Abstract
Burn injury is associated with profound inflammation and activation of the innate immune system involving γδ T-cells. Similarly, toll-like receptors (TLR) are associated with activation of the innate immune response; however, it is unclear whether TLR expression is altered in γδ T-cells after major burn injury. To study this, male C57BL/6 mice were subjected to burn injury (25% TBSA) and 1 or 7 days thereafter, blood and spleen cells were isolated and subjected to FACs analysis for TLRs and other phenotypic markers (γδ TCR, αβ TCR, CD69, CD120b). A marked increase in the number of circulating γδ T-cells was observed at 24 hr. post-burn (14% vs. 4%) and a higher percentage of these cells expressed TLR-2. TLR-4 expression was also increased post-burn, but to a lesser degree. These changes in TLR expression were not associated with altered CD69 or CD120b expression in γδ T-cells. The mobilization of, and increased TLR expression in, γδ T-cells was transient, as phenotypic changes were not evident at 7 days post-burn or in γδ T-cells from the circulation or spleen. The increases in TLR expression were not observed in αβ T-cells after burn injury. In conclusion, 24 hr. after burn injury mobilization of γδ T-cells with increased TLR expression was observed. This finding suggests that this unique T-cell population is critical in the innate immune response to injury, possibly through the recognition of danger signals by TLRs.
Keywords: trauma, SIRS, T-cell receptors, cytokines, chemokines
INTRODUCTION
Thermal injury remains a significant health problem. Despite advances in patient care, immunosuppression, increased susceptibility to sepsis, wound healing complications, and multiple organ failure remain major concerns in this severely compromised and unique patient population. Major burn injury is associated with an activation of the innate immune system and inflammation [1;2]. It is believed that the activation of the innate immune system contributes to the development of a systemic inflammatory response (SIRS) and subsequent multiple organ failure [1;3;4].
The involvement of γδ T-cells in a wide variety of disease processes is indicative of an important role for this T-cell subset in both innate and acquired immunity [5–7]. In this regard, studies from our laboratory suggest an important role for γδ T-cells in the immune response to thermal injury [8–11]. In these studies, increased activation of the circulating γδ T-cells was observed and the findings also indicated that γδ T-cells have a role in post-burn immune dysfunction, neutrophil-mediated tissue damage and wound healing. Normally, γδ T-cells are present in only small numbers in peripheral lymphoid tissues (i.e., spleen), but relatively abundant in the skin epithelia, intestine, uterus and tongue [5]. However, γδ T-cells have a phenotype of spontaneous activation and under pathological conditions they can quickly expand and infiltrate lymphoid compartments and other tissues [12;13].
The identification of a family of mammalian receptors related to Drosophila Toll has demonstrated how cells of the innate immune system are capable of recognizing and reacting to a wide variety of microbial antigens [14]. This family of receptors are known as Toll-like receptors (TLRs) and consists of 10 members that have shared structural homology and signaling components [15–17]. TLRs are primary sensors of microbial products and activate signaling pathways that lead to the induction of inflammatory genes. They belong to a family of IL-1R-related molecules that recognize conserved pathogen-associated molecules such as LPS (TLR-4) bacterial lipopeptide (TLR-2) [18]. Recent studies have shown that γδ T-cells can, upon activation express TLRs [19–21]. While studies have shown that TLRs and TLR responsiveness are involved in the post-burn inflammatory response by macrophages [1;18], it remains unknown whether TLRs are involved in the activation of γδ T-cells after major burn injury.
MATERIALS AND METHODS
Animals
C57BL/6 male mice (18–22 g; 8–10 weeks of age, Charles River Laboratories, Wilmington, MA) were used for all experiments. The mice were allowed to acclimatize in the animal facility for at least one week prior to experimentation. Animals were randomly assigned into either a thermal injury group or a sham treatment group. The experiments in this study were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham where the animal studies were performed. In addition, the animal experiments were performed in accordance with the National Institutes of Health guidelines for the care and handling of laboratory animals.
Thermal injury procedure
Mice received a scald burn as described previously [22]. Briefly, the mice were anesthetized by intra-peritoneal injection of ketamine/xylazine and the dorsal surface was shaved. The anesthetized mouse was placed in a custom insulated mold exposing 12.5% of their total body surface area along the right dorsum. The mold was immersed in 70°C water for 10 sec, producing a burn injury. The mouse was repositioned in the mold exposing the left dorsum, and the mold was re-immersed in 70°C water for 10 sec. The resulting injury covered 25% total body surface area, and previous studies have verified this injury to be a full-thickness burn, as defined by injury to the epidermal, dermal, and subdermal layers [23]. The mice were then resuscitated with 1 ml of Ringer’s lactate solution administered by intraperitoneal injection and returned to their cages. The cages were placed on a heating pad for 2 hr until the mice were fully awake, at which time they were returned to the animal facility. Sham treatment consisted of anesthesia and resuscitation with Ringer’s lactate solution only. The mice were not given analgesics post-procedure due to the profound immunomodulatory properties of NSAIDs and opiates [24–26].
