Abstract
To classify the defects of the skull base, we present a new concept that is intuitive, simple to use, and consistent with subsequent reconstructive procedures. The centers of defects are determined in the anterior (I) or middle (II) skull base. The defects are classified as localized in the defect's center (Ia, IIa) or extended horizontally (Ib, IIb) or vertically (Ic, IIc) from the defect's center. Accompanying defects of the orbital contents and skin are indicated by “O” and “S,” respectively. An algorithm for selecting subsequent reconstructive procedures was based on the classification. Using the new system, we retrospectively reclassified 90 skull base defects and examined how the defect classifications were related to the reconstructive flaps used and postoperative complications. All defects were reclassified with the new system without difficulty or omission. The mean correlation rate was high (88%) between the flaps indicated by the new classification and the flaps that had actually been used. The rate of postoperative complications tended to be higher with Ia, Ic, and IIb defects and combined defects. Our new classification concept can be used to classify defects and to help select flaps used for subsequent reconstructive procedures.
Keywords: skull base, skull base defect, classification, reconstructive surgery
Objectives
Neoplasms of the skull base are rare, and defects resulting after their resection remain a challenge for surgeons performing subsequent reconstructive procedures. Moreover, because of the high morbidity rate and complex goals of reconstruction, surgeons must exchange information among disciplines and institutions and compare outcomes based on classifications of skull base defects. In fact, multicenter studies1,2 have evaluated outcomes of skull base surgery but have focused on the results of tumor extirpation and patient survival, not on the outcomes of reconstructive procedures. A classification system for the skull base has been proposed by Irish et al3 but was designed to describe the location of primary disease, not that of skull base defects after tumor extirpation. The lack of an appropriate classification system for skull base defects has made it difficult for surgeons to compare or exchange information about perioperative surgical results, including postoperative complications, and the advantages and disadvantages of different reconstructive procedures.
Therefore, in the present report, we describe a new classification concept for skull base defects which we believe is simple, intuitive, easy to use, and consistent with flap selection and subsequent reconstructive procedures. In the present study, we assessed the usability of the classification for postablation defects of the skull base and examined the correspondence between our new classification and postoperative complications.
Design
A New Classification Concept
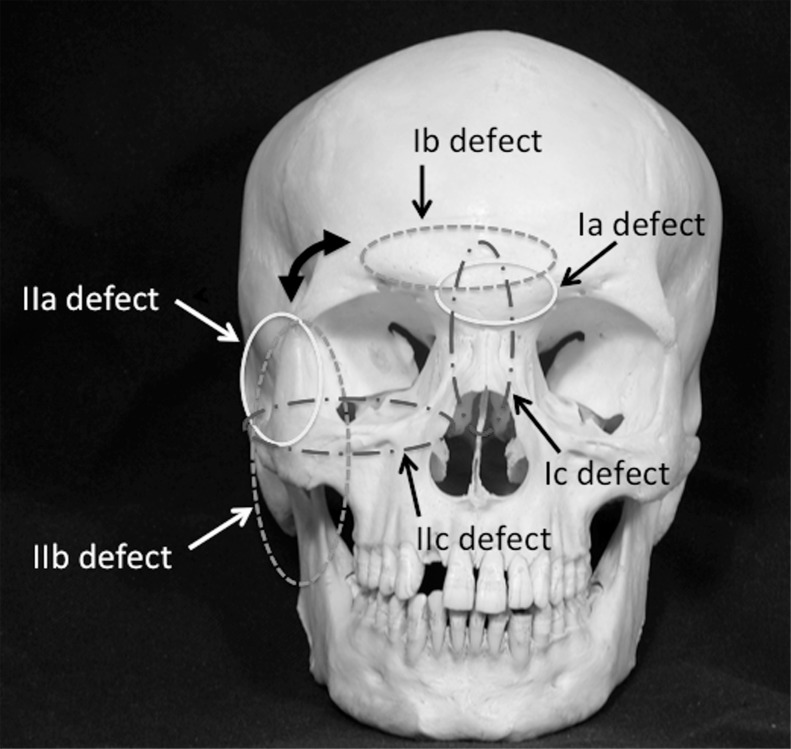
In our new classification concept (Table 1), the center of a defect of the anterior skull base or the middle skull base is defined. Then, the defect is further classified with three independent criteria: whether it is localized in its center, whether is extends horizontally from its center, or whether it extends vertically from its center. In other words, the defect is simply and intuitively classified, in relation to its defined center, as “localized,” “horizontally extended,” or “vertically extended.”
