Abstract
Bile duct damage is present in virtually all cholangiopathies, which share the biliary epithelial cells (i.e. cholangiocytes) as a common pathogenic target. Cholangiocyte cell death largely occurs through the process of apoptosis. In this review, we will summarize the mechanisms through which biliary damage occurs in a variety of animal and in vitro models, such as extrahepatic cholestasis induced by bile duct ligation (BDL), cytotoxin- and hepatotoxin-induced liver injury, and biliary atresia. Although we have increased our knowledge of the factors that regulate cholangiocyte cell death mechanisms during cholangiopathies, especially in experimental models, there is still a lack of effective treatment modalities for these biliary disorders. However, future studies will hopefully provide for new therapeutic modalities for the prevention or restoration of biliary mass and function lost during the progression of cholangiopathies.
Keywords: cholangiocyte, cholangiocarcinoma, apoptosis, cholestatic liver diseases
Introduction
The liver is composed of two types of epithelial cells, which are hepatocytes and cholangiocytes (i.e. biliary epithelial cells).1,2 While hepatocytes account for approximately 70% of the total liver mass, cholangiocytes contribute to 3 to 5% of the endogenous liver cell population.1 Cholangiocytes line the intra- and extrahepatic bile ducts of the biliary system, which is comprised of a series of interconnected tube like structures that drain bile from the liver and delivers it to the gallbladder or duodenum.1 Cholangiocytes modify the composition of bile that is secreted at the canalicular membranes of hepatocytes as it flows through the biliary system.1,3,4 This modification involves the secretion and absorption of water, electrolytes and other organic solutes from hepatocellular bile.1,2,4–9
Cholangiocytes are the target cells of a number of diseases termed cholangiopathies.1 This disease class is made up of inherited disorders (Alagille syndrome and cystic fibrosis (CF)), autoimmune disorders (primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), autoimmune cholangitis (AIC), allograft rejection, graft-versus-host disease (GVHD)), infections (cholangitis due to bacteria, fungi, parasites or viruses), drug-induced injury, ischemic injury, and diseases of unknown etiology (biliary atresia and idiopathic vanishing bile duct syndromes).10 Cholangiopathies are predominantly characterized by a bile duct-directed inflammatory response that leads to bile duct injury associated with biliary proliferation in the early stage of the disease course.10 If the biliary injury is chronic there will be increased bile duct loss (ductopenia), biliary fibrosis and the increased incidence of bile duct cancer (i.e. cholangiocarcinoma).10 This review summarizes the mechanisms responsible for non-neoplastic cholangiocyte proliferation and cell death.
Types of Cell Death
Cell death has been subdivided into three categories: apoptosis (Type I), autophagic cell death (Type II), and necrosis (Type III).11–13 A fine line exists between the two forms of programmed cell death, apoptosis (‘self-killing’) and autophagy (‘self-eating’) in that they share common pathways and are functionally linked.14–16 Apoptosis is the most investigated of the types of programmed cell death. Apoptosis results from the activation of a signaling cascade of catabolic enzymes, which lead to the destruction of cellular structures and organelles.17,18 At the conclusion of this process, morphologically changes occur that include cellular shrinkage, chromatin condensation and nuclear fragmentation.13
Apoptosis can be activated extrinsic and intrinsic pathways that lead to caspase-dependent cell death. The extrinsic pathway begins outside the cell through the activation of pro-apoptotic or death receptors.19 These death receptors are members of the tumor necrosis factor receptor (TNFR) superfamily, which includes TNF-receptor 1 (TNF-R1/p55/CD120a), Fas (CD95/APO-1), TNF-related apoptosis-inducing ligand receptor (TRAIL-R1/Death Receptor-4 (DR4), DR3 (APO-3/TRAMP/WSL-1/LARD), and TRAIL-R2(DR5/APO-2/KILLER).19,20 The ligands for death receptors include tumor necrosis factor-alpha (TNF-α), Apo2L/TRAIL and CD95L/FasL.20 The signaling mechanisms downstream of the activation of death receptors has been previously review.19 The key caspases activated by the extrinsic pathway are caspase 8 and 10.19 As its name suggests, the intrinsic pathway is initiated from within the cell. The intrinsic pathway is activated in response to cellular signals resulting from DNA damage, a defective cell cycle, detachment from the extracellular matrix, hypoxia, loss of cell survival factors, oxidative stress or other types of severe cell stress.21 The intrinsic pathway is characterized by the involvement of the mitochondria with mitochondrial outer membrane permeabilization and the release of mitochondrial cytochrome c.22 The release of cytochrome c stimulates the assembly of caspase-activating complex between caspase-9 and APAF1 (i.e. apoptosome).16 The role of the BH3-only proteins that participate in initiation of mitochondrial outer membrane permeabilization have been reviewed elsewhere.23 During DNA damage, activation of p53 can result in the transcriptional activation of the BH3 only proteins PUMA and NOXA, which can then promote mitochondrial outer membrane permeabilization via BAX and BAK channels.24 In addition, DNA damage can also activate caspase-2 in a complex of proteins that involves p53-induced protein with a death domain (PIDD) and RIP-associated protein with a death domain (RAIDD), which together is known as the piddosome.25,26 Activation of other cellular stress pathways can lead to the stimulation of apoptosis including the activation of caspase independent cell death that can result from factors that trigger lysosomal membrane permeabilization.27,28