Collection of cell populations
Anti-coagulated whole blood was obtained at 1 or 7 days after thermal injury or sham procedure by cardiac puncture. Splenocyte and MLN cells were collected at 7 days post-injury and cell suspensions prepared in PBS as described elsewhere [27;28].
Determination of cellular phenotype
Blood, Spleen or Mesenteric Lymph Node (MLN) cells were stained with a combination of antibodies (γδ TCR, αβ TCR, TLR-2, TLR-4, CD69, CD120b) conjugated to either FITC or PE to assess cellular phenotype. The manufacturer’s recommended methodology for each cell population was employed (BD Pharmingen). Appropriate isotype controls were included. FITC and PE were analyzed with a Becton-Dickinson LSR II flow cytometer (BD Biosciences, Mountain View, CA). Unless noted otherwise, the entire lymphocyte and monocyte populations (as determined for forward and side scatter) were gated in the analysis. A minimum of 20,000 events was collected and WinMIDI 2.8 software was used to analyze the results.
Analysis of cytokine levels
Plasma was collected and stored at −80° C until being assayed for TNF-α, IL-6, IL-10, KC and MCP-1. The Cytometric Bead Array (CBA; BD Pharmingen) and CBA Mouse Flex Sets were used for all analyses according to the manufacturer’s instructions with some modification. In brief, 25 μl of mixed capture beads were incubated with 25 μl of plasma for 1 hr at room temperature in the dark. Twenty-five μl of PE detection reagent was then added and incubated for 1 hr. The immunocomplexes were then washed twice with wash buffer and centrifuged at 200 × g for 5 min. Analysis was then carried out using the LSR II flow cytometer (BD Biosciences) and the data was processed with the accompanying FACSDiva and FCAPArray software.
Statistical analysis
Data are expressed as mean ± SEM. Comparisons were analyzed using ANOVA. A P value of < 0.05 was considered to be statistically significant for all analyses.
RESULTS
Effect of burn injury on circulating immune cells
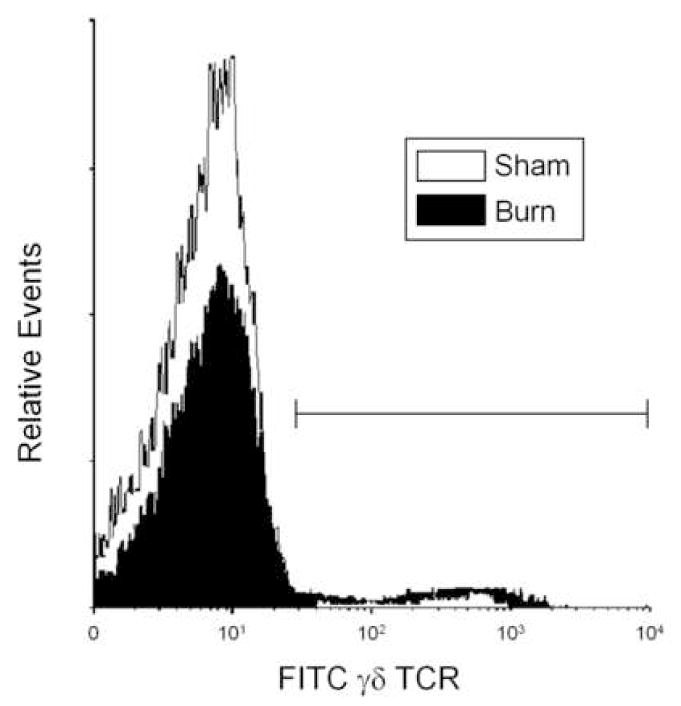
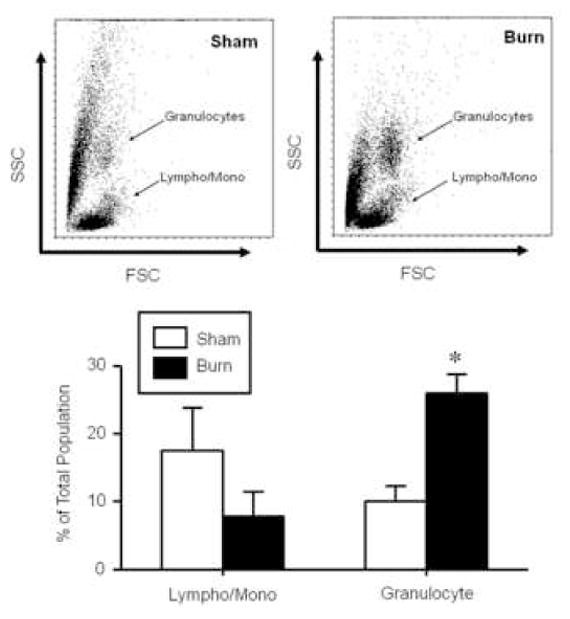
At 24 hrs after burn injury a profound change in the phenotype of the circulating immune cells was observed (Fig. 1). Based on the analysis of forward and side scatter the granulocyte and lymphocyte/monocyte populations could be identified. Approximately a 2-fold increase in the percentage of granulocytes was observed in the blood on burn injured mice as compared with shams (p<0.05). In parallel, an approximate 50% drop in the percentage of circulating lymphocytes and monocytes was observed; however, due to high variability between animals statistical significance was not observed. Further analysis of the cells in the lymphocyte and monocyte population revealed that the percentage of the cells positive for γδ TCR was significantly greater (p<0.05) in the burn group (9.9±1.5% vs. 18.9±3.6% for sham and burn, respectively; mean ± SEM; n=6/group. Fig. 2).
