Abstract
Background
Individuals with autism spectrum disorders (ASD) often struggle with complex tasks, such as those requiring divided attention (simultaneously completing two independent tasks) which also place high demands on working memory. Prior research shows that divided attention is impaired in adults and children with ASD, and is related to ASD and co-morbid attention deficit/hyperactivity disorder ADHD symptoms, but the impact on everyday functioning is unclear. Because ADHD symptoms are associated with poor divided attention and working memory performance in children with ASD, we also examined ADHD symptoms as moderators of divided attention performance.
Method
We examined performance on the Consonant Trigrams Test (CTT) between high-functioning 8–13-year-olds with ASD (n=28) and typically developing controls (n=18) matched on age and IQ. In the ASD group, we also correlated performance with ADHD symptoms and behavior ratings of everyday working memory.
Results
CTT performance in children with ASD was significantly worse than in matched controls. A significant correlation between CTT performance and everyday working memory was observed, but CTT performance was not related to co-morbid ADHD symptoms, in the ASD group.
Conclusion
Divided attention with high working memory demands is a relative weakness in children with high-functioning ASD, this weakness relates to everyday functioning, and it is independent from ADHD symptoms. That ADHD symptoms are not associated with divided attention performance is inconsistent with one prior investigation, which likely results from using different divided attention tasks in the two studies.
Individuals with autism spectrum disorders (ASD) often struggle with complex multi-step tasks. These tasks can be social in nature, such as participating in a ‘to and fro’ conversation, or they can be non-social, such as getting dressed. Many of these tasks require dividing attention, which involves completing two independent tasks simultaneously, or holding information about one task in working memory, while completing another task. The current study addresses a gap in our understanding of divided attention in children with ASD, as there have been only two pediatric studies (Kenworthy, Black, Harrison, Della Rosa, & Wallace, 2009a; Sinzig, Bruning, Morsch, & Lehmkuhl, 2008), and a few adult studies examining divided attention either within a single mode (Bogte, Flamma, Van Der Meere, & Van Engeland, 2009; García-Villamisar & Della Sala, 2002) or cross-modally (Ciesielski, Knight, Prince, Harris, & Handmaker, 1995; Sinzig et al., 2008), though none has examined the relation between task performance and everyday functioning.
One pediatric study on a clinic-referred, high-functioning ASD sample found that the ability to divide auditory attention was associated with reduced ASD symptoms (Kenworthy et al., 2009a). In particular, a significant negative correlation was reported between performance on an auditory divided attention task from the Tests of Everyday Attention for Children (Score! DT subtest) and ASD symptoms (social and communication domains from autism diagnostic measures). The Score! DT task requires a child to divide attention between counting beeps and listening to a newscast for a specific animal name. As such, it confounds language, versus more pure auditory processing requirements (i.e., identifying animal names versus counting beeps), with divided attention/working memory demands. Furthermore, this investigation did not address a recent concern that comorbid attention deficit/hyperactivity disorder (ADHD) symptoms moderate cognition in ASD (Corbett, Constantine, Hendren, Rocke, & Ozonoff, 2009; Sinzig et al., 2008; Yerys et al., 2009). Thus, new studies of cognition, particularly those related to executive control, need to control for the effects of ADHD symptoms. The other pediatric study examined divided attention in school-aged children with ADHD, children with ASD and clinically significant ADHD symptoms (ASD+ADHD), children with ASD but non-clinically significant ADHD symptoms (ASD−), and typically developing controls (Sinzig et al., 2008). Children were required to respond whenever a square was presented on a computer screen and when a high- or low-beep tone was repeated. The ADHD and ASD+ADHD groups provided fewer correct responses to the tone and shape identification tasks compared to controls. Furthermore, the ASD+ADHD group made more omissions (targets that did not receive a response) than the ASD− group. Taken together, these two pediatric studies suggest that there are divided attention impairments among children with ASD relative to typically developing children, and that ADHD symptoms may be the primary contributor to impaired performance.