On the other hand, autophagy (i.e. macroautophagy) represents a stress adaptation that avoids cell death while suppressing apoptosis in certain cellular conditions.15 However, in other stress conditions it represents an alternative cell death mechanism.15 Autophagy is a cellular mechanism that promotes the degradation of aging cytoplasmic proteins and intracellular organelles.15 Autophagy also plays a role in cellular adaptation to starvation by triggering self-catabolism to provide for the bioenergetic needs of the cell.29,30 The cellular signaling pathways that guide the cellular response to either autophagy as a survival or cell death mechanism and its relationship with apoptosis have been thoroughly reviewed elsewhere.11,14–16,27,31
The definition of necrosis is somewhat ambiguous in light of the fact that it is most often defined as a type of cell death that lacks features of apoptosis and autophagy, and is an uncontrolled mechanism.32 Whether or not necrosis is a controlled cell death mechanism remains controversial.32 However, necrosis can include signs of controlled processes such as mitochondrial dysfunction, ATP depletion and proteolysis by calpains and cathepsins.32
Types of Cell Death Observed in Cholangiopathies
Necrosis is usually the consequence of acute metabolic perturbations as those that occur in ischemia-reperfusion or acute drug-induced cellular toxicity.10 Bile duct necrosis is present in ischemic cholangiopathies, which predominantly affects the middle third of the common bile ducts, followed by the hepatic duct confluence, with intrahepatic involvement being the least common feature.33 In the liver, autophagy plays a key role in the regulation of energy balance and nutrients for basic cell functions as well as the removal of misfolded proteins and the turnover of organelles.34 The role of autophagy as a death mechanism has not been well addressed for biliary injury or cholestatic liver diseases. However, autophagy does play a role in autoimmunity in particular in the control of T lymphocyte homeostasis and potentially could be involved in immune-mediated liver diseases.32 On the other hand, apoptosis is thought to play a major role in cholestatic liver diseases such as PBC, PSC and biliary atresia.35,36 In immune-mediated liver diseases, such as PBC, PSC and autoimmune hepatitis, recent studies have indicated that programmed cell death ligands and circulating apoptotic markers might serve as diagnostic markers for these diseases.35,37 Apoptosis of cholangiocytes has been observed in a number of animal models of cholestasis and biliary injury.38–42 In light of these findings, our review will focus on apoptosis in non-neoplastic cholangiocytes.
Cholestatic Animal Models and Cholangiocyte Proliferation
A number of animal models that mimic cholestatic liver diseases and liver injury have been utilized to expand our knowledge concerning the mechanisms of cholangiocyte proliferation and bile duct damage.1,43–47 Of these models of bile duct injury, the bile duct ligated (BDL) model has been the most commonly used.4,8,44,47,48 In normal human and rodent liver, cholangiocytes are mitotically dormant and apoptosis is rare.1,47,49 BDL induces proliferation of cholangiocytes. Although cholangiocyte apoptosis is minimal, this model has proved valuable because it renders cholangiocytes more susceptible to injury.39,40,45,50,51 Proliferating cholangiocytes acquire a neuroendocrine phenotype and secrete and respond to a number of hormones, neuropeptides and neurotransmitters.52 The formation of a neuroendocrine compartment predominated by cholangiocytes represents a unique opportunity for cholangiocytes to regulate their own proliferation via autocrine pathways and for cholangiocytes to influence other nearby cell types, such as vascular endothelial cells, portal fibroblasts and hepatic stellate cells (HSC).52
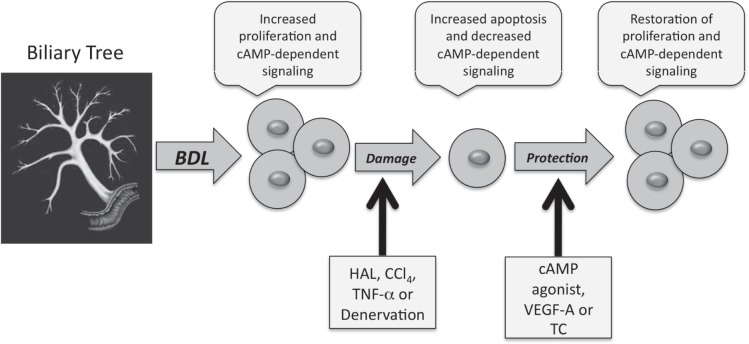
Of importance to both normal physiology and pathophysiology, cholangiocytes are the only cell types in the liver that express the secretin receptor (SR). Secretin stimulates ductal bile secretion by a series of coordinated events, which involves the elevation of intracellular 3′,5′-cyclic adenosine monophosphate (cAMP) leading to the activation of protein kinase A (PKA).53 Subsequently, PKA phosphorylates the cystic fibrosis transmembrane conductance regulator (CFTR) triggering the opening of Cl− channels leading to extrusion of Cl− at the apical membrane.54 The Cl− efflux from CFTR creates a Cl− gradient that favors the activation of the apically located Cl−/HCO3− exchanger,55 which results in secretin-stimulated bicarbonate-enriched bile.1,2,4,6,8 Several studies have revealed that SR expression is linked to cholangiocyte proliferative responses in animal models of biliary hyperplasia such as BDL, partial hepatectomy, chronic feeding of bile acids [e.g. taurocholic acid (TC)] and cirrhosis induced by chronic carbon tetrachloride (CCl4) administration and can