Table 1. Defect Classifications and Indicated Flaps.
| Defect Area | Classification | Indicated Flap |
|---|---|---|
| Anterior skull base (center = cribriform plate) | I | |
| Localized | Ia | Locoregional |
| Horizontally extended to orbital roof | Ib | Free |
| Vertically extended to deep sinonasal cavity | Ic | Locoregional |
| Middle skull base (center = infratemporal fossa) | II | |
| Localized | IIa | Locoregional |
| Horizontally extended to pterygoid muscles or mandible | IIb | Free |
| Vertically extended to maxillary sinus or epipharynx | IIc | Locoregional |
| Skin | Above + S | Free |
| Orbital contents | Above + O | Free |
| Combined defects | Multiple | Free |
I. Anterior skull base defects: For anterior skull base defects, the cribriform plate is defined as the center of the defect, which is then further classified as Ia, Ib, or Ic.
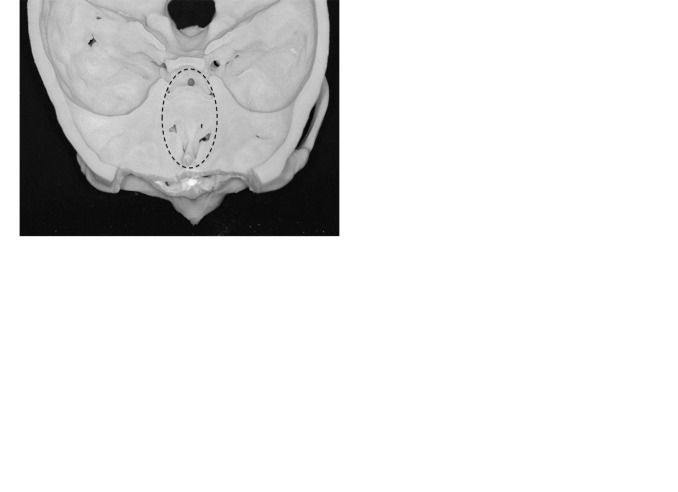
Ia: Defect localized to the cribriform plate (Fig. 1A)
Ib: Defect extends horizontally from the cribriform plate to the orbital roof (Fig. 1B)
Ic: Defect extends vertically from the cribriform plate to the deep part of the sinonasal cavity (Fig. 1C)
Figure 1.
(A, B, C) Defects Ia, Ib, and Ic.
II. Middle skull base defects; For middle skull base defects, the infratemporal fossa is defined as the center of the defect, which is then classified as IIa, IIb, or IIc (Fig. 1).
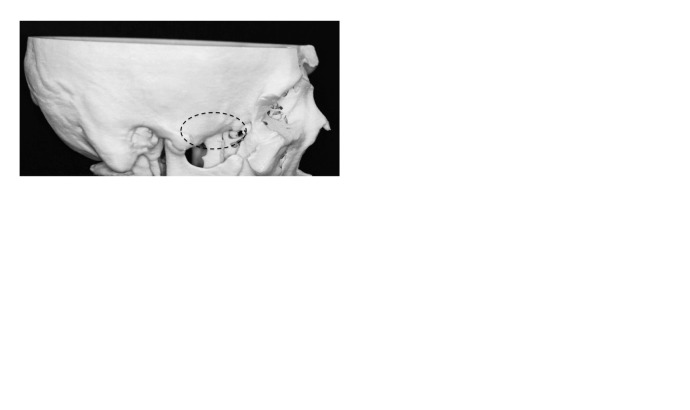
IIa: Defect localized to the infratemporal fossa (Fig. 2A)
IIb: Defect extends horizontally from the infratemporal fossa to the pterygoid muscle or mandible (Fig. 2B)
IIc: Defect extends vertically from the infratemporal fossa to the maxillary sinus or epipharynx (Fig. 2C)
Figure 2.