Figure 1. Effect of burn injury on circulating immune cell populations.
Blood was collected from mice at 24 hr after sham or burn injury. The lymphocyte/monocyte and granulocyte populations were identified by forward (FSC) and side scatter (SSC) and analyzed as a percentage of the total cell population. Data are mean ± SEM for n=6/group. * p<0.05 vs. respective sham group.
Figure 2. Effect of burn injury on circulating γδ T-cells.
Blood was collected from mice at 24 hr after sham or burn injury and stained for expression of γδ TCR as described in the materials and methods. The lymphocyte/monocyte population was identified by forward and side scatter and analyzed FITC γδ TCR. Bar indicates γδ TCR+ population. Data are representative of 6 experiments.
TLR expression by circulating T-cells early post-injury
At 24 hrs after burn injury the percentage of the γδ T-cells expressing TLR-2 or TLR-4 significantly increased as compared to sham animals (p<0.05; Fig. 3). A significantly greater proportion of the γδ T-cells in the burn group where TLR-2+ as compared with γδ T-cells that were not expressing TLR-2 (p<0.05; Fig 3A). Gamma-delta T-cells in the burn group had similar percentages for the TLR-4+ and TLR-4− populations (Fig. 3B). No such differences were observed in γδ T-cells from sham animals. In contrast to γδ T-cells, the expression of TLR-2 or TLR-4 on αβ T-cells at 24 hrs after burn injury was not different from that of sham animals (Fig. 4). Moreover, the majority of the αβ T-cells were negative for TLR expression.
Figure 3. TLR expression on circulating γδ T-cells.
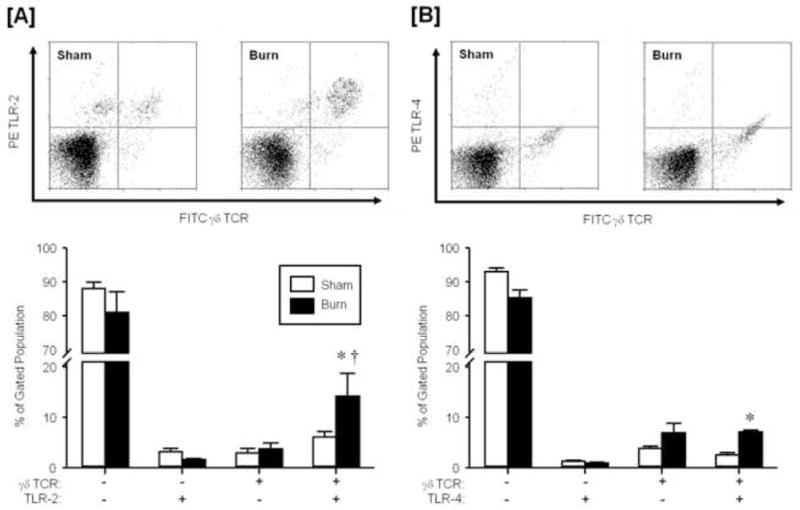
Blood was collected from mice at 24 hr after sham or burn injury and stained for expression of γδ TCR and TLR-2 [A] or TLR-4 [B] as described in the materials and methods. The lymphocyte/monocyte population was identified by forward and side scatter and analyzed FITC γδ TCR and PE TLR-2 or TLR-4. Data are mean ± SEM for n=4/group. * p<0.05 vs. respective sham group. † vs. respective gd TCR+ TLR-2− group.
Figure 4. TLR expression on circulating αβ T-cells.