serve as a surrogate marker for proliferative status and biliary damage.1,4,43,44,49,56 Proliferating cholangiocytes have increased SR expression while cholangiocytes that are damaged have lower levels of SR expression. Changes in the functional expression of this receptor have been suggested as a pathophysiological tool for evaluating changes in the degree of cholangiocyte growth/loss.1,7,40,49 In humans, SR expression is present in the biliary tract in normal bile ducts and ductules and the majority of cholangiocarcinomas, but is not present in hepatocytes or hepatocellular carcinoma.57,58 Consistent with animal models of cholestasis, SR expression was upregulated in ductular reactions in liver cirrhosis.58 No studies have determined yet whether SR expression can be a clinical therapeutic target. However, we have data demonstrating that cholangiocyte proliferation during cholestasis is heavily dependent upon the expression of SR. In SR knockout mice, biliary proliferation is dramatically reduced during extrahepatic cholestasis induced by BDL59 (and Alpini, G. unpublished data). Interestingly, cholangiocyte proliferation is predominantly regulated through the cAMP/PKA/ERK1/2-dependent signaling mechanisms, which is a critical mechanism involved in the activation of biliary epithelial cell damage and a mechanism that can be activated to protect cholangiocytes from damage under certain circumstances.38,46,48,60–65 A summary of the regulation of biliary damage that will be discussed in the following discussion is illustrated in Figure 1.
Figure 1.
Mechanisms of biliary damage during cholestasis. Bile duct ligation (BDL) induces the proliferation of cholangiocytes, which is associated with increased cAMP-dependent signaling mechanisms. Cholestasis induced by BDL renders cholangiocytes more sensitive to damage by hepatotoxins, hepatic artery ligation (HAL) and denervation. This damage is associated with increased cholangiocyte apoptosis and decreased cAMP-dependent signaling mechanisms. Administration of cAMP agonists (for CCl4 and denervation), VEGF-A (for HAL), and TC (for HAL and denervation) have been shown to restore cholangiocyte proliferation and cAMP dependent signaling mechanisms and prevent cholangiocyte apoptosis.
Mechanisms of Cholangiocyte Cell Death
Sympathetic and parasympathetic innervation
In rat liver, sympathetic and parasympathetic nerves are located around the hepatic artery, portal vein, and intrahepatic and extrahepatic biliary epithelium.66 Cholangiocytes have been shown to express α-1, α-2, β-1 and β-2 adrenergic receptor subtypes.64 The expression of these receptors is closely linked to the functional activity of cholangiocytes. The α-1 agonist, phenylephrine, stimulates secretin-induced choleresis of BDL rats through the activation of IP3/Ca2+-dependent PKC-α and PKC-βII.64 However, the α-2 agonist, UK14,304, modulates ductal bile secretion by decreasing secretin-stimulated choleresis of BDL rats by down-regulating cAMP/PKA/CFTR/Cl−/HCO3− exchanger (AE2)67 activity in cholangiocytes.61
Denervation of adrenergic terminal fibers of BDL rats by administration of 6-hydroxydopamine (6-OHDA) induces functional damage of the biliary system via the down-regulation of the cAMP-dependent signaling and the induction of cholangiocyte apoptosis.38 The functional damage and apoptosis induced by 6-OHDA was reversed by the administration of forskolin (adenylyl cyclase activator), clenbuterol (β-2 adrenergic agonist) and dobutamine (β-1 adrenergic agonist), which are all factors that stimulate adenylyl cyclase and elevate intracellular cAMP levels.38 Similar results were obtained when 6-OHDA treated BDL rats were fed the bile acid taurocholate for one week post 6-OHDA administration.68 Taurocholate feeding had previously been shown to increase cholangiocyte proliferation and secretion.56 Taurocholic acid prevented 6-OHDA-induced cholangiocyte apoptosis and restored cholangiocyte proliferation and secretin-stimulated ductal secretion through an AKT-dependent mechanism.68
Similar findings were observed with parasympathetic denervation by total vagotomy. Cholangiocytes express the M3 acetylcholine receptor and interruption of cholinergic innervation induces functional damage of cholangiocytes by apoptosis in BDL but not normal rats.5,47 Vagotomy-induced apoptosis was associated with decreased cAMP-dependent signaling and reduced cholangiocyte hyperplasia.47 In fact, chronic forskolin administration prevents vagotomy-induced damage of cholangiocytes in BDL rats. Chronic feeding of taurocholic acid also prevented vagotomy-induced apoptosis of cholangiocytes, which was dependent upon maintenance of ABAT (apical bile acid transporter) activity, down-regulation of caspase activity, and activation of PI3-kinase signaling.41
These studies clearly indicate the importance of the cAMP-dependent signaling mechanism in the prevention of cholangiocyte apoptosis and restoration of cholangiocyte proliferation in the absence of sympathetic and parasympathetic innervation. Cholangiocyte necrosis and autophagy have not been evaluated in the context of sympathetic and parasympathetic innervation. However, these findings indicate that the sympathetic and parasympathetic nervous systems play a key role in the regulation of biliary mass during cholestasis and that the modulation of these systems could potentially have a therapeutic effect in patients with early extrahepatic cholestasis.
