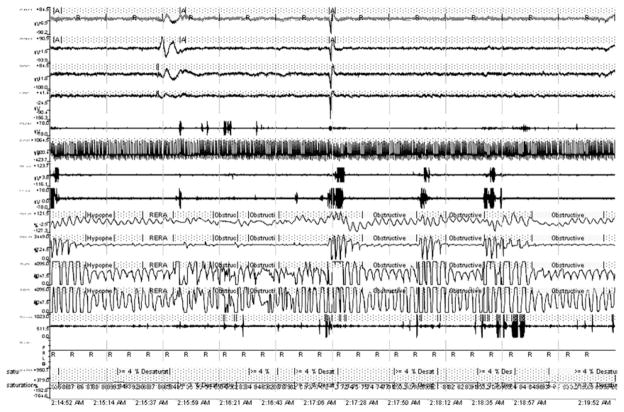
Breathing disorders during sleep are common in CHF.3–5 Sleep-disordered breathing (SDB) in CHF can be broadly classified as 2 types: central sleep apnea with Cheyne-Stokes breathing (CSA-CSB), and obstructive sleep apnea (OSA). The 2 can occur together. CSB is characterized by crescendo-decrescendo changes in tidal volume that result in central apneas (lack of airflow without respiratory effort) as shown in Fig. 1. OSA is characterized by repeated pharyngeal airway collapse during sleep, resulting in repetitive episodes of oxygen desaturation episodes despite ongoing respiratory effort, and arousals (Fig. 2). Three to four percent of women and 6% to 9% of men in the general population have OSA, when defined as an apnea-hypopnea index (AHI; number of apneas and hypopneas per hour of sleep) greater than 5 events per hour accompanied by daytime sleepiness or cardiovascular morbidity such as hypertension.6 CHF may contribute to SDB by various mechanisms, including increased pharyngeal wall edema and unstable ventilatory control (discussed in a later section). OSA may also impair cardiac function acutely by increasing afterload, caused by increased ventricular transmural pressure during ongoing respiratory efforts; and chronically by OSA’s association with increased sympathetic activity.7 OSA further complicates CHF by contributing to hypertension, myocardial infarction, stroke, and nocturnal arrhythmias.
Fig. 1.
Polysomnogram showing Cheyne-Stokes breathing. There are crescendo-decrescendo changes in tidal volume that result in central apneas (as shown in the chest and abdominal respiratory movements in the polysomnogram).
Fig. 2.
Polysomnogram showing obstructive sleep apnea. There are repetitive episodes of oxygen desaturation episodes despite ongoing respiratory effort (as shown by thoracic and abdominal respiratory movements), and arousals. Apnea exists when airflow is less than 20% of baseline for at least 10 seconds in adults. Hypopnea exists when airflow decreases at least 30% from baseline, there is diminished airflow lasting at least 10 seconds, at least 90% of the duration of diminished airflow is spent with airflow that is at least 30% less than baseline, and decreased airflow is accompanied by at least 4%oxyhemoglobin desaturation. Respiratory effort related arousals (RERAs) exist when there is a sequence of breaths that lasts at least 10 seconds, is characterized by increasing respiratory effort or flattening of the nasal pressure waveform, and leads to an arousal from sleep, but does not meet the criteria of an apnea or hypopnea.
In this article, the authors discuss the epidemiology of SDB in CHF, the pathophysiology of CSA and OSA in CHF, and the clinical consequences and management of SDB in CHF, including positive airway pressure (PAP).
EPIDEMIOLOGY OF SLEEP-DISORDERED BREATHING IN CONGESTIVE HEART FAILURE
Prevalence of SDB in CHF is difficult to estimate, as there are various criteria used to define sleep apnea, including the threshold of AHI, definition of hypopnea, and the criteria used to differentiate CSA from OSA. Most studies to date have focused on systolic (low ejection fraction) CHF. In the first study using polysomnography in systolic CHF patients by Javaheri and colleagues,3 the prevalence of SDB (defined by AHI ≥20 events/h) was 51% (40% CSA and 11% OSA). A large retrospective study in 450 subjects with systolic CHF reported a 61% prevalence of SDB using criteria of 15 events/h or greater. The prevalence of OSA was higher in this study (32%) than that of CSA (29%), likely due to the criteria used to define hypopnea or selection bias due to the retrospective nature of the study.5 Another study demonstrated a 55% prevalence of CSB in CHF patients with left ventricular ejection fraction (LVEF) less than 40%.8 Most of these studies were done before the current heart failure treatment guidelines were formulated and implemented, and did not necessarily not reflect current prevalence in the setting of widespread use of, for example, β-blockers in the treatment of CHF.
Recent studies have continued to show a high prevalence of SDB in patients with CHF treated with current guideline-based therapy. In the largest study done in systolic CHF patients treated with current guidelines, SDB was present in 76% of patients (40% had CSA and 36% had OSA)9—a prevalence and distribution generally confirmed by other studies.10–12 Between 1997 and 2004, the prevalences of OSA and CSA did not change significantly despite increased use of β-blockers and spironolactone, and an increase in LVEF. This relatively high prevalence of SDB in CHF does not reflect referral bias to sleep clinics: the prevalence was still 61% in 108 consecutive stable CHF patients on maximal therapy assessed in a heart failure clinic population, that is, with no referral bias to a sleep clinic (31% had CSA-CSB and 30% had OSA).13 Thus, SDB prevalence in CHF has remained high despite medical advances in the treatment of CHF. The sustained high prevalence of SDB appears to be independent of the effect of improved survival, and thus increasing age (a risk factor for the development of SDB), in this population.
To the authors’ knowledge, only one study has carefully investigated the prevalence of SDB in patients with stable diastolic (preserved ejection fraction) heart failure. In this study 55% of patients had SDB, predominantly OSA.14 The causal pathways mediating this association are unclear but given the strong link between OSA and hypertension, diastolic dysfunction may well be related to left ventricular hypertrophy resulting from hypertension. Unlike systolic heart failure, SDB may be a cause, rather than an effect, of diastolic heart failure.
Table 1 provides a summary of prevalence data in the literature. Prevalence of SDB ranges from 47% to 76% in systolic CHF. CSA is usually more common than OSA, particularly in studies in which men outnumber women. The factors associated with SDB in CHF in these epidemiologic studies are summarized in Table 2.