(A, B, C) Defects IIa, IIb, and IIc.
Note that each subclass (Ia, Ib, and Ic) of anterior skull base defect is rotated 90 degrees from the corresponding subclass of middle skull base defect (Fig. 3).
Figure 3.
The new classification system for defects of the anterior skull base (Ia, Ib, and Ic) and the middle skull base (IIa, IIb and IIC).
III: Posterior skull base defects: For posterior skull base defects, a region III classification that has previously been reported is used.3
IV. Defects of the skin or orbital contents and combined defects: If a defect of the skin or orbital contents is also present, an “S” or “O,” respectively, is added to each classification. Combined defects can be described with combinations of the classifications.
V. Reconstructive procedures: With this new classification system, appropriate reconstructive procedures are indicated as follows:
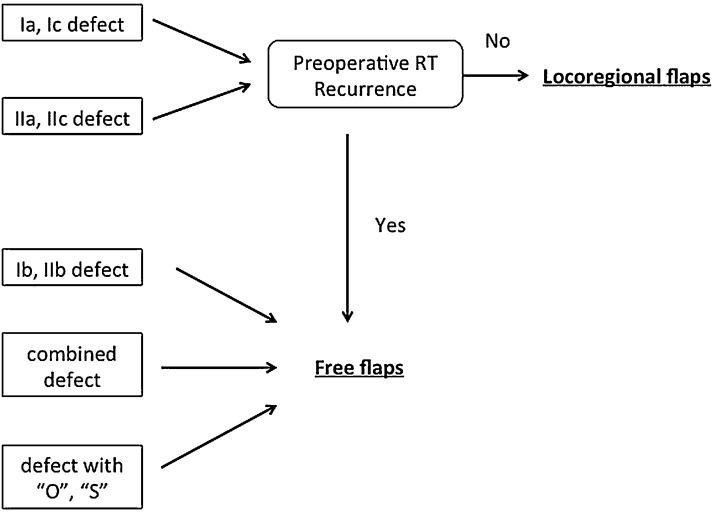
Defects of class Ia, Ic, IIa, or IIc should be reconstructed with a locoregional flap, such as a pericranial flap or a temporal muscle flap.
Defects of class Ib, IIb, “O,” or “S,” and combined defects should reconstructed with a free flap, such as an anterolateral thigh free flap or rectus abdominis myocutaneous free flap.
Defects of class III usually do not require any reconstructive procedure.
A free flap should be used if disease has recurred or if the patient has received preoperative radiotherapy.
Patients and Methods
We reviewed 90 patients who had undergone reconstructive surgery for skull base defects resulting from tumor ablation at the Department of Plastic and Reconstructive Surgery, Tokyo Medical and Dental University Hospital, from July 2000 through April 2010 and could be followed up for at least 1 year. The 90 patients (58 male and 32 female) ranged in age from 2 to 79 years (mean age, 43 years). Tumors were benign in 29 patients and malignant in 61 patients and arose in the anterior skull base in 53 patients, the middle skull base in 29 patients, the anterior-middle skull base in 7 patients, and the middle-posterior skull base in 1 patient. Our new system was used to classify the skull base defects in these patients.
Statistical analyses were performed with the Yates χ2 tests. Differences with p-value of 0.05 or less were considered significant.
Results
Of the 90 defects, 70 were single-class defects (such as Ia, Ib, Ic, IIa, IIb, and IIc), and the remaining 20 defects were combined defects. The pathologic diagnoses of the cases in each class are shown in Table 2.
Table 2. Pathological Diagnoses in Each Classification.