Ischemic injury
The function of the intrahepatic biliary epithelium is closely linked to its vascular supply the peribiliary vascular plexus (PBP).69 Alterations of the intra-hepatic biliary tree during cholestasis are associated architectural changes of the PBP.69 The PBP undergoes marked proliferation in order to support the increased nutritional and functional demands from proliferating bile ducts during bile duct ligation (BDL).70 Interestingly, the proliferation of the PBP occurs only after hyperplasia of the intrahepatic biliary epithelium during extrahepatic cholestasis.70 Apoptosis of cholangiocytes is also observed in BDL rats following hepatic artery ligation, which interrupts the main blood supply of the intrahepatic biliary epithelium.70 The dramatic loss of cholangiocytes was presumably due to lack of oxygen and nutrients to supply the increased biliary mass induced by bile duct ligation thus, making proliferating cholangiocytes more sensitive to apoptosis. Hepatic artery ligation was associated with the disappearance of the peribiliary vascular plexus (PBP) and decreased expression of vascular endothelial growth factor (VEGF) by cholangiopcytes.42 Administration of recombinant VEGF-A to BDL rats with hepatic artery ligation prevented cholangiocyte apoptosis due to a VEGF-A-dependent maintenance of the PBP and blood flow to cholangiocytes,42 which might have occurred through the formation of collaterals. In addition, administration of anti-VEGF antibodies to BDL rats was associated with a decrease in cholangiocyte proliferation and increased cholangiocyte apoptosis.42 This work highlights the importance of VEGF in the modulation of biliary mass and is supported by other studies that demonstrate that VEGF-A regulates cholangiocyte proliferation in an autocrine fashion during extrahepatic cholestasis.50 These studies suggest that administration of VEGF-A during ischemic periods such as during liver transplantation might reduce bile duct injury in humans.
Tumor necrosis factor-alpha (TNF-α)-induced and cytotoxic cell death
TNF-α is a pro-inflammatory mediator with the capacity to induce apoptosis. Cholangiocytes are the primary epithelial source of TNF-α in the liver, and biliary levels of TNF-α are increased in patients with cholangitis following biliary tract obstruction.71,72 TNF-α plays a critical role in epithelial cell injury as well as in immune-mediated cholangiocyte injury.73 Immune mediated injury has been implicated in the pathogenesis of PBC and PSC.1 In vitro studies have demonstrated that that TNF-α in combination with interleukin-1 (IL-1), IL-6 and interferon-γ, inhibits cAMP-dependent ductal secretion.74 TNF-α binds to TNF-receptor 1 (TNF-R1/p55/CD120a), which is part of the TNF superfamily of membrane death receptors.19 Death receptors are characterized by a cytoplasmic region termed, the death domain, which is required for apoptotic signaling.20 The mechanisms by which death receptors trigger apoptosis have been recently reviewed.19 We have previously shown that TNF-α, when administered in combination with actinomycin D, induces cholangiocyte apoptosis and loss of ductal secretion in BDL rats.45 In this study co-incubation with actinomycin D sensitized the cholangiocytes from BDL (but not normal cholangiocytes) to TNF-α toxicity.45 These findings suggest that during cholestasis proliferating cholangiocytes are more sensitive to the toxic effects of TNF-α. The bile acid, taurocholate, was shown to prevent TNF-α induced damage of cholangiocyte through the activation of the PI3K pathway.75
Human cholangiocytes express DR5, and TRAIL expression and apoptosis were shown to be significantly elevated in cholangiocytes of human PSC and PBC patients.76 Takeda et al have shown that TRAIL receptor 2/DR5 may be a key play in the regulation of cholestatic liver injury.76 In the study, they demonstrated that administration of agonistic anti-DR5 antibody triggered cholangiocyte apoptosis, induced cholangitis and cholestatic liver injury in B6 mice.76 BDL in the mice augmented DR5 expression and sensitized the mice to DR5-induced cholangitis with a histological presentation similar to PSC.76 Their findings suggest that TRAIL-mediated apoptosis may play an important role in the progression of chronic cholestasis. Recently, Feng and colleagues have reported an up-regulation of tumor necrosis factor related apoptosis-inducing ligand (TRAIL) receptors, death receptors (DR) DR4 and DR5, in an in vitro model of hypoxia/reoxygenation, a condition that may occur during the pathogenesis of liver diseases.77 The upregulation of DR4 and DR5 resulted in increased sensitivity to TRAIL-induced apoptosis in cholangiocytes.77
