Abstract
Vestibular schwannomas (VS) have a higher risk of recurrence following subtotal resection than following near-total resection. We measured tumor remnant growth volumetrically in an attempt to determine potential predictors for postoperative recurrence following subtotal resection. We reviewed the charts of patients who had undergone VS surgery between 1998 and 2007. Thirty patients had an incomplete resection. The principal outcome measure was change in tumor volume (TV) on serial imaging. At a median follow-up of 6.8 years, volumetric measurements showed that 12 patients (40%) developed further tumor growth, while 18 patients remained with stable residual disease. The median rate of growth was 0.53 cm3/year. Two-dimensional measurements confirmed growth in only eight of these patients. The postoperative residual TV correlated significantly with subsequent tumor growth (p = 0.038). All patients with residual volumes in excess of 2.5 cm3 exhibited recurrence. On univariate analysis, only postoperative TV was significantly associated with growth. Median time to failure was 21.5 months. This is the first report of volumetric measurements of VS tumor growth postoperatively. Volumetric measurements appear to be superior to two-dimensional measurements in documenting VS growth and patients with residual tumors >2.5 cm3 have a significantly higher rate of recurrence.
Keywords: vestibular schwannoma, acoustic neuroma, postoperative growth, volumetric measurement, partial resection
Vestibular schwannomas (VS) are tumors of the myelin-producing Schwann cells of the 8th cranial nerve. They are classified as benign, but their local growth can cause hearing loss, vestibular problems, and brainstem compression. Based on planimetric measurements, most VSs grow slowly. Because of this, the optimal postoperative management of an incompletely resected VS remains controversial. While some earlier reports1 described that the majority of incompletely resected VSs remained dormant, even after a long follow-up time, more recent studies have shown a substantially higher growth rate following incomplete resection.2,3,4 El-Kashlan et al5 reported that in 39 patients with incompletely removed VSs, tumor regrowth occurred in 17 (44%). Most regrowths occurred in patients with 90% or less of the lesion excised (based on the surgeon's intraoperative estimate). Bloch et al6 classified their 52 patients with incomplete resections into near-total (intra-operative tumor remnants no greater than 2 mm thick or 25 mm2 in area) or subtotal (any larger remnant) resections. Recurrences were observed in 1 of 33 (3%) patients with near-total resection versus 6 of 19 patients (32%) with subtotal resections.
All previous studies have assessed VS tumor growth by measuring the greatest dimension of the lesion. Volumetric measurement may be more accurate as tumors may grow in different directions than the planimetric axes. We set out to confirm the reports of high recurrence rates after incomplete resection of VS by being the first to serially document VS growth volumetrically.
Methods
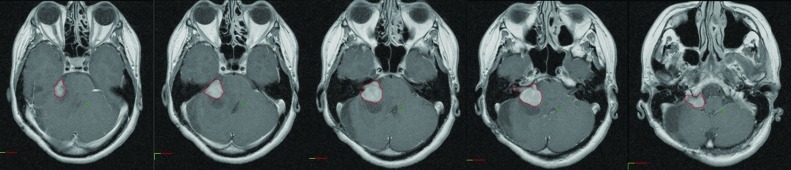
After obtaining Institutional Review Board approval, the charts of all patients having undergone resection of a VS by the same surgeon (AZ) at our institution between February 1998 and November 2007 were carefully reviewed. Patient demographic characteristics, extent of surgical resection, and adjuvant therapy were recorded. The preoperative and postoperative follow-up radiological studies were analyzed. All but two patients were followed by MRI. All magnetic resonance imaging (MRI) and/or computed tomography (CT) images were transferred to a commercially available radiotherapy treatment planning software (Varian Eclipse), where the tumors were contoured on each imaging slice and volumes were determined (Fig. 1). Thus, this represents a true volume calculation rather than volume estimation. The T1, gadolinium-enhanced MRI sequences were used for all measurements; most MRI studies were obtained in a standard fashion with a 1.5 T magnet using the internal auditory canal acquisition sequence with slice thickness of 2 mm and slice separation of 2.2 mm. However, images used for volumetric and planimetric measurements in this study ranged from slice thickness and separation of 5 mm (for older studies) to less than 1 mm. Imaging was obtained 6 months following the surgery and yearly thereafter. Linear measurements were also taken for each patient using the same images. Three linear measurements were taken: the largest diameter parallel to the petrous bone, the largest diameter perpendicular to the petrous bone, and the intracanalicular portion of the lesion. Two of the authors (SV and DM) performed all imaging review and measurements and DM was blinded to outcome data.
Figure 1.
Serial tumor contouring for volume definition.
We divided patients into two groups based on the extent of surgical resection. Those with a postoperative residual volume on imaging of less than 0.01 cm3 (the lower limit of the software's volume determination) were considered to have near-complete resections. Patients with postoperative volumes of 0.01 cm3 or greater were deemed to have an incomplete resection. Tumor growth was assessed by the increase in tumor volume on serial imaging. Outcomes were calculated from the date of surgery. The date of the first postoperative imaging modality where growth was documented was considered to be the time of recurrence. The following variables were analyzed for potential independent prediction of postoperative tumor growth: age at diagnosis, gender, planimetric tumor size prior and postsurgery, and pre- and postoperative tumor volumes.
