Proton Transfer Reaction-Mass Spectrometry (PTR-MS) analyses revealed that damaged Brassica roots emit sulfur-containing volatiles. B. nigra, B. juncea and B. napus emitted isothiocyanate markers, whereas B. rapa, B. oleracea, and B. carinata emitted methanethiol. These compounds can be used as markers for root damage by insect larvae and other below-ground herbivores.
Abstract
Background and aims
Plants damaged by herbivores emit a variety of volatile organic compounds (VOCs). Here we used proton-transfer reaction mass spectrometry (PTR-MS) as a sensitive detection method for online analysis of herbivore-induced VOCs. Previously, it was found that Brassica nigra plants emit several sulfur-containing VOCs when attacked by cabbage root fly (Delia radicum) larvae with m/z 60 as a marker for the formation of allylisothiocyanate from the glucosinolate sinigrin. We tested the hypothesis that m/z 60 emission occurs only in plants with sinigrin in their roots. Additionally, we tested the hypothesis that methanethiol, dimethylsulfide and dimethyldisulfide are only emitted after larval infestation.
Methodology
Proton-transfer reaction mass spectrometry was used to track sulfur-containing VOCs from six different species of Brassica over time. The roots were either artificially damaged or infested with cabbage root fly larvae. Glucosinolate profiles of the roots were analysed using high-pressure liquid chromatography and compared with VOC emissions.
Principal results
Brassica nigra, B. juncea and B. napus primarily emitted m/z 60 directly after artificial damage or root fly infestation. Sulfide and methanethiol emissions from B. nigra and B. juncea also increased after larval damage but much later (6–12 h after damage). Brassica rapa, B. oleracea and B. carinata principally emitted methanethiol after artificial and after larval damage. Brassica oleracea and B. carinata showed some increase in m/z 60 emission after larval damage. Comparison with root glucosinolate profiles revealed that sinigrin cannot be the only precursor for m/z 60.
Conclusions
The principal compound emitted after root damage is determined by the plant species, and not by damage type or root glucosinolate composition. Once determined, the principal compounds may be used as markers for identifying damaged or infested plants. Further analyses of plant enzymes involved in the breakdown of sulfur compounds is needed to reveal the origin of sulfur-containing VOCs from plants.
Introduction
Plants infested by herbivores emit a variety of volatile organic compounds (VOCs). In addition to ozone quenching and contributing to resistance to pathogens, VOCs also act indirectly in defence (Holopainen 2004). Herbivore-induced VOCs specifically attract natural enemies that kill or parasitize the herbivore, which may then reduce current and future herbivore damage to the plant (Dicke and Baldwin 2010). The role of VOCs as indirect defences has been studied in many plant–herbivore interactions. So far, most studies have primarily been based on above-ground interactions whereas interactions between below-ground herbivores and roots have received much less attention (van Dam 2009). One reason may be the obscurity of plant–herbivore interactions in the soil. It follows from this that the feeding activities of below-ground-feeding herbivores cannot be easily observed.