Table 1.
Clinical studies showing prevalence of sleep-disordered breathing (SDB) in congestive heart failure (CHF)
| First Author | Year | Number of CHF Patients | Characteristics of CHF Patients | Definition of SDB | Results |
|---|---|---|---|---|---|
| Javaheri3 | 1998 | 81 | LVEF <45% | AHI >20/h | 51% Prevalence of SDB (CSA 540%, OSA 511%) |
| Sin5 | 1999 | 450 | Known CHF, NYHA class II–IV | AHI ≥15/h | 61% Prevalence of SDB (CSA 529%, OSA 532%) |
| Lanfranchi8 | 1999 | 62 | LVEF ≤35% NYHA class II–III | Studied only CSA | 55% Prevalence of CSB |
| Oldenburg9 | 2007 | 700 | LVEF ≤40%, NYHA class ≥II | AHI ≥5/h | 76% Prevalence of SDB (CSA 540%, OSA 536%) |
| Yumino10 | 2009 | 218 | LVEF ≤45% | AHI ≥15/h | 47% Prevalence of SDB (CSA 521%, OSA 526%) |
| Schulz11 | 2007 | 203 | LVEF <40%, NYHA class II–III | AHI≥10/h | 71% Prevalence of SDB (CSA 528%, OSA 543%) |
| MacDonald13 | 2008 | 108 | LVEF <40%, NYHA class II–IV | AHI ≥15/h | 61% Prevalence of SDB (CSA 531%, OSA 530%) |
| Chan14 | 1997 | 20 | Diastolic CHF, NYHA class II–III | AHI ≥10/h | 55% Prevalence of SDB (OSA 535%, CSA 520%) |
| Padeletti12 | 2009 | 29 | Decompensated CHF (mean LVEF 5 20%) | AHI ≥15/h | 76% Prevalence of SDB, predominantly CSA-CSB |
Abbreviations: AHI, apnea-hypopnea index; CSA, central sleep apnea; CSB, Cheyne-Stokes breathing; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OSA, obstructive sleep apnea.
Table 2.
Factors associated with SDB in CHF
| Central Sleep Apnea | Obstructive Sleep Apnea |
|---|---|
| Severity of NYHA functional class | Obesity |
| Atrial fibrillation | Habitual snoring |
| Awake hypocapnia (PaCO2 <36 mm Hg) | |
| Nocturnal ventricular arrhythmias | |
| LVEF <20% |
PATHOPHYSIOLOGY OF SLEEP-DISORDERED BREATHING IN CONGESTIVE HEART FAILURE
Mechanisms of CSA-CSB in Heart Failure
Prolonged circulation time secondary to low cardiac output in systolic CHF was traditionally thought to be the main cause of CSB. It was hypothesized that delayed transmission of changes in arterial blood gases to central and peripheral chemoreceptors would cause ventilatory undershoot and overshoot. However, it has been shown that there is no difference in cardiac output, LVEF, or blood circulation time from the lungs to chemoreceptors in carefully matched CHF patients with and without CSA-CSB,15 challenging the hypothesis that prolonged circulatory time plays a key role in CSA-CSB in patients with CHF.
CSA in CHF is predominantly caused by instability of ventilatory control systems. Patients with CSA-CSB have increased peripheral and central chemoresponsiveness that promotes hyperventilation and hypocapnia.16–19 Patients with CSA-CSB in CHF typically have a chronic respiratory alkalosis due to hyperventilation during both wake-fulness and sleep. An important factor contributing to chronic hyperventilation is pulmonary vagal irritant receptor stimulation by pulmonary venous congestion.20–23 Hyperventilation often drives the PCO2 below the apneic threshold, leading to decreased central respiratory drive. Patients with CSA-CSB in CHF also have abnormal cerebrovascular reactivity to CO2 leading to a greater tendency to develop ventilatory undershoot in response to a greater degree of alkalosis than normal, causing central apnea.24
Mechanisms of OSA in Heart Failure
Patients with CSA-CSB in CHF have also been found to have obstructive apnea episodes at the end of central apneas in almost one-third of patients.25 Direct fiberoptic observation has shown episodes of upper airway narrowing and closure during central apneas.26 This narrowing could be the effect of reduced neural output to the upper airway muscles during the nadir of ventilation, in addition to an anatomically narrow upper airway.26
OSA alone can also occur in CHF. Like other patients with OSA, obesity and age are significant risk factors. Obesity may be a marker for narrowing of the upper airway because of deposition of pharyngeal fat or reduced end-expiratory lung volume. However, the prevalence of obesity in CHF patients is not very high, most patients being clinically overweight (mean body mass index [BMI; body weight divided by height squared] 28 kg/m2)3 and mildly obese.5 CHF patients may have increased risk for OSA due to extracellular fluid overload. Pharyngeal edema and narrowing may develop during supine sleep with redistribution of fluid from the legs. This observation was supported by the observed improvement in OSA after diuretic therapy in a group of patients with diastolic CHF and OSA.27 The mechanisms of SDB in CHF are summarized in Table 3.
Table 3.
Principal mechanisms contributing to SDB in CHF
DIAGNOSIS OF SLEEP-DISORDERED BREATHING IN CONGESTIVE HEART FAILURE
An in-laboratory overnight polysomnogram with an attendant technician is the gold standard for diagnosing SDB. CHF patients commonly have both central and obstructive apneas occurring together, although 1 of the 2 SDB patterns may predominate.3,5 Patients with CHF who report snoring, excessive daytime somnolence or fatigue, and poor sleep quality should have their sleep assessed by polysomnography in a sleep laboratory. Paroxysmal nocturnal dyspnea may actually reflect CSA-CSB, with an arousal during a period of hyperpnea. Polysomnography could also be considered in patients with heart failure who have nocturnal angina, recurrent arrhythmias, refractory heart failure symptoms, or in whom an abnormal respiratory pattern has been witnessed. Questionnaires like the Epworth Sleepiness Scale (ESS),28 the Berlin Questionnaire,29 and the Maislin Questionnaire30 can be used to screen patients for SDB; however, CHF patients typically do not report excessive daytime sleepiness. CHF patients have less subjective daytime sleepiness as measured by ESS despite having less total sleep time compared with subjects without CHF.