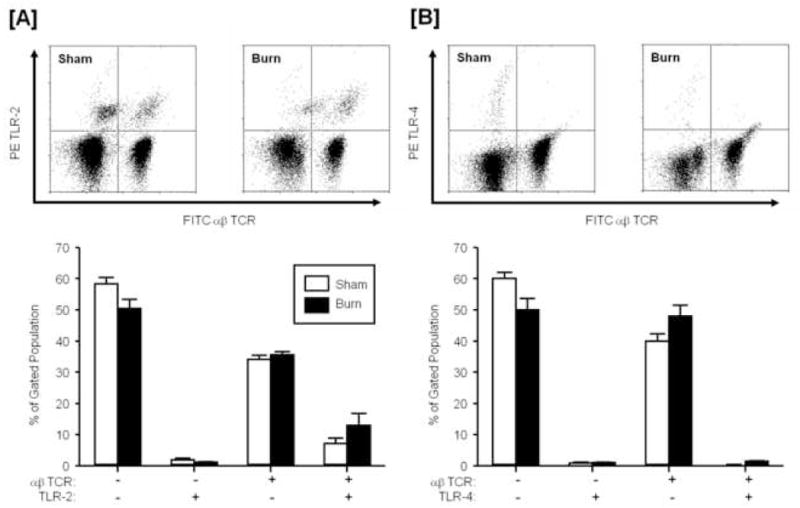
Blood was collected from mice at 24 hr after sham or burn injury and stained for expression of αβ TCR and TLR-2 [A] or TLR-4 [B] as described in the materials and methods. The lymphocyte/monocyte population was identified by forward and side scatter and analyzed FITC αβ TCR and PE TLR-2 or TLR-4. Data are mean ± SEM for n=4/group.
The circulating γδ T-cells were further analyzed for the expression of the T-cell activation markers CD69 and CD120b (p75 TNFR2). In contrast to TLR-2 and -4 expressions, expression of CD69 and CD120b was not different between the sham and burn groups (Table 1).
Table 1.
Expression of activation markers on circulating γδ T-cell at 24 hr post-injury
Data are expressed mean ± SEM of % of the gated lymphocyte population (n = 5–6/group).
p<0.05 as compared with sham.
Burn injury induction of a systemic inflammatory response
Burn injury induced a systemic inflammatory response at 24 hrs post-injury as evidenced by the increased plasma levels of IL-6 and KC as compared to sham animals (p<0.05; Fig. 5). Plasma levels of these inflammatory mediators was increased approximately 10–12-fold. In contrast, TNF-α, IL-10, and MCP-1 plasma levels were not different between sham and burn animals at 24 hrs post-injury.
Figure 5. The impact of burn injury on systemic cytokine and chemokine levels.
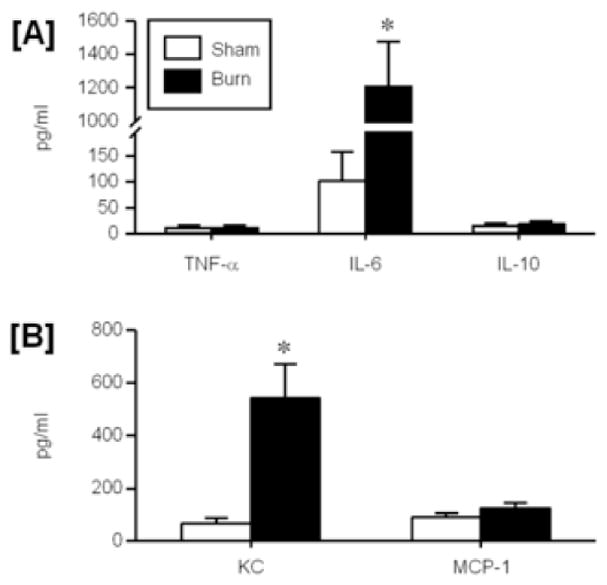
Plasma was collected from mice at 24 hr after sham or burn injury and assayed for the cytokines TNF-α, IL-6 and IL-10 [A] and the chemokines KC and MCP-1 [B] as described in the materials and methods. Data are mean ± SEM for n=5/group. * p<0.05 vs. respective sham group.
TLR expression by circulating tissue-fixed T-cells late post-injury
The increased expression of TLRs on circulating γδ T-cells appeared to be transient, as analysis of cells at 7 days post-injury revealed no differences between the sham and burn groups (Fig. 6A, 6B). Similarly, no differences in TLR expression were observed in circulating αβ T-cells at 7 days post-injury (Fig. 6C, 6D).
Figure 6. TLR expression on circulating T-cells late post-injury.