| Defect Class | No. of Cases | Diagnoses (No. of Cases) | Defect Class | No. of Cases | Diagnoses (No. of Cases) |
|---|---|---|---|---|---|
| Ia | 27 | Olfactory neuroblastoma (13), nasal cavity carcinoma (3), meningioma (2), ossifying fibroma (2), ethmoidal sinus carcinoma (2), pleomorphic adenoma (1), frontal sinus carcinoma (1), chondoromesenchymal hamartoma (1), cemento-ossifying fibroma (1), adenoid cystic carcinoma (1) | IIa | 15 | Trigeminal nerve-sheath tumor (8), mucocele (1), giant cell tumor (1), dermoid cyst (1), small cell carcinoma (1), angiofibroma (1), adenoid cystic carcinoma (1), rhabdomyosarcoma (1) |
| Ib | 4 | Angioepithelioma (1), meningioma (1), frontal sinus carcinoma (1), chordoma (1) | IIb | 12 | Rhabdomyosarcoma (3), synovial sarcoma (2), osteosarcoma (2), maxillary sinus carcinoma (2), fibrosarcoma (1), ameloblastoma (1), basal cell carcinoma (1) |
| Ic | 7 | Olfactory neuroblastoma (2), ethmoidal sinus carcinoma (1), malignant myoepithelioma (1), osteosarcoma (1), chondrosarcoma (1), trigeminal nerve-sheath tumor (1) | IIc | 5 | Trigeminal nerve-sheath tumor (2), angiofibroma (2), solitary fibroma (1) |
| Ib + IIa | 1 | Meningioma (1) | Ic + IIc | 1 | Adenoid cystic carcinoma (1) |
| Ib + IIc | 1 | Chordoma (1) | IIa + III | 1 | Endolymphatic sac tumor (1) |
| Ib + Ic + IIc | 1 | Angiofibroma (1) | |||
| Ib + Ic + S | 1 | Adenoid cystic carcinoma (1) | Ib + S | 1 | Undifferentiated carcinoma (1) |
| Ib + O | 5 | Rhabdomyosarcoma (2), osteosarcoma (1), ethmoidal sinus carcinoa (1), transitional cell carcinoma (1), adenoid cystic carcinoma (1) | Ib + Ic + O | 2 | Maxillary sinus adenoma (1), maxillary sinus carcinoma (1), small cell carcinoma (1) |
| Ic + O | 1 | Maxillary sinus carcinoma (1) | Ib + IIc + O | 1 | Maxillary sinus carcinoma (1) |
| Ib + Ic + S + O | 1 | Maxillary sinus carcinoma (1) | Ic + IIc + O | 1 | Maxillary sinus carcinoma (1) |
The type of flap indicated by the classification, the type of flap actually transferred, and the rates of concordance between them are shown in Table 3. All Ia, Ic, IIa, or IIc defects, except for those in patients who had received preoperative radiotherapy or in whom disease had recurred, were reconstructed with locoregional flaps. For defects in patients who had received preoperative radiation therapy (three patients with Ia defects and two patients with Ic defects) or in whom disease had recurred (in three patients with Ia defects and one patient with a IIa defect), free flap reconstruction was indicated and was, therefore, performed. Free flaps were used to reconstruct 3 of 4 cases of Ib defects, 11 of 12 cases of IIb defects, and 19 of 20 cases of combined defects. The remaining Ib defects (1 of 4) and IIb defects (1 of 12) were reconstructed with locoregional flaps; although free flaps had been planned, the defects were not large enough to require free flaps and could be reconstructed with locoregional flaps. One combined defect was reconstructed with a locoregional flap because the patient's general condition was poor and because a locoregional flap could be used to achieve the minimum degree of reconstruction required. The rate of correspondence between the flaps indicated and the flaps actually used ranged from 71 to 100% (mean, 88%).
Table 3. Correspondence between Flaps Indicated by the New Classification and Flaps Actually Transferred.
| Classification | No. of Cases | Flap Indicated | Flap Transferred | Flap Correspondence Rate (Transferred as Percentage of Indicated) (%) | |
|---|---|---|---|---|---|
| Locoregional | Free | ||||
| Ia | 27 | Locoregional | 21 | 6 | 89 |
| Ib | 4 | Free | 1 | 3 | 75 |
| Ic | 7 | Locoregional | 5 | 2 | 71 |
| IIa | 15 | Locoregional | 14 | 1 | 93 |
| IIb | 12 | Free | 1 | 11 | 92 |
| IIc | 5 | Locoregional | 5 | 0 | 100 |
| Combined defects | 20 | Free | 1 | 19 | 95 |
As for postoperative complication, overall postoperative complication rate did not significantly differ between Ia, Ib, and Ic defect, and IIa, IIb, and IIc defect. And also whether single-class defects or combined defects, postoperative complication rate did not significantly differ. Although complication rate did not reach the level of significant difference, several tendencies were observed regarding the postoperative complications in each classification (Table 4). In the anterior skull base, the complication rate tended to be higher with Ia and Ic defects than with Ib defects, and in the middle skull base, complication rates tended to be higher with IIb defects than with IIa or IIc defects; combined defects also had a high rate of postoperative complications. In addition, major complications (those requiring additional surgical treatment) tended to be observed with anterior skull base defects and combined defects, and minor complications (those treated with only conservative treatment, such as localized debridement, irrigation, antibiotics, and ointments) tended to be observed with middle skull base defects.