A variety of portable home polysomnograph devices are also available to diagnose SDB, but none is currently recommended.31,32 Home nocturnal oximetry has 85% sensitivity and 93% specificity to diagnose SDB in CHF, but may not distinguish between CSA and OSA accurately.33 Home respiratory telemonitoring might constitute a potential low-cost alternative to traditional polysomnography in the evaluation and management of SDB in CHF patients.34 Heart rate variation in response to apnea events has been used to diagnose SDB in patients with CHF.35 Thoracic impedance monitoring has been used to measure cardiac output variation in response to apnea events in CHF patients with SDB.36 These new techniques are not being used widely to diagnose SDB in CHF patients, and the authors await further data before giving any definitive recommendations.
PATHOPHYSIOLOGICAL CONSEQUENCES OF SLEEP-DISORDERED BREATHING IN CONGESTIVE HEART FAILURE
CSB results in recurrent hypoxemia, hypercapnia, and hypocapnia, and increased negative intrathoracic pressure. These changes cause release of inflammatory mediators, increases in transmural pressure in cardiac chambers, and diminished oxygen delivery to tissues. The mean overnight urinary norepinephrine excretion (UNE) level is significantly elevated in CHF patients with either OSA or CSA-CSB compared with those with CHF and no SDB, indicating increased overnight sympathetic activity.7 CSA-CSB is associated with nocturnal ventricular arrhythmias—premature ventricular contractions (PVCs), couplets, and ventricular tachycardia—which decrease significantly when CSA is suppressed using continuous positive airway pressure (CPAP).37 PVCs may be seen more in the hypercapnic phase of CSA than the apneic phase.38 In addition, atrial fibrillation is also common in CHF patients with CSA,3,39 although the causal relationship is unclear.
Whether these nocturnal arrhythmias or CSA-CSB affect outcome is unknown. Although patients with CSA-CSB and CHF have worse quality of life than those without CSA-CSB,40 CSA is not clearly associated with increased mortality in CHF patients.15,41 Thus, the data remain controversial as to whether CSA has independent prognostic utility in CHF.
OSA and potential relationships to cardiovascular function have been better studied. Ongoing respiratory efforts against an occluded upper airway cause intrathoracic pressure swings that increase systolic transmural pressure, increase left ventricular afterload, and thus reduce cardiac output.42 OSA has been shown to cause left ventricular systolic43,44 and diastolic45 dysfunction even in patients without a history of CHF, which improved after nocturnal CPAP treatment for 6 months. Increased sympathetic activity during OSA results in increased heart rate, vasoconstriction, and increased peripheral resistance.46 OSA is also a well-recognized independent risk factor for hypertension47 and coronary artery disease,48 which may worsen CHF. OSA may yield acceleration of atherosclerosis by causing inflammation and endothelial dysfunction. This finding is supported by the observation that OSA patients have high plasma C-reactive protein concentrations (in some but not all studies)49 and increased reactive oxygen species production in neutrophils.50 Thus OSA may exacerbate CHF through a variety of mechanisms.
TREATMENT OF SLEEP-DISORDERED BREATHING IN CONGESTIVE HEART FAILURE
The first step in the management plan for a patient with SDB and CHF should be optimization of CHF treatment. As discussed earlier, use of diuretics27 reduced OSA in CHF patients, and lowering of the pulmonary capillary wedge pressure can improve CSA. Captopril51 and carvedilol52,53 may also reduce CSA in CHF patients.
General measures such as avoiding supine sleep, attempting to reduce weight if obese, and avoidance of the use of benzodiazepines and alcoholic beverages before bedtime may decrease the likelihood of upper airway occlusion during sleep, thus reducing OSA in CHF patients. Treatment of OSA with a mandibular advancement device in mild to moderate stable CHF reduced AHI and plasma brain natriuretic peptide level, but there was no improvement in LVEF or health-related quality of life.54 Despite these alternative therapies, many patients will require PAP to eliminate apneas.
Positive Airway Pressure
Positive airway pressure devices have been successfully used to treat OSA in stable CHF patients. Nocturnal CPAP use in patients with CHF and OSA can result in reductions in left ventricular transmural pressure, which may increase LVEF.55 CPAP use has been shown to improve LVEF in some56–59 (but not all60) studies, improve quality of life, and to lower overnight UNE,57 daytime systolic blood pressure, and left ventricular end-systolic diameter58 in patients with stable systolic CHF and OSA. Negative results in one study may be explained by the low CPAP use (mean CPAP usage was 3.5 h/night). In a meta-analysis of randomized controlled trials comparing the effect of nocturnal CPAP therapy on LVEF in patients with stable systolic CHF and OSA, the pooled odds ratio for improvement in LVEF after CPAP therapy was 7.3.61 The effect is probably rapid: significant improvement in LVEF was also seen after 3 days of PAP therapy in patients with decompensated CHF and OSA.62–64 The results of the randomized controlled trials evaluating PAP therapy in CHF and OSA are summarized in Table 4.
Table 4.
Randomized controlled trials studying effect of positive airway pressure therapy in patients with CHF and obstructive sleep apnea
| First Author | Year | Number of CHF Patients | Intervention | Duration of Intervention | Control | Results |
|---|---|---|---|---|---|---|
| Egea56 | 2008 | 60 | CPAP | 3 mo | Sham CPAP | Improvement in LVEF |
| Mansfield57 | 2004 | 55 | CPAP | 3 mo | Medical therapy for CHF | Significant improvement in LVEF and QOL, reduction in overnight UNE |
| Kaneko58 | 2003 | 24 | CPAP | 1 mo | Medical therapy for CHF | Improvement in LVEF, reduction in LVESD |
| Smith60 | 2007 | 26 | Autotitrating CPAP | 6 wk | Sham CPAP | Improvement in daytime sleepiness, but not in LVEF or exercise capacity |
| Khayat62 | 2009 | 46 | Autotitrating CPAP | 3 d | Medical therapy for decompensated CHF | Significant improvement in LVEF |
Abbreviations: CPAP, continuous positive airway pressure; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; QOL, quality of life; UNE, urinary norepinephrine excretion.