Investigations of basic cognitive processes have demonstrated that working memory capacity plays a role in divided attention performance (Kane & Engle, 2000). Verbal working memory is most relevant for these auditory divided attention tasks, because they likely invoke verbal mediation for successful performance. A recent literature review of verbal working memory in ASD found that children and adults with ASD perform similarly to matched control groups on most n-back, digit-, letter- and word-span tasks (Kenworthy, Yerys, Anthony, & Wallace, 2008); however, we have found that the presence of ADHD symptoms moderates verbal working memory performance in ASD, such that greater working memory deficits are associated with greater ADHD symptom severity (Yerys et al., 2009). In a much more limited literature, there is also evidence that divided attention impairments may correlate with co-morbid ADHD symptoms in children with ASD (Sinzig et al., 2008). Additionally, ADHD symptoms have a significant relationship with real world behaviors that require working memory and divided attention in ADHD (Gioia, Isquith, Kenworthy, & Barton, 2002; Mahone et al., 2002) and in ASD (Matsushima et al., 2008; Yerys et al., 2009). The relationship between performance measures of divided attention with strong working memory demands and everyday working memory/divided attention has not been examined in ASD.
A ‘classic’ divided attention task is the Brown-Peterson technique (L. R. Peterson & M. J. Peterson, 1959), also known as the Consonant Trigrams Test (CTT), which requires individuals to listen to a sequence of three letters and then a number (e.g., “LBQ 88”). The individual must retain the letters in working memory while simultaneously subtracting three from the number (e.g., “88 85 82) during a delay, and then recite the letter sequence. The CTT clearly requires splitting attention between two concurrent tasks. It also relies on working memory, as individuals are required to maintain information (three letters) in the face of interfering stimuli that require mental manipulation (counting backwards). It has adequate sensitivity for detecting subtle impairments, as demonstrated in an investigation of individuals with good recovery from closed head injuries (Stuss et al., 1985). One avenue that has not been explored previously is whether CTT performance relates to real world behaviors for clinical groups, particularly behaviors that rely heavily upon working memory and divided attention.
The current study’s purpose is to investigate: (a) divided attention (and working memory) with limited language demands in high-functioning children with ASD and age-, IQ-, gender ratio, and socioeconomic status matched typically developing (TYP) controls; (b) the relationship between a divided attention task with strong working memory demands and everyday working memory in high-functioning children with ASD; and (c) the impact of comorbid ADHD symptoms on divided attention and everyday working memory behaviors in high-functioning children with ASD. In the current study, we use a child-friendly version of the CTT, which requires children to count backward by one instead of three (Paniak, Miller, Murphy, Andrews, & Flynn, 1997), and we collect informant ratings of everyday working memory behaviors and ADHD symptoms to explore whether the proposed working memory components of CTT are ecologically valid and/or are moderated by ADHD symptoms. We predicted that: (a) the ASD group would score lower than matched controls on the CTT, due to its divided attention demands; (b) better CTT performance would relate to fewer reported working memory impairments in everyday settings, as the CTT is hypothesized to draw upon both divided attention and working memory abilities; (c) poorer CTT performance would correlate with ADHD symptoms.
Method
Twenty-eight children with high-functioning ASD and 18 TYP children participated in the study. Children were recruited through the local community via advertisements and a hospital’s outpatient clinic specializing in ASD and neuropsychological assessment. Children with ASD received a clinical diagnosis (Autism n=11, Asperger’s Syndrome n=12, Pervasive Developmental Disorder-Not Otherwise Specified [PDD-NOS] n=5) based on DSM-IV-TR criteria (American Psychiatric Association, 2000). They also met the criteria for ‘broad ASD’ based on scores from the ADI or ADI-R (Le Couteur et al., 1989; Lord, Rutter, & Le Couteur, 1994) and/or the ADOS (Lord et al., 2000) following criteria established by the NICHD/NIDCD Collaborative Programs for Excellence in Autism (Lainhart et al., 2006); Twenty-three children received both the ADI/ADI-R and the ADOS while five received one of the two measures. Children with ASD were screened through a parent phone interview and excluded if they had any history of comorbid genetic, psychiatric, or neurological disorders (e.g., Fragile X syndrome, Tourette’s syndrome, etc.), with the exception of significant ADHD symptoms, which were quantified with the parent version of the ADHD Rating Scale-IV: Home Verson (DuPaul, Power, Anastopoulos, & Reid, 1998). The American Psychiatric Association does not currently allow ADHD to be diagnosed in conjunction with ASD (American Psychiatric Association, 2000). Anti-psychotic or anti-epileptic medication usage was grounds for exclusion; stimulant medications were withheld at least 24 hours prior to testing (n=5). TYP children were screened and excluded if they or a first-degree relative had developmental, language, learning, neurological, or psychiatric disorders or psychiatric medication usage, or if the child had a known genetic disorder. This resulted in the exclusion of two participants (original n=20). All participants were required to have a Full Scale IQ≥80, as measured by a Wechsler Intelligence scale (Wechsler Intelligence Scale for Children–3rd Edition, Wechsler Intelligence Scale for Children–4th Edition, or Wechsler Abbreviated Scale of Intelligence; Wechsler, 1991, 1999, 2003). Groups did not differ in terms of age, IQ, gender ratio, race/ethnicity, or socioeconomic status based on the Four-Factor index (Hollingshead, 1975), but did significantly differ in everyday working memory ratings and ADHD symptoms (see Table 1).