TNF-α has been implicated in the pathogenesis of biliary atresia, which is a fibrosis/inflammatory cholangiopathy that obstructs the extrahepatic bile ducts in infants.36 Apoptosis is thought to play a key role in the progression of biliary atresia. In a mouse rotavirus model of biliary atresia, the biliary epithelium undergoes an extensive activation of early apoptosis. This increase in apoptosis was associated with increased expression of caspase 1 and 4, interferon-γ (IFNγ)-related and TNFα-related gene expression.36 Simultaneous exposure of cholangiocytes to IFNγ and TNFα decreased cell viability.36 Blockade of caspase activity in vivo decreased the extent of injury to the biliary epithelium and supports the role of apoptosis in the pathogenesis of biliary atresia in animal models.36
PBC is characterized by sustained macrophage infiltration suggesting that these immune cells may mediate the destruction of bile ducts.78 Activation of CD40 on cholangiocytes by soluble CD154 induces apoptosis in vitro.79 Co-incubation of human cholangiocytes with activated liver-derived macrophages stimulated CD40-dependent secretion of proinflammatory cytokines and apoptosis of cholangiocytes, which suggest that macrophages play a role in the destruction of bile ducts through CD40 in liver disease pathogenesis.80 Recently, Shimoda and colleagues have shown that chemokine-adhesion molecule CX3CL1 (fractalkine) plays a role in bile duct destruction in PBC.81 Their data indicate that TNF-α and CX3CL1, induced by toll-like receptor ligand, participate in processes that lead to the recruitment of lymphoid cells into the portal tracts characteristic of chronic nonsuppurative destructive cholangitis of PBC.81
Hepatoxin-induced biliary damage
As mentioned early, the bile ducts of animals with BDL are more sensitive to damage. However, in the CCl4 model of hepatotoxin induced liver damage both normal and BDL cholangiocytes are susceptible to damage.39,40 Administration of an acute dose of CCl4 to normal or BDL rats induces apoptosis of large cholangiocytes39,40 (which line large ducts).82,83 Small cholangiocyte (which line small ducts)82,83 were resistant to injury and proliferated to compensate for the loss of functionally active large cholangiocytes.39,40 Recently, it has been shown that exendin-4 (a long acting analogue of glucagon-like peptide-1 (GLP-1)) prevents cholangiocyte apoptosis in rats with BDL treated with CCl4, which was due to exendin-4 ability to counteract the activation of the mitochondrial pathway of apoptosis.84 On the other hand, chronic feeding of the hepatotoxin, α-napthylisothiocyanate (ANIT), induces cholangiocyte proliferation in both small and large cholangiocytes.51 Apoptosis is observed in small and large cholangiocytes upon withdrawal of the diet allowing for the regression of biliary mass.51
Conclusion and Future Directions
Over the previous 20 years, we have significantly increased our understanding of the mechanisms involved in cholangiocyte death. Cholangiocyte apoptosis plays a key role in the pathogenesis of many cholangiopathies such as PBC and biliary atresia. In most models of biliary damage, proliferating cholangiocytes are more sensitive to factors that activate apoptosis (Fig. 1). New therapies based upon the inhibition of cholangiocyte apoptosis (i.e. biliary damage) should prove beneficial for sustaining biliary mass in cholangiopathies that result in the loss of cholangiocytes, such as PBC and biliary atresia. Due to the high probability that a large proportion of cholangiopathies are autoimmune in nature, more studies that address how cholangiocytes interact with the immune system and immune cells will be required for a complete understanding of the pathogenesis of these devastating biliary tract diseases. In addition, evaluation of the role of autophagy and its relationship with apoptotic programmed cell death is needed for a complete understanding of how cell death mechanisms participate in the pathogenesis of cholangiopathies.
Acknowledgments
Portions of the studies discussed here were supported partly by a grant award from Scott and White and NIH RO1 DK081442 to Shannon Glaser, by the Dr. Nicholas C. Hightower Centennial Chair of Gas-troenterology from Scott and White, the VA Research Career Scientist Award, a VA Merit Award and the NIH grants DK76898, DK58411 and DK62975 to Gianfranco Alpini, by University and Federate Athenaeum funds from University of Rome “La Sapienza” to Eugenio Gaudio.
Abbreviations
- AIC
autoimmune cholangitis
- ANIT
α-napthylisothiocyanate
- BDL
bile duct ligation
- cAMP
3′,5′-cyclic monophosphate
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CCl4
carbon tetrachloride
- GLP-1
glucagon-like peptide-1
- GVHD
graft-versus-host disease
- HSC
hepatic stellate cells
- IFNγ
Interferon-gamma
- PBC
primary biliary cholangitis
- PBP
peribiliary vascular plexus
- PKA
protein kinase A
- PSC
primary sclerosing cholangitis
- SR
secretin receptor
- TC
taurocholic acid
- TNFα
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
Footnotes
This article is available from http://www.la-press.com.
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors report no conflicts of interest.