In various studies with a follow-up period of 1 to 3 months, CPAP was an effective treatment for CHF with CSA-CSB, improving SDB,65–67 LVEF,65,66,68 and inspiratory muscle strength,66 and decreasing urinary norepinephrine levels.67 CPAP treatment was associated with a 60% relative risk reduction in mortality-cardiac transplantation rate in patients on CPAP therapy for a mean of 2.2 years.65 In another study with longer follow-up, LVEF and sleep quality were significantly improved after 3 months of CPAP use in CHF patients, and the improvement persisted after 12 months.69
The Canadian Continuous Positive Airway Pressure Trial for Congestive Heart Failure Patients with Central Sleep Apnea (CANPAP) studied 258 CHF patients with CSA with nocturnal CPAP for 2 years (n = 128) against a control group without CPAP (n = 130).68 The CPAP group had the expected decrease in AHI, but improvements were also seen in UNE levels, mean nocturnal oxygen saturation, LVEF, and 6-minute walk distance compared with the control group after 3 months. However, these changes did not lead to differences between the 2 groups in terms of number of hospitalizations, quality of life, or transplant-free survival (primary outcome). There was actually an early increase in mortality in the CPAP-treated group, although later survival was better then the control group. A major finding of this trial is that CSA suppression by CPAP was not adequate (CPAP reduced the mean AHI to 19 events per hour of sleep, which remained above the trial inclusion threshold of 15 events/h). In a post hoc analysis, the patients whose CSA was suppressed below 15 events/h with CPAP treatment experienced a greater increase in LVEF and did have better transplant-free survival at 3 months compared with control subjects.70 These results suggested that in CHF patients, CPAP might improve both LVEF and heart transplant–free survival if CSA is suppressed soon after its initiation. However, the improvement in apnea may simply be a marker of less severe underlying disease, emphasizing the need for further randomized trials.
Other technologies exist for treatment of apneas in CHF, although outcome data such as with CPAP are not yet available. For example, bilevel ventilation has been found to be equally as effective as CPAP in improving sleep quality, New York Heart Association functional class, and circulation time in patients with CSA and CHF.71 Flow-targeted dynamic bilevel positive airway pressure (BPAP) was found to reduce AHI to a significantly lower level than untreated, CPAP, or fixed BPAP groups, and also reduced AHI below threshold level of 15 events/h in all CHF patients who had residual CSA after treatment with CPAP and fixed BPAP.72 Adaptive servoventilation (ASV) is a new technology that adjusts the delivered pressure support according to the measured air flow of the patient. Use of ASV in systolic CHF patients with CSA caused a significant reduction in the AHI73,74 and UNE level,74 and improved LVEF,73 quality of life, and cardiopulmonary exercise testing parameters.75 ASV suppressed CSA-CSB better than CPAP and nocturnal O2 in CHF patients.76 Several ongoing clinical trials are further investigating the effect of ASV on various parameters including survival, LVEF, exercise capacity, and quality of life in patients with CHF and CSA or CPAP-refractory sleep apnea.77–79 The CANPAP II study has recently been funded to test the hypothesis that these newer devices may be useful in treating SDB in CHF. However, at the present time hard outcome data are generally lacking for these newer devices. A summary of controlled studies evaluating use of PAP in CHF patients with CSA is shown in Table 5.
Table 5.
Controlled trials studying effect of positive airway pressure therapy in patients with CHF and central sleep apnea/Cheyne-Stokes breathing
| First Author | Year | Number of CHF Patients | Intervention | Duration of Intervention | Control | Results |
|---|---|---|---|---|---|---|
| Sin65 | 2000 | 66 | CPAP | 3 mo | Medical therapy for CHF | Improvement in LVEF, reduction in combined mortality-cardiac transplantation rate |
| Granton66 | 1996 | 17 | CPAP | 3 mo | Medical therapy for CHF | Improvement in LVEF and inspiratory muscle strength (MIP) |
| Naughton67 | 1995 | 35 | CPAP | 1 mo | Medical therapy for CHF | Reduction in UNE and PNE level |
| Bradley68 | 2005 | 258 | CPAP | 3 mo | Medical therapy for CHF | Improvement in exercise capacity, LVEF and mean oxygen saturation; no difference in survival after 2 years follow-up. Better heart transplant–free survival in CSB-suppressed group in post hoc analysis80 |
| Köhnlein71 | 2002 | 35 | Bilevel ventilation, CPAP | 2 wk | Crossover design | Significant and equivalent improvement in sleep quality, circulation time, and NYHA class with both bilevel ventilation and CPAP |
| Arzt70 | 2008 | 14 | BPAP, CPAP, dynamic BPAP | Consecutive night use of all 3 PAP devices | BPAP, CPAP | Dynamic BPAP suppressed CA-CSB better than CPAP or BPAP |
| Pepperell74 | 2003 | 30 | ASV | 1 mo | Subtherapeutic level of ASV | Improvement in LVEF; reduction in serum BNP and urinary catecholamine excretion |
| Hastings75 | 2008 | 19 | ASV | 6 mo | Medical therapy for CHF | Improvement in LVEF and QOL |
| Teschler76 | 2001 | 14 | ASV, BPAP, CPAP, nocturnal O2 | 1 night | Crossover design | ASV suppressed CSA-CSB better than CPAP and nocturnal O2, but not BPAP; LVEF not measured |
Abbreviations: ASV, adaptive seroventilation; BNP, brain natriuretic peptide; BPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; CSB, Cheyne-Stokes breathing; LVEF, left ventricular ejection fraction; MIP, maximal inspiratory pressure; NYHA, New York Heart Association; PNE, peripheral norepinephrine excretion; QOL, quality of life; UNE, urinary norepinephrine excretion.
These studies show the importance of keeping a high index of suspicion for SDB in patients with CHF. PAP devices thus may be used in CHF patients with SDB who have symptoms of excessive daytime sleepiness or other comorbidities like hypertension; however, their use to improve CHF outcome (including survival and LVEF) is not recommended at present. It is clear that further research is needed in this area.
Nocturnal Oxygen Therapy
Nocturnal oxygen therapy has been shown to reduce central sleep apnea in CHF. Treatment with oxygen was found to improve exercise capacity,80–83 decrease overnight urinary norepinephrine secretion,80 and decrease muscle sympathetic activity84 in CHF patients with CSA. In addition, nocturnal oxygen also improved LVEF and quality of life in CHF patients.82,83
Supplemental CO2 Therapy
Adding dead space (as a form of supplemental CO2) using a facemask attached to a cylinder of adjustable volume improved CSA and sleep quality in CHF patients.85 Several researchers have investigated either inspiring CO2 or adding dead space, but these approaches are rarely used clinically. Inspired CO2 can lead to considerable insomnia, and was classically used by psychiatrists to induce panic attacks in diagnostic testing. Thus, these treatment approaches remain theoretical at the present time.