Table 1.
Participant demographics and mean ratings by parents and guardians
| Total N | TYP 18 | ASD1 28 | p-value |
|---|---|---|---|
| Chronological Age (Years) | |||
| M (SD) | 11.07 (1.32) | 10.89 (1.50) | .69 |
| Range | 9.39–13.81 | 8.50–13.66 | |
| Full Scale IQ2 | |||
| M (SD) | 118.89 (12.94) | 113.86 (15.49) | .26 |
| Range | 100–140 | 85–143 | |
| Gender (male/female) | 13/5 | 21/7 | .83 |
| Family Socioeconomic Status | |||
| M (SD) | 23.78 (9.14) | 25.25 (11.93) | .66 |
| Race3 | |||
| AI/AN4 | 0 | 1 | .41 |
| Black/African American | 0 | 2 | |
| Caucasian | 15 | 20 | |
| Other | 1 | 4 | |
| Missing | 2 | 1 | |
| ADI/ADI-R5 | |||
| Social | -- | 19.76 (5.44) | -- |
| Communication | -- | 16.20 (4.90) | -- |
| Repetitive Behaviors | -- | 6.12 (2.52) | -- |
| ADOS6 | |||
| Communication | -- | 3.68 (1.97) | -- |
| Social | -- | 8.12 (2.95) | -- |
| ADHD Rating Scale7 | |||
| Total Raw Score M (SD) | 3.67 (2.72) | 24.92 (14.21) | <.001 |
| Range | 0–9 | 2–54 | |
| BRIEF – Working Memory | |||
| T-Score M (SD) | 43.11 (6.97) | 65.36 (11.49) | <.001 |
| Range | 36–58 | 39–87 | |
A total of 5 children with ASD were missing either the ADI/ADI-R or ADOS, but not
both. Also, these 5 children are not the same children who received one of the WISCs
rather than the WASI (see superscript 2).
Weschler Intelligence scale (Wechsler Intelligence Scale for Children–3rd Edition,
Wechsler Intelligence Scale for Children–4th Edition, or Wechsler Abbreviated Scale of
Intelligence)
No children of Asian, Native Hawaiian or Other Pacific Islander descent participated.
American Indian or Alaskan Native
ADI/ADI-R= Autism Diagnostic Interview (n= 25)
ADOS= Autism Diagnostic Observation Schedule (n=25)
TYP Group: n=15; ASD Group: n=25
Children completed CTT (Paniak et al., 1997), while parents completed the ADHD Rating Scale-Parent Edition (DuPaul et al., 1998), and the Behavior Rating Inventory of Executive Function-Parent Form (BRIEF; (Gioia, Isquith, Guy, & Kenworthy, 2000). This CTT is a pediatric version of the Brown-Peterson technique (L. R. Peterson & M. J. Peterson, 1959) and is normed on a sample of over 700 8–15-year-olds. In a series of trials, children are presented with three consonant letters followed by a number. They must count backwards from that number during a variable delay (0, 3, 9, or 18 seconds), after which they are asked to state the letters. The primary metric of performance for each trial is the correct number of letters recalled. Standard scores can be derived for each delay interval and the total score. The total score was chosen as the dependent variable due to its good reliability; the normed data were near ceiling in the lowest delay conditions, which created widely distributed scores when a child missed a single item (e.g., providing a correct response to 14 of 15 items for a 9-year-old in the 0-second delay condition resulted in a standard score of 66). The ADHD Rating Scale assesses severity in inattention and hyperactivity/impulsivity symptoms. This 18-item questionnaire yields two domains: inattention and hyperactivity/impulsivity. For each question, parents use a 0–3 scale to rate the participant. A higher score indicates greater symptom severity. The total raw score was the dependent variable of interest. The BRIEF is an informant report of executive function in everyday situations comprising eight subdomains. Results are reported as T-scores. Higher scores indicate greater impairment; T-scores≥65 (i.e., 1.5 SDs≥ the mean) indicate clinically significant ratings. The Working Memory subscale of the BRIEF was the dependent variable of interest. All measures were part of a larger study examining the cognitive profile of ASD. Consent from parents/guardians of children and assent from the children were obtained within the guidelines of the hospital’s Institutional Review Board, which approved the study.