Table 4. Defect Classifications and Postoperative Complications.
| Defect Class |
No. of Cases | Major Complicationsa | Minor Complicationsb | Overall Complication Rate (%) | ||
|---|---|---|---|---|---|---|
| No. | Type | No. | Type | |||
| Ia | 27 | 3 | Total flap necrosis, intracranial hematoma, titanium mesh removal | 3 | Partial flap necrosis, subcutaneous abscess, cerebrospinal fluid leakage | 22 |
| Ib | 4 | 0 | 0 | 0 | ||
| Ic | 7 | 2 | Epidural abscess | 0 | 28 | |
| IIa | 15 | 0 | 1 | Chronic fistula | 6 | |
| IIb | 12 | 1 | Total flap necrosis | 2 | Titanium mesh exposure, partial flap necrosis | 25 |
| IIc | 5 | 0 | 0 | 0 | ||
| Combined defects | 20 | 3 | Partial flap necrosis, epidural abscess, intracranial hematoma | 0 | 15 | |
Major complications were those requiring additional surgical treatment.
Minor complications were those treated with only conservative treatment, such as localized debridement, irrigation, antibiotics, and ointments.
Discussion
Skull base reconstruction with the transfer of either locoregional flaps or free flaps has become a safe and widely accepted surgical procedure after tumor ablation.4,5,6,7,8 For further advances and innovation in skull base reconstruction, researchers require a common standard for evaluating and discussing the end results of reconstructive procedures after tumor ablation. The first step toward this goal is establishing a defect-classification system that is simple, intuitive, and easy to use. Several defect classifications for skull base surgery have previously been proposed. For example, Urken et al9 have proposed a system in which skull base defects are classified according to the condition of the dura, mucosa, skin, bone, cavities, nerves, and carotid artery. However, we believe that because of its large number of variables, this classification is burdensome and complicated to use and prevents defects from being understood visually and intuitively. Yamamoto et al10 have proposed a classification concept for skull base defects in which the details of the defect around the orbital contents are fully addressed, but this classification appears to be suitable only for anterior skull base defects caused by extended maxillectomy involving the skull base and not for those caused by procedures performed via the transcranial approach. Pusic et al11 have proposed an alternative defect classification with accompanying flap selection. Their classification focuses on the surrounding tissues resected along with the primary tumor. It is simple and easy to use but the reconstructive procedures are limited to the transfer of free flaps, and the recipient vessels to be selected are limited to the neck region, with none from the temporal region. Therefore, this classification did not include all types of skull base defect.
To address these problems, we developed a new classification concept that: (1) is based on the center of the defect in the skull base region; (2) corresponds to both the transcranial and craniofacial approaches; (3) is simple, intuitive, and easy to use; and (4) is consistent with reconstructive procedures and the selection of either locoregional or free flaps. With this classification concept in the present retrospective study all defects, including those caused by resection via either the transcranial or craniofacial approach, were classified without difficulty or exception. Furthermore, the mean rate of correspondence was high (88%) between flaps indicated by the classification and flaps that had actually been used for reconstruction. In other words, by classifying defects with our new system, the most appropriate flap for reconstruction can be selected. According to these outcomes, we have proposed an algorithm for selecting appropriate reconstructive procedures in accordance with our new classification concept (Fig. 4).
Figure 4.
An algorithm for subsequent skull base reconstructive procedures based on the new defect classification.