Theophylline
Oral theophylline therapy for 5 days in stable CHF patients (LVEF <45%) improved SDB in a study,86 although the mechanism by which theophylline improves CSA in CHF is unclear. At therapeutic serum concentrations, theophylline competes with adenosine at some of its receptor sites. In the central nervous system, adenosine is a respiratory depressant, and theophylline stimulates respiration by antagonizing adenosine. It is therefore conceivable that an increase in ventilation as a result of treatment with theophylline results in a decreased number of episodes of central apnea during sleep.87 In addition, theophylline is likely a potent inotrope in the short term, when the agent is given to methylxanthine-naïve hearts. Because there are no controlled long-term studies, theophylline is not commonly used to treat CSA, perhaps in part due to its arrhythmogenic potential.
Acetazolamide
Use of acetazolamide orally before bedtime daily for 6 nights resulted in significant reduction in AHI and improved sleep quality.16 Acetazolamide is a mild diuretic and thus may decrease pulmonary venous congestion in CHF, which contributes to CSA. In addition, acetazolamide induces nonanion gap metabolic acidosis, which stimulates breathing; this results in reduction in the apneic threshold to PCO2, which decreases the likelihood of developing central sleep apnea.88
Atrial Overdrive Pacing
Atrial overdrive pacing (AOP) in patients with central or obstructive sleep apnea who had received permanent atrial-synchronous ventricular pacemakers for symptomatic sinus bradycardia decreased apneas and hypopneas significantly in a single study.89 However, another study failed to show any beneficial effect.90 Atrial overdrive pacing exerted a mild effect on apnea-hypopnea events in CHF patients with OSA, but the results were less effective than for CPAP therapy.91
Left Ventricular Assist Device
Correction of CSA and significant improvement in exercise capacity and symptoms after implantation of a left ventricular assist device in CHF patients was reported in one study.92
Cardiac Resynchronization Therapy
Cardiac resynchronization therapy (CRT), which improves cardiac output by coordinating right and left ventricular contraction, leads to a reduction of CSA and to increased sleep quality in patients with CHF and SDB.93 CRT also improved cardiac function and reduced the AHI in patients with both OSA and CHF.94 CRT combined with AOP resulted in a significant but minor additional improvement of CSA compared with CRT alone.95 Both AOP and CRT may reduce SDB by increasing cardiac output, thus decreasing pulmonary venous congestion. Decrease in pharyngeal wall edema may also decrease OSA in these patients. The ongoing Impact of Resynchronization Therapy on Sleep Disordered Breathing in Advanced Congestive Heart Failure (IMPACT) trial is further investigating the effect of CRT with or without AOP on SDB and sleep quality in patients with CHF and SDB.96
Cardiac Transplantation
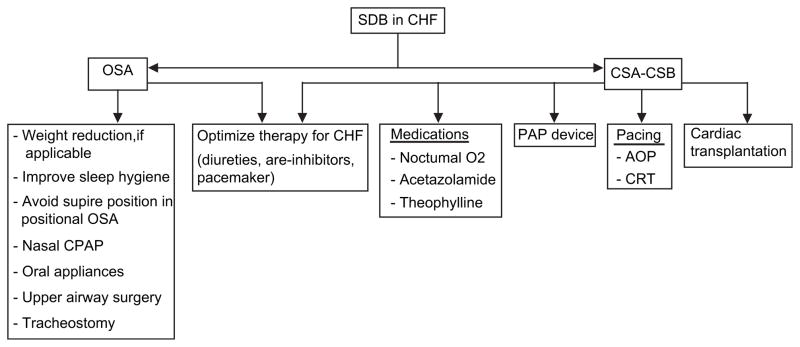
Thirteen CHF patients with CSA were studied before and after successful cardiac transplantation, there was improvement in SDB after 6 months of follow-up, but CSA persisted in 3 patients and 4 patients acquired OSA after 13 months.97 A similar study confirmed that 36% of CHF patients who had successful cardiac transplantation developed OSA, likely due to weight gain and fat deposition secondary to the use of steroids for immunosuppression. A suggested algorithm for managing SDB in CHF is shown in Fig. 3.
Fig. 3.
A suggested algorithm for management of sleep-disordered breathing in congestive heart failure.
PERIODIC LIMB MOVEMENTS AND CONGESTIVE HEART FAILURE
The prevalence of periodic limb movements (PLMs; defined as a PLM index >5 events/h) in adult systolic CHF patients was 19% in one study.98 The proportion of stable CHF patients with moderately severe PLM (>25/h) was significantly higher (52%) than control subjects (11%) in another study by Hanly and colleagues.99 Proposed hypotheses for the high prevalence of PLMs in CHF patients are (a) electrolyte and acid-base abnormalities associated with CHF, and (b) stimulation of a spinal reflex in conjunction with cortical inhibition during sleep secondary to reduced peripheral blood flow due to CHF.100 The clinical relevance or prognostic value of these findings is unknown.
SUMMARY
CHF and SDB are common diseases, and they frequently exist together. CHF may contribute to SDB, and SDB in turn further worsens CHF. CHF patients with SDB have a worse prognosis and may have increased mortality. Optimum treatment of CHF as per guidelines is the first step in the management of SDB in CHF. PAP may improve AHI as well as LVEF in CHF patients with OSA. Positive pressure ventilation may also improve LVEF and survival in the subgroup of CHF patients in whom CSA is adequately suppressed. Newer devices may be more successful than conventional positive pressure ventilation in suppressing CSA in CHF; however, long-term clinical trials are required to show efficacy of these novel treatments in improving survival in CHF patients with SDB.
Acknowledgments
A.M. has received consulting and/or research funding from Philips, Pfizer, NMT, Apnex, Cephalon, Itamar, Sepracor, Restore/Medtronic, Ethicon, SGS, Novartis. He has an established investigator award from the American Heart Association, and is Principal Investigator on NIH HL73146 R01 HL085188-01A2 R01 HL090897-01A2 K24 HL 093218 – 01 A1.