The CTT data violated Levene’s test for homogeneity of variances, so a Welch’s t-test was used to assess group differences. Pearson’s r was used to examine the relationship between CTT performance and ADHD symptoms and everyday working memory behaviors in the ASD group.
Results & Discussion
The CTT total score fell in the Average range for both groups, but the TYP group scored significantly higher (which signifies better performance) than the ASD group, t(44)=3.15, p≤.001. There was a large effect size associated with this group difference (Cohen’s d=1.03). This finding reveals divided attention as a relative weakness for high-functioning children with ASD. One of the more striking observations was the ASD group’s standard deviation relative to that of the TYP group (see Figure 1), reflecting the considerable variability in the ASD group’s CTT performance. There was not a significant relationship between CTT performance and ADHD symptoms in the ASD group, r(25)=−.13, p=.55, therefore ADHD symptoms were not included as a covariate. A significant correlation was found between the CTT total score and the Working Memory subscale from the BRIEF in the ASD group, r(28)=−.38, p<.05. The overall pattern of findings was not different when the five children taking stimulant medication were removed from analyses (data not shown).
Figure 1.
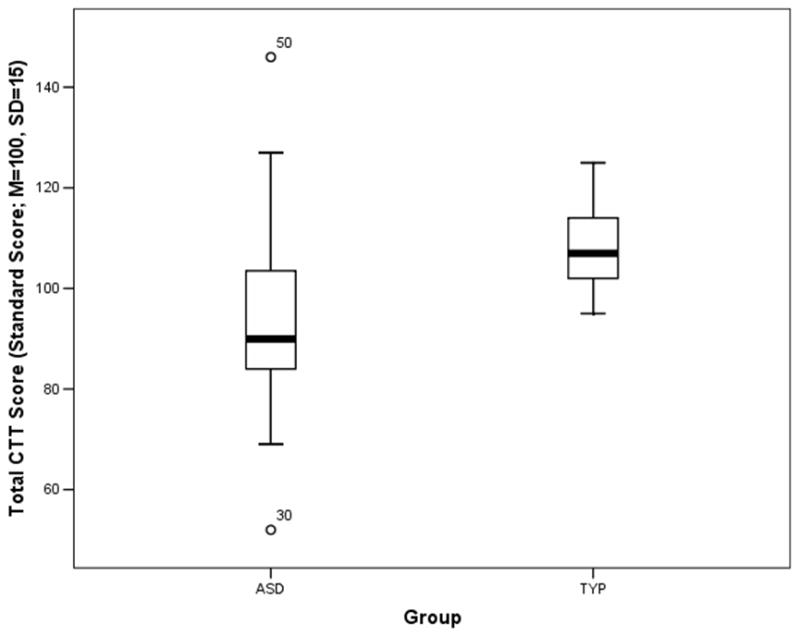
This boxplot figure shows the total performance standard score (M=100, SD=15) on the consonant trigrams test (CTT) for the autism spectrum disorder (ASD) and typically-developing matched control (TYP) groups. The ASD group scores significantly lower than the TYP group. Note the large variability in the whiskers of the ASD group’s boxplot.
There is limited evidence about divided attention with strong verbal working memory demands in children with ASD (Kenworthy et al., 2009a; Sinzig et al., 2008); our results converge with prior investigations documenting divided attention weaknesses in an ASD group, however we diverge from a prior study in that our findings are independent from ADHD symptoms. We also extend earlier work by examining the relationship between divided attention difficulties in ASD and everyday working memory while controlling for ADHD symptoms. Unlike ADHD symptomatology, everyday working memory correlates significantly with CTT performance in the ASD group, providing ecological validity for this documented divided attention/working memory weakness (Burgess et al., 2006; Kenworthy et al., 2008). The CTT was developed as a divided attention task and not a working memory task, (Peterson & Peterson, 1959), however recent investigations demonstrate relationships between the two processes (Kane & Engle, 2000). The correlation between the CTT and an ecologically valid measure of working memory support this relationship beyond performance-based measures.