We observed a specific tendency between postoperative complications and types of defect with our new classification system, namely Ia, Ic, and IIb defects, and combined defects were associated with high rates of postoperative complications. A possible reason that Ia defects had a complication rate higher than did other defects is that Ia defects were the most common. Combined defects also tended to be complex and extensive; therefore, the associated complication rate might be expected to be higher. The high complication rates with Ic and IIb defects are more difficult to explain. However, we speculate that because Ic defects are extended vertically, i.e., deeper into the sinonasal cavity, which contains flora from the aerodigestive tract, they are more likely to be infected than are other types of defect. Furthermore, IIb defects are transversely extended and involve the mandible and the pterygoid plexus and muscles; the large dead spaces can easily lead to hematomas and might, therefore, increase the rate of postoperative complications.
We believe the observed relation between our new classification and postoperative complications is significant, because it implies that our new classification concept could be improved. In particular, the high rates of postoperative complications with Ia, Ic, and IIb defects suggest that our algorithm for selecting reconstructive procedures on the basis of the new classification should be reconsidered and, possibly, revised.
Conclusion
Our new classification concept can be used to classify skull base defects and might be used to select the most appropriate type of flap to use for reconstruction. We believe our new classification concept will also be useful for evaluating outcomes of and exchanging information about skull base reconstruction.
Acknowledgment
This work was supported by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor, and Welfare of Japan.
References
- 1.Gil Z, Patel S G, Cantu G. et al. Outcome of craniofacial surgery in children and adolescents with malignant tumors involving the skull base: an international collaborative study. Head Neck. 2009;31(3):308–317. doi: 10.1002/hed.20958. [DOI] [PubMed] [Google Scholar]
- 2.Ganly I, Patel S G, Singh B. et al. Craniofacial resection for malignant tumors involving the skull base in the elderly: an international collaborative study. Cancer. 2011;117(3):563–571. doi: 10.1002/cncr.25390. [DOI] [PubMed] [Google Scholar]
- 3.Irish J C, Gullane P J, Gentili F. et al. Tumors of the skull base: outcome and survival analysis of 77 cases. Head Neck. 1994;16(1):3–10. doi: 10.1002/hed.2880160103. [DOI] [PubMed] [Google Scholar]
- 4.Hanasono M M, Sacks J M, Goel N, Ayad M, Skoracki R J. The anterolateral thigh free flap for skull base reconstruction. Otolaryngol Head Neck Surg. 2009;140(6):855–860. doi: 10.1016/j.otohns.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Gil Z, Abergel A, Leider-Trejo L. et al. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base. 2007;17(1):25–37. doi: 10.1055/s-2006-959333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu E S, Kraus D, Bui D T. et al. Anterior and middle cranial fossa skull base reconstruction using microvascular free tissue techniques: surgical complications and functional outcomes. Ann Plast Surg. 2008;60(5):514–520. doi: 10.1097/SAP.0b013e3181715707. [DOI] [PubMed] [Google Scholar]
- 7.Thurnher D, Novak C B, Neligan P C, Gullane P J. Reconstruction of lateral skull base defects after tumor ablation. Skull Base. 2007;17(1):79–88. doi: 10.1055/s-2006-959338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yano T, Tanaka K, Kishimoto S, Iida H, Okazaki M. Reliability of and indications for pericranial flaps in anterior skull base reconstruction. J Craniofac Surg. 2011;22(2):482–485. doi: 10.1097/SCS.0b013e318207b714. [DOI] [PubMed] [Google Scholar]
- 9.Urken M L, Catalano P J, Sen C, Post K, Futran N, Biller H F. Free tissue transfer for skull base reconstruction analysis of complications and a classification scheme for defining skull base defects. Arch Otolaryngol Head Neck Surg. 1993;119(12):1318–1325. doi: 10.1001/archotol.1993.01880240054007. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Minakawa H, Kawashima K. et al. Experience with 24 cases of reconstructive anterior skull base surgery: classification and evaluation of postoperative facial appearance. Skull Base Surg. 2000;10(2):65–70. doi: 10.1055/s-2000-7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pusic A L, Chen C M, Patel S, Cordeiro P G, Shah J P. Microvascular reconstruction of the skull base: a clinical approach to surgical defect classification and flap selection. Skull Base. 2007;17(1):5–15. doi: 10.1055/s-2006-959331. [DOI] [PMC free article] [PubMed] [Google Scholar]