References
- 1.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA Guidelines for the evaluation and management of chronic heart failure in the adult: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2001;104(24):2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Kenechaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97(21):2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 4.Tremel F, Pépin JL, Veale D, et al. High prevalence and persistence of sleep apnoea in patients referred for acute left ventricular failure and medically treated over 2 months. Eur Heart J. 1999;20(16):1201–9. doi: 10.1053/euhj.1999.1546. [DOI] [PubMed] [Google Scholar]
- 5.Sin DD, Fitzgerald F, Parker JD, et al. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solin P, Kaye DM, Little PJ, et al. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest. 2003;123(4):1119–26. doi: 10.1378/chest.123.4.1119. [DOI] [PubMed] [Google Scholar]
- 8.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–40. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 9.Oldenburg O, Lamp B, Faber L, et al. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9(3):251–7. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail. 2009;15(4):279–85. doi: 10.1016/j.cardfail.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Schulz R, Blau A, Börgel J, et al. Sleep apnoea in heart failure. Eur Respir J. 2007;29(6):1201–5. doi: 10.1183/09031936.00037106. [DOI] [PubMed] [Google Scholar]
- 12.Padeletti M, Green P, Mooney AM, et al. Sleep disordered breathing in patients with acutely decompensated heart failure. Sleep Med. 2009;10(3):353–60. doi: 10.1016/j.sleep.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald M, Fang J, Pittman SD, et al. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4(1):38–42. [PMC free article] [PubMed] [Google Scholar]
- 14.Chan J, Sanderson J, Chan W, et al. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest. 1997;111(6):1488–93. doi: 10.1378/chest.111.6.1488. [DOI] [PubMed] [Google Scholar]
- 15.Findley LJ, Zwillich CW, Ancoli-Israel S, et al. Cheyne-Stokes breathing during sleep in patients with left ventricular heart failure. South Med J. 1985;78(1):11–5. doi: 10.1097/00007611-198501000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bradley TD. Crossing the threshold: implications central sleep apnea. Am J Respir Crit Care Med. 2002;165(9):1203–4. doi: 10.1164/rccm.2203016. [DOI] [PubMed] [Google Scholar]
- 17.Javaheri S. Acetazolamide improves central sleep apnea in congestive heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173(2):234–7. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 18.Solin P, Roebuck T, Johns DP, et al. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162(6):2194–200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 19.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341(13):949–54. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 20.Solin P, Bergin P, Richardson M, et al. Influence of pulmonary capillary wedge pressure on central sleep apnea in heart failure. Circulation. 1999;99(12):1574–9. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 21.Lorenzi-Filho G, Azevedo ER, Parker JD, et al. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J. 2002;19(1):37–40. doi: 10.1183/09031936.02.00214502. [DOI] [PubMed] [Google Scholar]
- 22.Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973;53(1):159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- 23.Oldenburg O, Bitter T, Wiemer M, et al. Pulmonary capillary wedge pressure and pulmonary arterial pressure in heart failure patients with sleep-disordered breathing. Sleep Med. 2009;10(7):726–30. doi: 10.1016/j.sleep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Xie A, Skatrud JB, Khayat R, et al. Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med. 2002;165(3):371–8. doi: 10.1164/rccm.200406-807OC. [DOI] [PubMed] [Google Scholar]
- 25.Dowdell WT, Javaheri S, McGinnis W. Cheyne-Stokes respiration presenting a sleep apnea syndrome: clinical and polysomnographic features. Am Rev Respir Dis. 1990;141(4 Pt 1):871–9. doi: 10.1164/ajrccm/141.4_Pt_1.871. [DOI] [PubMed] [Google Scholar]
- 26.Badr S, Toiber F, Skatrud J, et al. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995;78(5):1806–15. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 27.Bucca CB, Brussino L, Battisti A, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132(2):440–6. doi: 10.1378/chest.07-0311. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epsworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 30.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18(3):158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 31.Chesson AL, Jr, Berry RB, Pack A. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep. 2003;26(7):907–13. doi: 10.1093/sleep/26.7.907. [DOI] [PubMed] [Google Scholar]
- 32.American Thoracic Society/American College of Chest Physicians/American Academy of Sleep medicine Taskforce Steering Committee. Executive summary on the systematic review and practice parameters for portable monitoring in the investigation of suspected sleep apnea in adults. Am J Respir Crit Care Med. 2004;169(10):1160–3. doi: 10.1164/rccm.169.1160. [DOI] [PubMed] [Google Scholar]
- 33.Sériès F, Kimoff RJ, Morrison D, et al. Prospective evaluation of nocturnal oximetry for detection of sleep-related breathing disturbances in patients with chronic heart failure. Chest. 2005;127(5):1507–14. doi: 10.1378/chest.127.5.1507. [DOI] [PubMed] [Google Scholar]
- 34.Mortara A, Pinna GD, Johnson P, et al. Home telemonitoring in heart failure patients: the HHH study (Home or Hospital in Heart Failure) Eur J Heart Fail. 2009;11(3):312–8. doi: 10.1093/eurjhf/hfp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tateishi O, Shouda T, Sakai T, et al. Apnea-related heart rate variability in congestive heart failure patients. Clin Exp Hypertens. 2003;25(3):183–9. doi: 10.1081/ceh-120019150. [DOI] [PubMed] [Google Scholar]
- 36.Yasuda Y, Umezu A, Horihata S, et al. Modified thoracic impedance plethysmography to monitor sleep apnea syndromes. Sleep Med. 2005;6(3):215–24. doi: 10.1016/j.sleep.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101(4):392–7. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 38.Leung RS, Diep TM, Bowman ME, et al. Provocation of ventricular ectopy by Cheyne-Stokes respiration in patients with heart failure. Sleep. 2004;27(7):1337–43. doi: 10.1093/sleep/27.7.1337. [DOI] [PubMed] [Google Scholar]
- 39.Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol. 2006;106(1):21–8. doi: 10.1016/j.ijcard.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 40.Carmona-Bernal C, Ruiz-García A, Villa-Gil M, et al. Quality of life in patients with congestive heart failure and central sleep apnea. Sleep Med. 2008;9(6):646–51. doi: 10.1016/j.sleep.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Javaheri S, Shukla R, Zeigler H, et al. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49(20):2028–34. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 42.Luo Q, Zhang HL, Tao XC, et al. Impact of untreated sleep apnea on prognosis of patients with congestive heart failure. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.03.050. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Bradley TD, Hall MJ, Ando S, et al. Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest. 2001;119(6):1827–35. doi: 10.1378/chest.119.6.1827. [DOI] [PubMed] [Google Scholar]