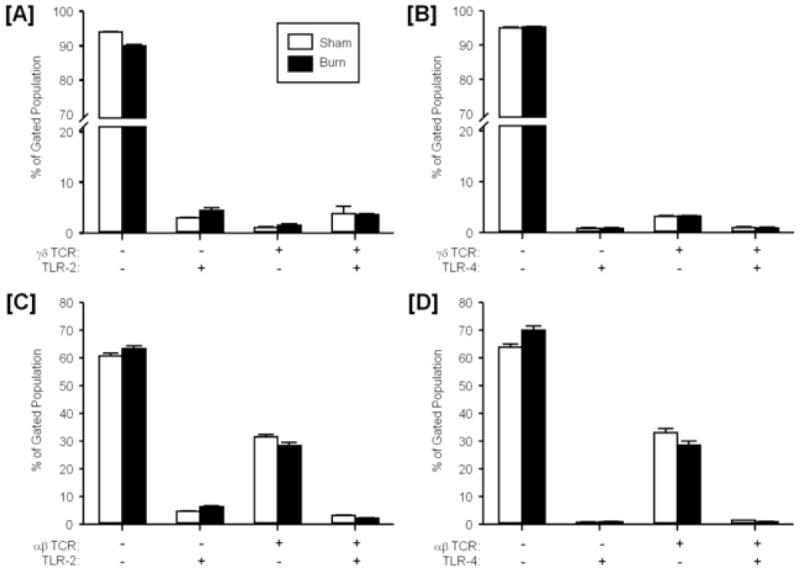
Blood was collected from mice at 7 days after sham or burn injury and stained for expression of γδ TCR and TLR-2 [A], γδ TCR and TLR-4 [B], αβ TCR and TLR-2 [C] and γδ TCR and TLR-4 [D] as described in the materials and methods. The lymphocyte/monocyte population was identified by forward and side scatter and analyzed FITC TCR and PE TLR-2 or TLR-4. Data are mean ± SEM for n=4/group.
T-cells isolated from the spleen and MLN were assessed for and TLR-2 or TLR-4 expression. The data presented in Table 2 indicates that the vast majority of the tissue-fixed T-cells were of the αβ TCR phenotype. The expression TLR-2 or TLR-4 on either T-cell population was negligible, irrespective of sham treatment or burn injury
Table 2.
Expression of TLRs on splenic and MLN T-cells at 7 days post-injury
| γδ TCR+ | γδ TCR+ TLR-2+ |
γδ TCR+ TLR-4+ |
αβ TCR+ | αβ TCR+ TLR-2+ |
αβ TCR+ TLR-4+ |
|
|---|---|---|---|---|---|---|
|
|
||||||
| Spleen (n=4) | ||||||
| Sham | 2.5±0.9a | 1.1±0.4 | 0.3±0.1 | 30.9±2.1 | 1.2±0.1 | 0.4±0.1 |
| Burn | 1.8±0.5 | 0.8±0.2 | 0.2±0.1 | 28.4±0.6 | 1.0±0.2 | 0.4±0.1 |
| MLN (n=3) | ||||||
| Sham` | 1.7±0.1 | 0.4±0.1 | 0.8±0.1 | 49.2±0.3 | 0.4±0.1 | 1.6±0.3 |
| Burn | 1.7±0.3 | 0.3±0.1 | 0.3±0.1 | 54.3±0.6 | 0.8±0.1 | 3.7±0.3 |
Data are expressed mean ± SEM of % of the gated lymphocyte population.
DISCUSSION
Major injuries, such as burn injury, induce a profound immunoinflammatory response that ultimately can predispose the host to various types of opportunistic infections. Toll-like receptors (TLRs) can recognize conserved microbial antigens and/or host stress or danger signals induced by injury. Thus, injury-induced alterations in TLR expression or responsiveness by the injured host may contribute to the development of infectious complications and the potential for the development of multiple system organ failure and death. The studies presented here were conducted to determine whether changes in TLR expression by γδ T-cells after burn injury were associated with the inflammatory response. We have made a number of important observations concerning the post-burn immunoinflammatory response. First, a profound change in the circulating immune cell populations was observed relatively early post-burn (i.e., 24 hr) that was associated with an increased circulating γδ T-cells, granulocytes and a systemic inflammatory response. Secondly, these γδ T-cells were primed for TLR reactivity, as evidenced by increased expression of TLR-2 and TLR-4. Third, these changes in TLR expression were specific for the γδ TCR T-cell subset in the circulation and transient, since it was not observed in the spleen, MLN or blood at 7 days post-injury.