SPSS (version 10.0, SPSS Inc, Chicago, IL) was used for all statistical analyses. In cases where the increase in volume was small and debatable, the Wilcoxon Signed Rank Test (p <0.1) was used to determine if growth had occurred. The Student's t-test was used to compare the median preoperative and postoperative volumes of patients with stable residuals to those of patients who exhibited tumor growth. Spearman's correlation coefficient was used to determine the correlation between tumor growth and pre-operative and postoperative tumor volumes. We used one-way ANOVA to evaluate the relationship between tumor growth and the following six variables: age, gender, preoperative linear dimension, postoperative linear dimension, preoperative tumor volume, and postoperative tumor volume.
Results
A total of 60 patients were identified. Of these, 20 patients were either followed at another institution (no follow-up information) or had incomplete follow-up imaging and were excluded. Thus a total of 40 patients were available for the analysis. Of these, 10 patients, 7 men and 3 women, had near-complete resections (residual volumes less than 0.01 cm3). Their median age was 64 years (range: 33 to 78). The median preoperative tumor volume of this group was 1.32 cm3 (range: 0.15 to 12.27). All 10 patients were followed with MRI, and none showed demonstrable disease or tumor recurrence at a median follow-up of 6.3 years (range: 2.5 to 9.75).
The 30 patients who had incomplete resections were the main study population. Table 1 shows the demographic characteristics of these patients. At a median follow-up of 6.8 years (range: 1.9 to 10.5), 12 patients (40%) showed subsequent tumor growth on volumetric measurement, whereas 18 patients (60%) remained with stable residual disease. In the recurring lesions, the median rate of growth was 0.53 cm3/year (range: 0.01 to 3.81). Planimetric measurements (two-dimensional) confirmed growth in only eight patients (26.6%), whereas enlargement was missed in four. The median time to failure was 21.5 months (range: 4 to 29). Of the 12 patients in which postoperative growth was documented, 5 underwent re-excision, 4 underwent adjuvant radiotherapy, and 3 are still under observation.
Table 1. Characteristics of Patients with Incomplete Resection.
| Parameter | Number of Patients | |
|---|---|---|
| Median age (range) | 59 years (29–82) | |
| Gender | ||
| Male | 16 | |
| Female | 14 | |
| Median preoperative linear dimension (range) | 2.92 cm (0.86–6.45) | |
| Median postoperative linear dimension (range) | 1.48 cm (0.41–4.33) | |
| Median preoperative volume (range) | 9.48 cm3 (0.28–67.91) | |
| Median postoperative volume (range) | 0.91 cm3 (0.03–54.14) | |
Patients who showed tumor recurrence had mean postoperative tumor volumes that were significantly greater (p = 0.041) than those patients with stable residuals (Table 2). The postoperative residual tumor volume also correlated significantly with subsequent tumor growth (Spearman's correlation coefficient p = 0.038). All patients with tumor residual volumes in excess of 2.5 cm3 exhibited further tumor growth.
Table 2. Comparison of Mean Preoperative and Postoperative Planimetric and Volumetric Measurements as a Function of Tumor Growth.
| Tumor Status | Number of Patients | Mean Preoperative Measurement | Mean Postoperative Measurement | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Planimetric | p-Valuea | Volumetric | p-Valuea | Planimetric | p-Valuea | Volumetric | p-Valuea | ||
| Stable Residual | 18 | 3.09 cm | 0.853 | 11.54 cm3 | 0.327 | 1.54 cm | 0.326 | 1.30 cm3 | 0.041 |
| Tumor Growth | 12 | 3.20 cm | 18.97 cm3 | 1.91 cm | 11.24 cm3 | ||||
Student's t-test.
Univariate analysis demonstrated that only postoperative tumor volume (p <0.05) was significantly associated with growth. Gender, age, preoperative and postoperative planimetric dimensions, and preoperative volume had no significant association with tumor growth.
Discussion
By measuring VS tumor volumes sequentially, we found that 40% of incompletely resected tumors regrew. There was a statistically significant relationship between the volume of the residual tumor and regrowth. In this series, all tumors with residual volumes greater than 2.5 cm3 grew, suggesting that the volume of residual tumor is the most important prognostic variable for tumor regrowth.
This study suggests that volumetric measurements were superior to two-dimensional measurements in determining that growth had occurred. This makes sense because volume is an important metric. For instance, the volume of a sphere is proportional to the cube of the radius, so that small changes in radius or diameter (two-dimensional) will translate into a large difference in volume. In four cases, growth was not suspected from serial linear measurements but became clear when serial volumes were calculated. Also, preoperative and postoperative linear measurements had no association with tumor growth, whereas postoperative volume did, again showing volume to be a more powerful indicator of growth.