- 44.Shahar E, Whitney CW, Redline S, et al. Sleep disordered breathing and cardiovascular disease: cross-sectional results of The Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 45.Arias MA, García-Río F, Alonso-Fernández A, et al. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112(3):375–83. doi: 10.1161/CIRCULATIONAHA.104.501841. [DOI] [PubMed] [Google Scholar]
- 46.Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283(14):1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 48.Butt M, Dwivedi G, Khair O, et al. Obstructive sleep apnea and cardiovascular disease. Int J Cardiol. 2010;139(1):7–16. doi: 10.1016/j.ijcard.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105(21):2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 50.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162(2 Pt 1):566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 51.Walsh JT, Andrews R, Starling R, et al. Effects of captopril and oxygen on sleep apnoea in patients with mild to moderate congestive cardiac failure. Br Heart J. 1995;73(3):237–41. doi: 10.1136/hrt.73.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamura A, Kawano Y, Kadota J, et al. Carvedilol reduces the severity of central sleep apnea in chronic heart failure. Circ J. 2009;73(2):295–8. doi: 10.1253/circj.cj-08-0678. [DOI] [PubMed] [Google Scholar]
- 53.Tamura A, Kawano Y, Naono S, et al. Relationship between beta-blocker treatment and the severity of central sleep apnea in chronic heart failure. Chest. 2007;131(1):130–5. doi: 10.1378/chest.06-0919. [DOI] [PubMed] [Google Scholar]
- 54.Eskafi M, Cline C, Nilner M, et al. Treatment of sleep apnea in congestive heart failure with a dental device: the effect on brain natriuretic peptide and quality of life. Sleep Breath. 2006;10(2):90–7. doi: 10.1007/s11325-006-0053-2. [DOI] [PubMed] [Google Scholar]
- 55.Johnson CB, Beanlands RS, Yoshinaga K, et al. Acute and chronic effects of continuous positive airway pressure therapy on left ventricular systolic and diastolic function in patients with obstructive sleep apnea and congestive heart failure. Can J Cardiol. 2008;24(9):697–704. doi: 10.1016/s0828-282x(08)70668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Egea CJ, Aizpuru F, Pinto JA, et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Med. 2008;9(6):660–6. doi: 10.1016/j.sleep.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Mansfield DR, Gollogly NC, Kaye DM, et al. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169(3):361–6. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 58.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 59.Tkacova R, Rankin F, Fitzgerald FS, et al. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998;98(21):2269–75. doi: 10.1161/01.cir.98.21.2269. [DOI] [PubMed] [Google Scholar]
- 60.Smith LA, Vennelle M, Gardner RS, et al. Autotitrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J. 2007;28(10):1221–7. doi: 10.1093/eurheartj/ehm131. [DOI] [PubMed] [Google Scholar]
- 61.Sharma B, Karbowitz S, Feinsilver S. A meta-analysis of randomized controlled trials evaluating the effect of nocturnal continuous airway pressure (CPAP) therapy on ejection fraction in patients with systolic congestive heart failure and obstructive sleep apnea. Sleep. 2009;32:A175. [Google Scholar]
- 62.Khayat RN, Abraham WT, Patt B, et al. In-hospital treatment of obstructive sleep apnea during decompensation of heart failure. Chest. 2009;134(4):991–7. doi: 10.1378/chest.09-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krachman SL, D’Alonzo GE, Berger TJ, et al. Comparison of oxygen therapy with nasal continuous positive airway pressure on Cheyne-Stokes respiration during sleep in congestive heart failure. Chest. 1999;116(6):1550–7. doi: 10.1378/chest.116.6.1550. [DOI] [PubMed] [Google Scholar]
- 64.Yasuma F. Effects of continuous positive airway pressure on Cheyne-Stokes breathing in congestive heart failure. Nihon Kokyuki Gakkai Zasshi. 2002;40(10):801–5. [in Japanese] [PubMed] [Google Scholar]
- 65.Sin DD, Logan AG, Fitzgerald FS, et al. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000;102(1):61–6. doi: 10.1161/01.cir.102.1.61. [DOI] [PubMed] [Google Scholar]
- 66.Granton JT, Naughton MT, Benard DC, et al. CPAP improves inspiratory muscle strength in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1996;153(1):277–82. doi: 10.1164/ajrccm.153.1.8542129. [DOI] [PubMed] [Google Scholar]
- 67.Naughton MT, Benard DC, Liu PP, et al. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152(2):473–9. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 68.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 69.Yasuma F. Cheyne-Stokes respiration in congestive heart failure: continuous positive airway pressure of 5–8 cm H2O for 1 year in five cases. Respiration. 2005;72(2):198–201. doi: 10.1159/000084053. [DOI] [PubMed] [Google Scholar]
- 70.Arzt M, Floras JS, Logan AG, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115(25):3173–80. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 71.Köhnlein T, Welte T, Tan LB, et al. Assisted ventilation for heart failure patients with Cheyne-Stokes respiration. Eur Respir J. 2002;20(4):934–41. doi: 10.1183/09031936.00.02622001. [DOI] [PubMed] [Google Scholar]
- 72.Arzt M, Wensel R, Montalvan S, et al. Effects of dynamic bilevel positive airway pressure support on central sleep apnea in men with heart failure. Chest. 2008;134(1):61–6. doi: 10.1378/chest.07-1620. [DOI] [PubMed] [Google Scholar]
- 73.Oldenburg O, Schmidt A, Lamp B, et al. Adaptive servoventilation improves cardiac function in patients with chronic heart failure and Cheyne-Stokes respiration. Eur J Heart Fail. 2008;10(6):581–6. doi: 10.1016/j.ejheart.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Pepperell JC, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168(9):1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 75.Hastings PC, Vazir A, Meadows GE, et al. Adaptive servoventilation in heart failure patients with sleep apnea: a real world study. Int J Cardiol. 2010;139(1):17–24. doi: 10.1016/j.ijcard.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 76.Teschler H, Döhring J, Wang YM, et al. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614–9. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 77.Zhang XL. [Accessed December 12, 2009];Treatment of Cheyne Stokes respiration with adaptive servo ventilation and bilevel ventilators in patients with chronic heart failure. Available at: http://www.ClinicalTrials.gov. Identifier: NCT00725595.