TLRs are cell surface molecules that participate in innate immunity by recognizing conserved motifs on pathogens These pathogen-associated molecular patterns (PAMP) are displayed in a variety of microorganisms [17;29], as well as endogenous danger/damage associated molecular pattern molecules (DAMP’s) [30]. Increased TLR reactivity by macrophages has been implicated in a number of immunopathological aspects of burn injury [1;15;18]. These studies have primarily focused on responses to the TLR-4 ligand LPS. This enhanced TLR reactivity appears to be in part related to increased expression of intracellular TLR-4-MD2, rather than cell surface expression of the receptor complex [18]. Our laboratory and others [18;31;32] have also shown TLR-4 reactivity is associated with an increase in p38 MAPK activity. Nonetheless, TLR signaling pathways converge in the activation of the transcription factor NF-κB, which controls the expression of an array of inflammatory associated genes [30]. In contrast to macrophages, the analysis of TLR expression by T-cells post-injury has been very limited. A recent study by Cairns et al. [33] have shown that late post-burn injury (i.e., 14 days) increased expression of TLR-4 was evident on splenic T-cells. The current findings are consistent with the work Cairns et al. with regard to T-cell expression of TLRs; however, TLR expression was seen earlier, only in the circulation and specific for the γδ T-cell subset. This apparent discrepancy is most likely related to the time post-injury used for analysis (i.e., 1–7 days vs. 14 days). Moreover, while patient studies indicate mobilization and activation of γδ T-cells in SIRS patients at 24 hr (i.e., 0–1 day) [34], it remains to be determined whether changes in γδ T-cell TLR expression occur earlier than 24 hrs. post-injury, as mobilization of this cell population has been observed at 3–4 hr after burn injury or sepsis in mouse injury models [9;35].
Gamma-delta T cells responses have observed in disease states of by an infectious and non-infectious nature, suggesting that this unique T-cell subset has a primary role in immunoregulation and the protection of host tissues against the damaging side-effects of immune responses [36]. There are a number of features which distinguish γδ T-cells from “traditional” αβ T-cells. Most γδ T-cells have a phenotype of spontaneous activation with a rapid turnover rate [37] and quickly expand upon activation by pathogens or inflammatory stimuli [13]. A critical role for γδ T-cells has been demonstrated with regard to inflammation, pro-inflammatory cytokine (i.e., TNF-α) production [38] and tissue repair [39]. They also can regulate Th1-type and Th2-type T-cell phenotypes and establishment of protective CD8+ responses [40]. A clinical study has demonstrated activated γδ T-cells are present in the circulation of SIRS patients [34]. We have also shown that early post-burn (3 hr) in our murine model there is a mobilization of γδ T-cells to the circulation [9]. In that study the circulating γδ T-cells were in an primed state as evidenced by increased CD120b expression [41]. In contrast, our findings at 24 hr post-burn indicate that, while increased number of γδ T-cells are present in the circulation, their phenotype has changed and the cells, in a classical sense, are not activated, since CD120b and CD69 expression was unaltered. While the increased expression of TLR-2 and TLR-4 suggests that these γδ T-cells may be more reactive towards PAMPs and DAMPs [29;30], it remains to be determined whether this phenotypic change significantly alters the response to TLR ligands post-burn. Chemokine receptors are expressed on both circulating and tissue γδ T-cells that allow them to extravasate and migrate to sites of tissue damage or infectious foci. Gamma-delta T-cells can also produce chemokines and thereby contribute to the recruitment of additional γδ T-cells [9;42;43]. It can be suggested that injury induces an early activation and migration of γδ T-cells from epithelial rich tissue to the lymph/circulation and subsequently to burn injured tissue to help control inflammation and/or initiate the healing process. Previous studies have support such a concept for γδ T-cells under other pathological conditions [44;45]. Moreover, this process appears to be, in part, TLR-dependent [46]. Nonetheless, it remains to be yet determined whether or not such trafficking of γδ T-cells occurs post-burn.
While the regulation of TLR expression is not clearly understood [47], cell surface TLR expression is likely to be limited to avoid excessive activation. Thus, the transient nature of the post-burn increase in γδ T-cell TLR expression is likely to be protective. Studies have shown that TLR expression is transiently upregulated on monocytes in response to LPS or TNF-α [48;49]. Moreover, the activation of TLRs can induce the expression of genes that regulate cell migration [50]. Thus, the post-burn changes in γδ T-cell TLR expression may be related to the early inflammatory response (i.e., TNF-α) and important in their mobilization and migration.
The findings presented here further support a role for γδ T-cell in post-burn immunopathology. Previous studies demonstrated high mortality in mice lacking γδ T-cells during the initial 48 h post-burn period [8], a role for γδ T-cells in post-burn end organ injury [9], and an important role in post-burn wound healing [10;11]. We have extended these observations by demonstrating a potential role for TLRs in the γδ T-cells response to burn injury. It is likely that the activation and migration of γδ T-cell during the initial post-injury period (i.e., 24 hr.) is overall protective to the host, most likely via regulation of inflammation and initiation of healing processes. Nonetheless, additional studies are needed to develop a more comprehensive understanding of this unique T-cell subset and their role in post-injury immunopathology.