References
- 1.Alpini G, Prall R, LaRusso N. The pathobiology of biliary epithelia. In: Arias I, Boyer J, Chisari F, Fausto N, Jakoby W, Schachter D, Shafritz D, editors. The Liver; Biology and Pathobioloty. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 421–435. [Google Scholar]
- 2.Tietz PS, Alpini G, Pham LD, Larusso NF. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol. 1995;269:G110–8. doi: 10.1152/ajpgi.1995.269.1.G110. [DOI] [PubMed] [Google Scholar]
- 3.Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–66. [PubMed] [Google Scholar]
- 4.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–78. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvaro D, Alpini G, Jezequel AM, Bassotti C, Francia C, Fraioli F, et al. Role and mechanisms of action of acetylcholine in the regulation of rat cholangiocyte secretory functions. J Clin Invest. 1997;100:1349–62. doi: 10.1172/JCI119655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvaro D, Cho WK, Mennone A, Boyer JL. Effect of secretion on intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1993;92:1314–25. doi: 10.1172/JCI116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaser S, Francis H, Demorrow S, Lesage G, Fava G, Marzioni M, et al. Heterogeneity of the intrahepatic biliary epithelium. World J Gastroenterol. 2006;12:3523–36. doi: 10.3748/wjg.v12.i22.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glaser SS, Rodgers RE, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, et al. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol. 1997;273:G1061–70. doi: 10.1152/ajpgi.1997.273.5.G1061. [DOI] [PubMed] [Google Scholar]
- 9.Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–25. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 10.Xia X, Demorrow S, Francis H, Glaser S, Alpini G, Marzioni M, et al. Cholangiocyte injury and ductopenic syndromes. Semin Liver Dis. 2007;27:401–12. doi: 10.1055/s-2007-991516. [DOI] [PubMed] [Google Scholar]
- 11.Lockshin RA, Zakeri Z. Apoptosis, autophagy, and more. Int J Biochem Cell Biol. 2004;36:2405–19. doi: 10.1016/j.biocel.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc) 2005;70:231–9. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 13.Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12(Suppl 2):1463–7. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- 14.Lleo A, Invernizzi P, Selmi C, Coppel RL, Alpini G, Podda M, et al. Autophagy: highlighting a novel player in the autoimmunity scenario. J Autoimmun. 2007;29:61–8. doi: 10.1016/j.jaut.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 16.Allan LA, Clarke PR. Apoptosis and autophagy: Regulation of caspase-9 by phosphorylation. FEBS J. 2009;276:6063–73. doi: 10.1111/j.1742-4658.2009.07330.x. [DOI] [PubMed] [Google Scholar]
- 17.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 18.Green DR. Apoptotic pathways: ten minutes to dead. Cell. 2005;121:671–4. doi: 10.1016/j.cell.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23:1625–37. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 21.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 22.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 23.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 24.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 25.Cuenin S, Tinel A, Janssens S, Tschopp J. p53-induced protein with a death domain (PIDD) isoforms differentially activate nuclear factor-kappaB and caspase-2 in response to genotoxic stress. Oncogene. 2008;27:387–96. doi: 10.1038/sj.onc.1210635. [DOI] [PubMed] [Google Scholar]
- 26.Tinel A, Janssens S, Lippens S, Cuenin S, Logette E, Jaccard B, et al. Autoproteolysis of PIDD marks the bifurcation between pro-death caspase-2 and pro-survival NF-kappaB pathway. EMBO J. 2007;26:197–208. doi: 10.1038/sj.emboj.7601473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5:886–97. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- 28.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–30. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 29.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–48. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 30.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–48. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 31.Apel A, Zentgraf H, Buchler MW, Herr I. Autophagy-A double-edged sword in oncology. Int J Cancer. 2009;125:991–5. doi: 10.1002/ijc.24500. [DOI] [PubMed] [Google Scholar]
- 32.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Deltenre P, Valla DC. Ischemic cholangiopathy. Semin Liver Dis. 2008;28:235–46. doi: 10.1055/s-0028-1085092. [DOI] [PubMed] [Google Scholar]
- 34.Yin XM, Ding WX, Gao W. Autophagy in the liver. Hepatology. 2008;47:1773–85. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 35.Kremer AE, Rust C, Eichhorn P, Beuers U, Holdenrieder S. Immune-mediated liver diseases: programmed cell death ligands and circulating apoptotic markers. Expert Rev Mol Diagn. 2009;9:139–56. doi: 10.1586/14737159.9.2.139. [DOI] [PubMed] [Google Scholar]
- 36.Erickson N, Mohanty SK, Shivakumar P, Sabla G, Chakraborty R, Bezerra JA. Temporal-spatial activation of apoptosis and epithelial injury in murine experimental biliary atresia. Hepatology. 2008;47:1567–77. doi: 10.1002/hep.22229. [DOI] [PubMed] [Google Scholar]