Unlike most previous studies that tended to use the surgeon's intraoperative assessment for the completeness of the tumor resection, our definition was based on the first available postoperative image. This was done primarily in the attempt to have an objective, third-party measure. However, using imaging to define extent of surgical excision has its problems. El-Kashlan et al5 have shown that in some of their patients who underwent incomplete resections as assessed by the surgeon, no residual tumor could be visualized on the postoperative scan. This could be partially explained by the fact that these authors mainly used CT scans, with their well-known poor sensitivity for posterior fossa structures, to follow their patients. In our series, all patients with near-complete resections by our volumetric assessment were followed and none were found to recur.
The use of postoperative imaging to define the extent of surgery can be somewhat imprecise because many follow-up images show postoperative changes, such as dural enhancement, which are often difficult to distinguish from a tumor remnant. In this review, we often made use of the extensive clinical experience of our senior neuroradiologist (DM) to make this distinction. Admittedly, however, later follow-up imaging studies influenced the designation and classification of such imaging phenomena. For example, an area of postoperative enhancement was more likely to be considered dural enhancement if it was present only on the first postoperative MRI, and was not present on subsequent scans. An alternative explanation for this, although unlikely, could be a true tumor remnant which actually shrank and disappeared, leading to misclassification in this example of an incompletely resected lesion.
Our observation that all residuals greater than 2.5 cm3 recurred, leads to the question of what to do with large residual tumors. Re-excision can be an option, although reoperation may carry significant risks. Alternatively, these patients can be treated with stereotactic radiosurgery techniques or fractionated stereotactic radiation therapy. Iwai et al7 have reported their experience treating 14 patients with large ANs with surgery followed by adjuvant radiosurgery. In this group, 11 patients had either stable disease or decrease in residual tumor size, while 3 exhibited tumor growth with only 1 patient requiring re-excision following radiosurgery.
Our study is limited by the relatively small number of patients and its retrospective nature. A larger, prospective study could provide more precise information on the potential value of assessing the rate of volumetric growth in these patients with large residual tumors. For instance, if a fast volumetric growth is detected, perhaps a closer follow-up imaging routine could be established or even immediate adjuvant stereotactic radiosurgery or surgery could be performed. A further difficulty we encountered lay in the determination that tumor regrowth had occurred. In most cases, follow-up imaging clearly demonstrated increases in volume that were large in magnitude (over 1 cm3) and consistent in later scans. However, in some instances, the increase in volume or linear dimension was small and inconsistent. In these cases, the Wilcoxon Signed Rank test was performed on the differences in volume or diameter between subsequent imaging studies, to determine whether these differences could be due to chance alone. An alternative solution would have been to perform tests of interobserver reproducibility to obtain an objective assessment of measurement variability against which growth could be compared.
In conclusion, this study confirmed that incompletely resected VSs have a high rate of regrowth. Our results suggest that the likelihood of postoperative VS growth is related to residual volume and that volumetric growth measurement is more accurate than linear measurement. The routine use of volumetric measurements may detect tumor growth at an earlier stage and could have potential clinical implications in the management of patients with large residual tumors in the postoperative setting. Future studies are needed to confirm our findings and to assess the most appropriate management algorithm and adjuvant treatment for large residual tumors.
Acknowledgment
The authors would like to thank William Parker and Suzanna Darvasi for their technical support to this project.
References
- 1.Silverstein H, McDaniel A, Norrell H, Wazen J. Conservative management of acoustic neuroma in the elderly patient. Laryngoscope. 1985;95(7 Pt 1):766–770. [PubMed] [Google Scholar]
- 2.Kemink J L, Langman A W, Niparko J K, Graham M D. Operative management of acoustic neuromas: the priority of neurologic function over complete resection. Otolaryngol Head Neck Surg. 1991;104(1):96–99. doi: 10.1177/019459989110400117. [DOI] [PubMed] [Google Scholar]
- 3.Comey C H, Jannetta P J, Sheptak P E, Joh H D, Burkhart L E. Staged removal of acoustic tumors: techniques and lessons learned from a series of 83 patients. Neurosurgery. 1995;37(5):915–920, discussion 920–921. doi: 10.1227/00006123-199511000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Roberson J B, Brackmann D E, Hitselberger W E. Acoustic neuroma recurrence after suboccipital resection: management with translabyrinthine resection. Am J Otol. 1996;17(2):307–311. [PubMed] [Google Scholar]
- 5.El-Kashlan H K, Zeitoun H, Arts H A, Hoff J T, Telian S A. Recurrence of acoustic neuroma after incomplete resection. Am J Otol. 2000;21(3):389–392. doi: 10.1016/s0196-0709(00)80049-6. [DOI] [PubMed] [Google Scholar]
- 6.Bloch D C, Oghalai J S, Jackler R K, Osofsky M, Pitts L H. The fate of the tumor remnant after less-than-complete acoustic neuroma resection. Otolaryngol Head Neck Surg. 2004;130(1):104–112. doi: 10.1016/S0194-5998(03)01598-5. [DOI] [PubMed] [Google Scholar]
- 7.Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol. 2003;59(4):283–289, discussion 289–291. doi: 10.1016/s0090-3019(03)00025-9. [DOI] [PubMed] [Google Scholar]