- 78.Hetland A. [Accessed December 12, 2009];Chronic heart failure—Cheyne Stokes respiration—CS2 (3C-Study) Available at: http://www.ClinicalTrials.gov. Identifier: NCT00563693.
- 79.Teschler H, Cowie M. [Accessed December 12, 2009];Treatment of predominant central sleep apnoea by adaptive servo ventilation in patients with heart failure (Serve-HF) Available at: http://www.ClinicalTrials.gov. Identifier: NCT00733343.
- 80.Staniforth AD, Kinnear WJ, Starling R, et al. Effect of oxygen on sleep quality, cognitive function and sympathetic activity in patients with chronic heart failure and Cheyne-Stokes respiration. Eur Heart J. 1998;19(6):922–8. doi: 10.1053/euhj.1997.0861. [DOI] [PubMed] [Google Scholar]
- 81.Sasayama S, Izumi T, Seino Y, et al. Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and Cheyne-Stokes respiration. Circ J. 2006;70(1):1–7. doi: 10.1253/circj.70.1. [DOI] [PubMed] [Google Scholar]
- 82.Sasayama S, Izumi T, Matsuzaki M, et al. Improvement of quality of life with nocturnal oxygen therapy in heart failure patients with central sleep apnea. Circ J. 2009;73(7):1255–62. doi: 10.1253/circj.cj-08-1210. [DOI] [PubMed] [Google Scholar]
- 83.Toyama T, Seki R, Kasama S, et al. Effectiveness of nocturnal home oxygen therapy to improve exercise capacity, cardiac function and cardiac sympathetic nerve activity in patients with chronic heart failure and central sleep apnea. Circ J. 2009;73(2):299–304. doi: 10.1253/circj.cj-07-0297. [DOI] [PubMed] [Google Scholar]
- 84.Andreas S, Bingeli C, Mohacsi P, et al. Nasal oxygen and muscle sympathetic nerve activity in heart failure. Chest. 2003;123(2):366–71. doi: 10.1378/chest.123.2.366. [DOI] [PubMed] [Google Scholar]
- 85.Khayat RN, Xie A, Patel AK, et al. Cardiorespiratory effects of added dead space in patients with heart failure and central sleep apnea. Chest. 2003;123(5):1551–60. doi: 10.1378/chest.123.5.1551. [DOI] [PubMed] [Google Scholar]
- 86.Javaheri S, Parker TJ, Wexler L, et al. Effect of theophylline on sleep-disordered breathing in heart failure. N Engl J Med. 1996;335(8):562–7. doi: 10.1056/NEJM199608223350805. [DOI] [PubMed] [Google Scholar]
- 87.Cherniack NS. Sleep apnea and its causes. J Clin Invest. 1984;73(6):1501–6. doi: 10.1172/JCI111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakayama H, Smith CA, Rodman JR, et al. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med. 2002;165(9):1251–60. doi: 10.1164/rccm.2110041. [DOI] [PubMed] [Google Scholar]
- 89.Garrigue S, Bordier P, Jaïs P, et al. Benefit of atrial pacing in sleep apnea syndrome. N Engl J Med. 2002;346(6):404–12. doi: 10.1056/NEJMoa011919. [DOI] [PubMed] [Google Scholar]
- 90.Lüthje L, Unterberg-Buchwald C, Dajani D, et al. Atrial overdrive pacing in patients with sleep apnea with implanted pacemaker. Am J Respir Crit Care Med. 2005;172(1):118–22. doi: 10.1164/rccm.200409-1258OC. [DOI] [PubMed] [Google Scholar]
- 91.Sharafkhaneh A, Sharafkhaneh H, Bredikus A, et al. Effect of atrial overdrive pacing on obstructive sleep apnea in patients with systolic heart failure. Sleep Med. 2007;8(1):31–6. doi: 10.1016/j.sleep.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 92.Vazir A, Hastings PC, Morrell MJ, et al. Resolution of central sleep apnoea following implantation of a left ventricular assist device. Int J Cardiol. 2010;138(3):317–9. doi: 10.1016/j.ijcard.2008.06.072. [DOI] [PubMed] [Google Scholar]
- 93.Sinha AM, Skobel EC, Breithardt OA, et al. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004;44(1):68–71. doi: 10.1016/j.jacc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 94.Skobel EC, Sinha AM, Norra C, et al. Effect of cardiac resynchronization therapy on sleep quality, quality of life, and symptomatic depression in patients with chronic heart failure and Cheyne-Stokes respiration. Sleep Breath. 2005;9(4):159–66. doi: 10.1007/s11325-005-0030-1. [DOI] [PubMed] [Google Scholar]
- 95.Stanchina ML, Ellison K, Malhotra A, et al. The impact of cardiac resynchronization therapy on obstructive sleep apnea in heart failure patients: a pilot study. Chest. 2007;132(2):433–9. doi: 10.1378/chest.06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shalaby A. [Accessed December 12, 2009];Impact of resynchronization therapy on sleep disordered breathing in advanced congestive heart failure (IMPACT) Available at: http://www.ClinicalTrials.gov. Identifier: NCT00521534.
- 97.Mansfield DR, Solin P, Roebuck T, et al. The effect of successful heart transplant treatment of heart failure on central sleep apnea. Chest. 2003;124(5):1675–81. doi: 10.1378/chest.124.5.1675. [DOI] [PubMed] [Google Scholar]
- 98.Javaheri S, Abraham WT, Brown C, et al. Prevalence of obstructive sleep apnoea and periodic limb movement in 45 subjects with heart transplantation. Eur Heart J. 2004;25(3):260–6. doi: 10.1016/j.ehj.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 99.Hanly PJ, Zuberi-Khokhar N. Periodic limb movements during sleep in patients with congestive heart failure. Chest. 1996;109(6):1497–502. doi: 10.1378/chest.109.6.1497. [DOI] [PubMed] [Google Scholar]
- 100.Skomro R, Silva R, Alves R, et al. The prevalence and significance of periodic leg movements during sleep in patients with congestive heart failure. Sleep Breath. 2009;13(1):43–7. doi: 10.1007/s11325-008-0207-5. [DOI] [PubMed] [Google Scholar]