- 37.Berg CP, Stein GM, Keppeler H, Gregor M, Wesselborg S, Lauber K. Apoptosis-associated antigens recognized by autoantibodies in patients with the autoimmune liver disease primary biliary cirrhosis. Apoptosis. 2008;13:63–75. doi: 10.1007/s10495-007-0157-6. [DOI] [PubMed] [Google Scholar]
- 38.Glaser S, Alvaro D, Francis H, Ueno Y, Marucci L, Benedetti A, et al. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol Gastrointest Liver Physiol. 2006;290:G813–26. doi: 10.1152/ajpgi.00306.2005. [DOI] [PubMed] [Google Scholar]
- 39.LeSage GD, Benedetti A, Glaser S, Marucci L, Tretjak Z, Caligiuri A, et al. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology. 1999;29:307–19. doi: 10.1002/hep.510290242. [DOI] [PubMed] [Google Scholar]
- 40.LeSage GD, Glaser SS, Marucci L, Benedetti A, Phinizy JL, Rodgers R, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol. 1999;276:G1289–301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 41.Marzioni M, LeSage GD, Glaser S, Patel T, Marienfeld C, Ueno Y, et al. Taurocholate prevents the loss of intrahepatic bile ducts due to vagotomy in bile duct-ligated rats. Am J Physiol Gastrointest Liver Physiol. 2003;284:G837–52. doi: 10.1152/ajpgi.00398.2002. [DOI] [PubMed] [Google Scholar]
- 42.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Franchitto A, et al. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G307–17. doi: 10.1152/ajpgi.00507.2005. [DOI] [PubMed] [Google Scholar]
- 43.Alpini G, Elias I, Glaser SS, Rodgers RE, Phinizy JL, Robertson WE, et al. gamma-Interferon inhibits secretin-induced choleresis and cholangiocyte proliferation in a murine model of cirrhosis. J Hepatol. 1997;27:371–80. doi: 10.1016/s0168-8278(97)80184-5. [DOI] [PubMed] [Google Scholar]
- 44.Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol. 1998;274:G767–75. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 45.Alpini G, Ueno Y, Tadlock L, Glaser SS, LeSage G, Francis H, et al. Increased susceptibility of cholangiocytes to tumor necrosis factor-alpha cytotoxicity after bile duct ligation. Am J Physiol Cell Physiol. 2003;285:C183–94. doi: 10.1152/ajpcell.00497.2002. [DOI] [PubMed] [Google Scholar]
- 46.Glaser SS, Ueno Y, DeMorrow S, Chiasson VL, Katki KA, Venter J, et al. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest. 2007;87:914–26. doi: 10.1038/labinvest.3700602. [DOI] [PubMed] [Google Scholar]
- 47.LeSage EG, Alvaro D, Benedetti A, Glaser S, Marucci L, Baiocchi L, et al. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191–9. doi: 10.1016/s0016-5085(99)70567-6. [DOI] [PubMed] [Google Scholar]
- 48.Francis H, Franchitto A, Ueno Y, Glaser S, DeMorrow S, Venter J, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest. 2007;87:473–87. doi: 10.1038/labinvest.3700533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lesage G, Glaser SS, Gubba S, Robertson WE, Phinizy JL, Lasater J, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–44. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 50.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–82. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 51.Lesage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, et al. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–90. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- 52.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–31. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 53.Alvaro D, Mennone A, Boyer JL. Role of kinases and phosphatases in the regulation of fluid secretion and Cl-/HCO3-exchange in cholangiocytes. Am J Physiol. 1997;273:G303–13. doi: 10.1152/ajpgi.1997.273.2.G303. [DOI] [PubMed] [Google Scholar]
- 54.McGill JM, Basavappa S, Gettys TW, Fitz JG. Secretin activates Cl-channels in bile duct epithelial cells through a cAMP-dependent mechanism. Am J Physiol. 1994;266:G731–6. doi: 10.1152/ajpgi.1994.266.4.G731. [DOI] [PubMed] [Google Scholar]
- 55.Martinez-Anso E, Castillo JE, Diez J, Medina JF, Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–6. [PubMed] [Google Scholar]
- 56.Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, et al. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–86. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- 57.Onori P, Wise C, Gaudio E, Franchitto A, Francis H, Carpino G, et al. Secretin inhibits cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of secretin receptor. Int J Cancer. 2009 doi: 10.1002/ijc.25028. [DOI] [PubMed] [Google Scholar]
- 58.Korner M, Hayes GM, Rehmann R, Zimmermann A, Scholz A, Wiedenmann B, et al. Secretin receptors in the human liver: expression in biliary tract and cholangiocarcinoma, but not in hepatocytes or hepatocellular carcinoma. J Hepatol. 2006;45:825–35. doi: 10.1016/j.jhep.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 59.Lam I, Glaser S, Gaudio E, Ueno Y, Chow B, Antonella V, et al. Knockout of secretin receptor decreases the proliferation of large cholangiocytes in bile duct ligated (BDL) mice. Hepatology. 2008;48:416A. [Google Scholar]
- 60.Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, et al. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol. 2009;297:G11–26. doi: 10.1152/ajpgi.00025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Francis H, LeSage G, DeMorrow S, Alvaro D, Ueno Y, Venter J, et al. The alpha2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am J Physiol Cell Physiol. 2007;293:C1252–62. doi: 10.1152/ajpcell.00031.2007. [DOI] [PubMed] [Google Scholar]