Acknowledgments
These studies were presented in part at the 30th Annual Conference on Shock, Baltimore, MD and completed in part while MGS was a faculty member at the University of Alabama at Birmingham, Birmingham, AL. Support was provided by National Institutes of Health grant GM079122, AI049960 and Department of Defense grant PRO034212. We thank Drs. Bjoern Thobe and Bill Hubbard for their assistance in the completion of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 2.Schneider DF, Glenn CH, Faunce DE. Innate lymphocyte subsets and their immunoregulatory roles in burn injury and sepsis. J Burn Care Res. 2007;28:365–79. doi: 10.1097/BCR.0B013E318053D40B. [DOI] [PubMed] [Google Scholar]
- 3.Saffle JR, Sullivan JJ, Tuohig GM, Larson CM. Multiple organ failure in patients with thermal injury. Crit Care Med. 1993;21:1673–83. doi: 10.1097/00003246-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TT, Gilpin DA, Meyer NA, Herndon DN. Current treatment of severely burned patients. Ann Surg. 1996;223:14–25. doi: 10.1097/00000658-199601000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Ann Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 6.Havran WL, Jameson JM, Witherden DA. Epithelial cells and their neighbors. III. Interactions between intraepithelial lymphocytes and neighboring epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G627–G630. doi: 10.1152/ajpgi.00224.2005. [DOI] [PubMed] [Google Scholar]
- 7.Lopez RD. Human gammadelta-T cells in adoptive immunotherapy of malignant and infectious diseases. Immunol Res. 2002;26:207–21. doi: 10.1385/IR:26:1-3:207. [DOI] [PubMed] [Google Scholar]
- 8.Schwacha MG, Ayala A, Chaudry IH. Insights into the role of γδ T-lymphocytes in the immunopathogenic response to thermal injury. J Leuko Biol. 2000;67:644–50. [PubMed] [Google Scholar]
- 9.Toth B, Alexander M, Daniel T, Chaudry IH, Hubbard WG, Schwacha MG. The role of γδ T cells in the regulation of neutrophil-mediated tissue damage after thermal injury. J Leukoc Biol. 2004;76:545–52. doi: 10.1189/jlb.0404219. [DOI] [PubMed] [Google Scholar]
- 10.Alexander M, Daniel T, Chaudry IH, Choudhry M, Schwacha MG. T-cells of the γδ T-cell receptor lineage play an important role in the postburn wound healing process. J Burn Care Res. 2006;27:18–25. doi: 10.1097/01.bcr.0000188325.71515.19. [DOI] [PubMed] [Google Scholar]
- 11.Daniel T, Thobe BM, Chaudry IH, Choudhry MA, Hubbard WJ, Schwacha MG. Regulation of the postburn wound inflammatory response by gammadelta T-cells. Shock. 2007;28:278–83. doi: 10.1097/shk.0b013e318034264c. [DOI] [PubMed] [Google Scholar]
- 12.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–6. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 13.Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165:2643–50. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 15.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, Mannick JA, Lederer JA. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–83. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 16.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 17.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–8. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 18.Maung AA, Fujimi S, Miller L, Macconmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:568–73. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 19.Shimura H, Nitahara A, Ito A, Tomiyama K, Ito M, Kawai K. Up-regulation of cell surface Toll-like receptor 4-MD2 expression on dendritic epidermal T cells after the emigration from epidermis during cutaneous inflammation. J Dermatol Sci. 2005;37:101–10. doi: 10.1016/j.jdermsci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Deetz CO, Hebbeler AM, Propp NA, Cairo C, Tikhonov I, Pauza CD. Gamma interferon secretion by human Vgamma2Vdelta2 T cells after stimulation with antibody against the T-cell receptor plus the Toll-Like receptor 2 agonist Pam3Cys. Infect Immun. 2006;74:4505–11. doi: 10.1128/IAI.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–48. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Alexander M, Chaudry IH, Schwacha MG. Relationships between burn size, immunosuppression, and macrophage hyperactivity in a murine model of thermal injury. Cell Immunol. 2002;220:63–9. doi: 10.1016/s0008-8749(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 23.Schwacha MG, Knoferl MW, Chaudry IH. Does burn wound excision after thermal injury attenuate subsequent macrophage hyperactivity and immunosuppression? Shock. 2000;14:623–8. doi: 10.1097/00024382-200014060-00009. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Hsu B. The role of macrophages (Mφ) and PGE-2 in postburn immunosuppression. Burns. 1992;18:132–6. doi: 10.1016/0305-4179(92)90010-r. [DOI] [PubMed] [Google Scholar]
- 25.Bryant HU, Bernton EW, Holaday JW. Immunomodulatory effects of chronic morphine treatment: pharmacologic and mechanistic studies. NIDA Res Monogr. 1990;96:131–49. [PubMed] [Google Scholar]