Our findings of divided attention weaknesses independent from ADHD symptoms are distinguishable from previous findings of ASD+ADHD impairments in a cross-modal divided attention task (Sinzig et al., 2008). One potential explanation for this difference in findings may lie in differences in the specific task demands. The cross-modal nature of the divided attention task -auditory and visual discrimination tasks - used by Sinzig and colleagues (2008) requires engaging in two simple detection tasks that do not interfere at the sensory level. The CTT requires verbal encoding/maintenance over a delay in which children must count backwards by one. The second task (counting) directly impedes the child’s ability to engage in rehearsal of the letter string, and this additional feature of CTT may drive the differences in findings. Finally, our finding of extreme variability observed in the ASD group converges with previous investigations into cognitive and motor function (Geurts et al., 2008; Jones & Klin, 2009; Müller, Kleinhans, Kemmotsu, Pierce, & Courchesne, 2003), and future investigations will need to address genetic and neural underpinnings to explain the variability of cognition.
This study has several limitations. The confound of working memory and divided attention demands in the CTT task limits our ability to pinpoint the area of difficulty in ASD, and the reciprocal nature of correlations prevents causal interpretations of the relationship between the CTT and everyday working memory. However, this confound of multiple demands is common in neuropsychological research, as evidenced by the demands for sustained attention (Sinzig et al., 2008) and language (Kenworthy, Black, Harrison, Della Rosa, & Wallace, 2009b) inherent in divided attention tasks used in children with ASD. We can say that while past literature suggests intact verbal working memory in ASD without ADHD (Kenworthy et al., 2008; Yerys et al., 2009) weaknesses are observed with divided attention tasks with working memory demands, such as the CTT. Furthermore, the CTT relates to everyday working memory behaviors, and we speculate that targeting divided attention for intervention may have a cascading effect on these real world behaviors. An additional limitation is that our dependent measure only captured performance on one of the two CTT tasks, which is consistent with CTT administration (Paniak et al., 1997). Because we only measured performance on one task, it is unclear if children performed both tasks well or sacrificed performance on the counting task to perform well on the other letter memory task. Also, the ASD group was high-functioning with a mean IQ near the High-Average range; thus, our findings may not hold for lower-functioning children with ASD. Finally, 25% of the ASD group participating in the current study also took part in our prior study (Kenworthy et al., 2009a); however, the divided attention measures and dependent variables are independent across the two studies.
These findings pinpoint a cognitive skill relevant to outcomes and interventions. Real world associations have been elusive in past investigations of non-social cognition, other than intelligence, in ASD (Kenworthy et al., 2009a). Future research should: identify tasks that parse divided attention and working memory as much as possible; and more thoroughly investigate divided attention in ASD at the neural level and as an intervention target. This could include examining the developmental trajectory and neural correlates of divided attention skills in children with ASD. Finally, divided attention may serve as a lab-based index of poor “responsivity to multiple cues”, a targeted area of intervention in several ASD treatments, such as pivotal response training (Koegel, Koegel, & McNerney, 2001).
Acknowledgments
We thank the children and families that offered their time and energy for the current study. One author (LK) receives financial compensation from the BRIEF. There are no conflicts of interest, financial or otherwise, for the remaining authors involved directly or indirectly with this manuscript. This work was supported by the Frederick and Elizabeth Singer Foundation, the Gudelsky Foundation, and the Studies for the Advancement of Autism Research and Treatment (STAART: NIMH U54 MH066417) for supporting data collection. BEY, and in part this work, was supported by the Intellectual and Developmental Disabilities Research Center at Children’s National Medical Center (NIH IDDRC P30HD40677) and the General Clinic Research Center (NIH GCRC M01-RR13297). GLW was supported by the Intramural Program of the NIH, National Institute of Mental Health.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4. American Psychiatric Publishing, Inc; 2000. [Google Scholar]
- Bogte H, Flamma B, Van Der Meere J, Van Engeland H. Divided attention capacity in adults with autism spectrum disorders and without intellectual disability. Autism: The International Journal of Research and Practice. 2009;13(3):229–243. doi: 10.1177/1362361309103793. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Forbes C, Costello A, Coates LM, Dawson DR, Anderson ND, et al. The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. Journal of the International Neuropsychological Society: JINS. 2006;12(2):194–209. doi: 10.1017/S1355617706060310. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Knight JE, Prince RJ, Harris RJ, Handmaker SD. Event-related potentials in cross-modal divided attention in autism. Neuropsychologia. 1995;33(2):225–246. doi: 10.1016/0028-3932(94)00094-6. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research. 2009;166(2–3):210–22. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. Guilford Press; New York: 1998. [Google Scholar]