- 62.Fava G, Ueno Y, Glaser S, Francis H, Demorrow S, Marucci L, et al. Thyroid hormone inhibits biliary growth in bile duct-ligated rats by PLC/IP(3)/Ca(2+)-dependent downregulation of SRC/ERK1/2. Am J Physiol Cell Physiol. 2007;292:C1467–75. doi: 10.1152/ajpcell.00575.2006. [DOI] [PubMed] [Google Scholar]
- 63.Marzioni M, Alpini G, Saccomanno S, de Minicis S, Glaser S, Francis H, et al. Endogenous opioids modulate the growth of the biliary tree in the course of cholestasis. Gastroenterology. 2006;130:1831–47. doi: 10.1053/j.gastro.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 64.LeSage GD, Alvaro D, Glaser S, Francis H, Marucci L, Roskams T, et al. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology. 2004;40:1116–27. doi: 10.1002/hep.20424. [DOI] [PubMed] [Google Scholar]
- 65.Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, et al. cAMP stimulates the secretory and proliferative capacity of the rat intra-hepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–37. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 66.Reilly FD, McCuskey PA, McCuskey RS. Intrahepatic distribution of nerves in the rat. Anat Rec. 1978;191:55–67. doi: 10.1002/ar.1091910106. [DOI] [PubMed] [Google Scholar]
- 67.Banales JM, Arenas F, Rodriguez-Ortigosa CM, Saez E, Uriarte I, Doctor RB, et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43:266–75. doi: 10.1002/hep.21042. [DOI] [PubMed] [Google Scholar]
- 68.Marzioni M, Ueno Y, Glaser S, Francis H, Benedetti A, Alvaro D, et al. Cytoprotective effects of taurocholic acid feeding on the biliary tree after adrenergic denervation of the liver. Liver Int. 2007;27:558–68. doi: 10.1111/j.1478-3231.2007.01443.x. [DOI] [PubMed] [Google Scholar]
- 69.Gaudio E, Pannarale L, Ripani M, Onori P, Riggio O. The hepatic microcirculation in experimental cirrhosis. A scanning electron microscopy study of microcorrosion casts. Scanning Microsc. 1991;5:495–502. discussion 502–3. [PubMed] [Google Scholar]
- 70.Gaudio E, Onori P, Pannarale L, Alvaro D. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology. 1996;111:1118–24. doi: 10.1016/s0016-5085(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 71.Loffreda S, Rai R, Yang SQ, Lin HZ, Diehl AM. Bile ducts and portal and central veins are major producers of tumor necrosis factor alpha in regenerating rat liver. Gastroenterology. 1997;112:2089–98. doi: 10.1053/gast.1997.v112.pm9178702. [DOI] [PubMed] [Google Scholar]
- 72.Rosen HR, Winkle PJ, Kendall BJ, Diehl DL. Biliary interleukin-6 and tumor necrosis factor-alpha in patients undergoing endoscopic retrograde cholangiopancreatography. Dig Dis Sci. 1997;42:1290–4. doi: 10.1023/a:1018822628096. [DOI] [PubMed] [Google Scholar]
- 73.Patel T, Roberts LR, Jones BA, Gores GJ. Dysregulation of apoptosis as a mechanism of liver disease: an overview. Semin Liver Dis. 1998;18:105–14. doi: 10.1055/s-2007-1007147. [DOI] [PubMed] [Google Scholar]
- 74.Spirli C, Nathanson MH, Fiorotto R, Duner E, Denson LA, Sanz JM, et al. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001;121:156–69. doi: 10.1053/gast.2001.25516. [DOI] [PubMed] [Google Scholar]
- 75.Ueno Y, Francis H, Glaser S, Demorrow S, Venter J, Benedetti A, et al. Taurocholic acid feeding prevents tumor necrosis factor-alpha-induced damage of cholangiocytes by a PI3K-mediated pathway. Exp Biol Med (Maywood) 2007;232:942–9. [PubMed] [Google Scholar]
- 76.Takeda K, Kojima Y, Ikejima K, Harada K, Yamashina S, Okumura K, et al. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci U S A. 2008;105:10895–900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng L, Pang L, Guo Y, Ke N, Li S, Wei L, et al. Hypoxia/reoxygenation up-regulates death receptor expression and enhances apoptosis in human biliary epithelial cells. Life Sci. 2009 doi: 10.1016/j.lfs.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 78.Fava G, Glaser S, Francis H, Alpini G. The immunophysiology of biliary epithelium. Semin Liver Dis. 2005;25:251–64. doi: 10.1055/s-2005-916318. [DOI] [PubMed] [Google Scholar]
- 79.Ahmed-Choudhury J, Williams KT, Young LS, Adams DH, Afford SC. CD40 mediated human cholangiocyte apoptosis requires JAK2 dependent activation of STAT3 in addition to activation of JNK1/2 and ERK1/2. Cell Signal. 2006;18:456–68. doi: 10.1016/j.cellsig.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 80.Alabraba EB, Lai V, Boon L, Wigmore SJ, Adams DH, Afford SC. Coculture of human liver macrophages and cholangiocytes leads to CD40-dependent apoptosis and cytokine secretion. Hepatology. 2008;47:552–62. doi: 10.1002/hep.22011. [DOI] [PubMed] [Google Scholar]
- 81.Shimoda S, Harada K, Niiro H, Taketomi A, Maehara Y, Tsuneyama K, et al. CX3CL1 (fractalkine): A signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2009 doi: 10.1002/hep.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1064–74. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- 83.Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–43. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 84.Marzioni M, Alpini G, Saccomanno S, Candelaresi C, Venter J, Rychlicki C, et al. Exendin-4, a glucagon-like peptide 1 receptor agonist, protects cholangiocytes from apoptosis. Gut. 2009;58:990–7. doi: 10.1136/gut.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]