- 26.Alexander M, Daniel T, Chaudry IH, Schwacha MG. Opiate analgesics contribute to the development of post-injury immunosuppression. J Surg Res. 2005;129:161–8. doi: 10.1016/j.jss.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Daniel T, Alexander M, Hubbard WJ, Chaudry IH, Choudhry MA, Schwacha MG. Nitric oxide contributes to the development of a post-injury Th2 T-cell phenotype and immune dysfunction. J Cell Physiol. 2006;208:418–27. doi: 10.1002/jcp.20677. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Schwacha MG, Chaudry IH, Choudhry MA. A role of PP1/PP2A in mesenteric lymph node T cell suppression in a two-hit rodent model of alcohol intoxication and injury. J Leukoc Biol. 2006;79:453–62. doi: 10.1189/jlb.0705369. [DOI] [PubMed] [Google Scholar]
- 29.Aderem A. Role of Toll-like receptors in inflammatory response in macrophages. Crit Care Med. 2001;29:S16–S18. doi: 10.1097/00003246-200107001-00008. [DOI] [PubMed] [Google Scholar]
- 30.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 31.Chen XL, Xia ZF, Wei D, Han S, Ben DF, Wang GQ. Role of p38 mitogen-activated protein kinase in Kupffer cell secretion of the proinflammatory cytokines after burn trauma. Burns. 2003;29:533–9. doi: 10.1016/s0305-4179(03)00147-5. [DOI] [PubMed] [Google Scholar]
- 32.Alexander M, Daniel T, Chaudry IH, Schwacha MG. MAP kinases differentially regulate the expression of macrophage hyperactivity after thermal injury. J Cell Physiol. 2004;201:35–44. doi: 10.1002/jcp.20050. [DOI] [PubMed] [Google Scholar]
- 33.Cairns B, Maile R, Barnes CM, Frelinger JA, Meyer AA. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. J Trauma. 2006;61:293–8. doi: 10.1097/01.ta.0000228969.46633.bb. [DOI] [PubMed] [Google Scholar]
- 34.Matsushima A, Ogura H, Fujita K, Koh T, Tanaka H, Sumi Y, Yoshiya K, Hosotsubo H, Kuwagata Y, Shimazu T, Sugimoto H. Early activation of γδ T lymphocytes in patients with severe systemic inflammatory response syndrome. Shock. 2004;22:11–5. doi: 10.1097/01.shk.0000129203.84330.b3. [DOI] [PubMed] [Google Scholar]
- 35.Chung CS, Watkins L, Funches A, Lomas-Neira J, Cioffi WG, Ayala A. Deficiency of gammadelta T lymphocytes contributes to mortality and immunosuppression in sepsis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1338–R1343. doi: 10.1152/ajpregu.00283.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O’Brien R. Immunoregulatory functions of gamma delta T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 37.Tough DF, Sprent J. Lifespan of gamma/delta T cells. J Exp Med. 1998;187:357–65. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura H, Emoto M, Hiromatsu K, Yamamoto S, Matsuura K, Gomi H, Ikeda T, Itohara S, Yoshikai Y. The role of γδ T cells in priming macrophages to produce tumor necrosis factor-α. Eur J Immunol. 1995;25:1465–8. doi: 10.1002/eji.1830250551. [DOI] [PubMed] [Google Scholar]
- 39.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–9. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 40.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–42. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim EY, Teh HS. TNF type 2 receptor (p75) lowers the threshold of T cell activation. J Immunol. 2001;167:6812–20. doi: 10.4049/jimmunol.167.12.6812. [DOI] [PubMed] [Google Scholar]
- 42.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human gammadelta T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol. 2002;23:14–8. doi: 10.1016/s1471-4906(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 43.Boismenu R, Feng L, Xia YY, Chang JC, Havran WL. Chemokine expression by intraepithelial γδ T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157:985–92. [PubMed] [Google Scholar]
- 44.Egan PJ, Kimpton W, Seow HF, Bowles VM, Brandon MR, Nash AD. Inflammation-induced changes in the phenotype and cytokine profile of cells migrating through skin and afferent lymph. Immunology. 1996;89:539–46. doi: 10.1046/j.1365-2567.1996.d01-776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van RI, Rutten VP, Charleston B, Smits M, van Eden W, Koets AP. Massive, sustained gammadelta T cell migration from the bovine skin in vivo. J Leukoc Biol. 2007;81:968–73. doi: 10.1189/jlb.0506331. [DOI] [PubMed] [Google Scholar]
- 46.Penido C, Vieira-de-Abreu A, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Role of monocyte chemotactic protein-1/CC chemokine ligand 2 on gamma delta T lymphocyte trafficking during inflammation induced by lipopolysaccharide or Mycobacterium bovis bacille Calmette-Guerin. J Immunol. 2003;171:6788–94. doi: 10.4049/jimmunol.171.12.6788. [DOI] [PubMed] [Google Scholar]
- 47.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–55. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 49.Muzio M, Bosisio D, Polentarutti N, D’amico G, Stoppacciaro A, Mancinelli R, van’t Veer C, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 50.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]