- García-Villamisar D, Della Sala S. Dual-task performance in adults with autism. Cognitive Neuropsychiatry. 2002;7(1):63–74. doi: 10.1080/13546800143000140. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Grasman RPPP, Verté S, Oosterlaan J, Roeyers H, van Kammen SM, Sergeant JA. Intra-individual variability in ADHD, autism spectrum disorders and Tourette’s syndrome. Neuropsychologia. 2008;46(13):3030–41. doi: 10.1016/j.neuropsychologia.2008.06.013. S0028-3932(08)00259-5. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy S, Kenworthy L. BRIEF: Behavior Rating Inventory of Executive Function. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Gioia GA, Isquith PK, Kenworthy L, Barton RM. Profiles of everyday executive function in acquired and developmental disorders. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2002;8(2):121–37. doi: 10.1076/chin.8.2.121.8727. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Jones W, Klin A. Heterogeneity and homogeneity across the autism spectrum: the role of development. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(5):471–473. doi: 10.1097/CHI.0b013e31819f6c0d. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity, proactive interference, and divided attention: limits on long-term memory retrieval. Journal of Experimental Psychology Learning, Memory, and Cognition. 2000;26(2):336–358. doi: 10.1037//0278-7393.26.2.336. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Black D, Harrison B, Della Rosa A, Wallace G. Are Executive Control Functions Related to Autism Symptoms in High-Functioning Children? Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2009a;15(5):425–440. doi: 10.1080/09297040802646983. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Black DO, Harrison B, Della Rosa A, Wallace GL. Are executive control functions related to autism symptoms in high-functioning children? Child Neuropsychology. 2009b;15(5):425–440. doi: 10.1080/09297040802646983. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Anthony LG, Wallace GL. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychology Review. 2008;18(4):320–38. doi: 10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel R, Koegel L, McNerney E. Pivotal areas in intervention for autism. Journal of Clinical Child Psychology. 2001;30(1):19–32. doi: 10.1207/S15374424JCCP3001_4. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, Deutsch CK, et al. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. American Journal of Medical Genetics Part A. 2006;140(21):2257–74. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: a standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19(3):363–87. doi: 10.1007/BF02212936. 2793783. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–23. 11055457. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–85. doi: 10.1007/BF02172145. 7814313. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Cirino PT, Cutting LE, Cerrone PM, Hagelthorn KM, Hiemenz JR, Singer HS, et al. Validity of the behavior rating inventory of executive function in children with ADHD and/or Tourette syndrome. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists. 2002;17(7):643–662. [PubMed] [Google Scholar]
- Matsushima N, Miyawaki D, Tsuji H, Takahashi K, Horino A, Kawaguchi T, Suzuki F, et al. Evaluation of attention-deficit/hyperactivity disorder symptoms in male children with high-functioning pervasive developmental disorders. Osaka City Medical Journal. 2008;54(1):1–10. [PubMed] [Google Scholar]
- Müller R, Kleinhans N, Kemmotsu N, Pierce K, Courchesne E. Abnormal variability and distribution of functional maps in autism: an FMRI study of visuomotor learning. The American Journal of Psychiatry. 2003;160(10):1847–62. doi: 10.1176/appi.ajp.160.10.1847. 14514501. [DOI] [PubMed] [Google Scholar]
- Paniak C, Miller H, Murphy D, Andrews A, Flynn J. Consonant Trigrams Test for children: Development and norms. Clinical Neuropsychologist. 1997;11(2):198–200. [Google Scholar]
- Peterson LR, Peterson MJ. Short-term retention of individual verbal items. Journal of Experimental Psychology. 1959;58:193–198. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Bruning N, Morsch D, Lehmkuhl G. Attention profiles in autistic children with and without comorbid hyperactivity and attention problems. Acta Neuropsychiatrica. 2008;20(4):207–215. doi: 10.1111/j.1601-5215.2008.00292.x. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Ely P, Hugenholtz H, Richard MT, LaRochelle S, Poirier CA, Bell I. Subtle neuropsychological deficits in patients with good recovery after closed head injury. Neurosurgery. 1985;17(1):41–47. doi: 10.1227/00006123-198507000-00007. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Scales of Intelligence. 3. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scales of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. Wechsler Scales of Intelligence. 4. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research: Official Journal of the International Society for Autism Research. 2009;2(6):322–333. doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]