Recently, it has been found that indirect defence responses involving herbivore-induced VOCs and natural enemies of root herbivores also occur below ground (van Tol et al. 2001; Neveu et al. 2002; Rasmann et al. 2005). Traditionally, techniques based on gas chromatography (GC), such as gas chromatography-mass spectrometry (GC-MS), have been used to analyse herbivore-induced VOCs. The disadvantage is that gas chromatography-based techniques have limited sensitivity. The VOCs emitted from the plant must first be accumulated on a trap for some time—usually minutes to hours—before they can be analysed. This limits the ability for dynamic profiling of herbivore-induced VOC emissions. Proton-transfer reaction mass spectrometry (PTR-MS) has emerged as a useful tool for online VOC analysis by allowing real-time detection of trace gases from various chemical groups of the order of seconds at (sub) parts per billion (ppb) levels (Lindinger et al. 1998). Briefly, the PTR-MS instrument uses proton-transfer reactions of H3O+ with trace gas compounds to ionize a neutral molecule chemically. The ionized product is then analysed by a quadrupole mass spectrometer and detected as the MH+ ion (M = molecular weight of the molecule) according to their mass-to-charge ratio (m/z). As a primary condition, the instrument detects those compounds that have a proton affinity higher than water (166.5 kcal mol−1). Unsaturated and aromatic hydrocarbons as well as most oxygenated VOCs (aldehydes, ketones, alcohols, acids, etc.)—with the exception of some light alkanes—are included in this category. The common inorganic constituents of air, oxygen, nitrogen and carbon dioxide, possess proton affinities lower than that of water and cannot be measured (Hansel et al. 1995; de Gouw et al. 2003). In comparison with conventional techniques such as GC-MS, PTR-MS has a high sensitivity down to the sub-ppbv range (parts per billion by volume, 1 : 109) and a fast response time (seconds), which allow real-time measurements without the need for sample pre-concentration. Owing to these advantages, it has become a powerful tool for the analysis of VOCs in many fields including plant research (Brilli et al. 2011; Ruuskanen et al. 2011; Danner et al. 2012), food and flavour research (Biasioli et al. 2011), environmental research (de Gouw and Warneke 2007) and breath analysis (Cristescu et al. 2011). There are a few examples where PTR-MS has been used for screening plant VOCs induced after herbivory, but these studies primarily focused on above-ground emissions (Schaub et al. 2010; Ruuskanen et al. 2011) or measured root VOCs from in vitro grown plants (Steeghs et al. 2004). Only recently has it been acknowledged that PTR-MS can also be used to detect the activities of ‘invisible’ root herbivores in vivo on the basis of root-emitted VOCs (Crespo et al. 2012; Danner et al. 2012). As such, this opens new opportunities to screen for infested plants in fundamental ecological studies as well as in applied research.
The genus Brassica (Brassicaceae) contains many economically important crops, such as cabbage, broccoli and oil seed rape (Ahuja et al. 2010). Upon herbivore attack, Brassica plants emit complex blends of VOCs, including alcohols, ketones, aldehydes, esters, terpenoids, sulfides, carboxylic acids, nitriles and isothiocyanates (ITC) (Geervliet et al. 1997). The latter two compound classes are breakdown products of glucosinolates, a class of plant-produced organic compounds that are typical secondary metabolites of Brassicaceae (Bones and Rossiter 2006). Over 120 different glucosinolate structures have been identified to date (Fahey et al. 2001). Glucosinolates have limited biological activity themselves but, upon plant damage, for example by herbivore feeding, they are hydrolysed by thioglucosidase enzymes known as myrosinases. As a result, a variety of volatile hydrolysis products, including ITC, nitriles, epithionitriles and thiocyanates, are formed (Bones and Rossiter 2006; Halkier and Gershenzon 2006). The product formed by this reaction chiefly depends on the chemical structure of the glucosinolates present in the plant, the reaction conditions (pH) and the presence or absence of additional enzymes that modify the outcome of the reaction (Wittstock and Halkier 2002; Bones and Rossiter 2006). The glucosinolate–myrosinase defence system is distributed throughout the plant but the levels vary from organ to organ. Roots, for example, have higher glucosinolate levels than shoots, and also contain a specific glucosinolate, gluconasturtiin, that is generally lacking from above-ground organs (van Dam et al. 2009). Myrosinase-containing cells have been found to be present in the roots, confirming that the ‘mustard oil bomb’ components are all available in below-ground organs as well (McCully et al. 2008; Kissen et al. 2009).
Delia radicum, the cabbage root fly, is a major pest of Brassica crops. Females lay their eggs in batches near plant stems and, after hatching, the larvae crawl down to feed on the roots until they pupate in the soil (Neveu et al. 2002). Chromatography-mass spectrometry based analyses of Brassica nigra plants showed that infestation by D. radicum larvae increased the emissions of dimethyldisulfide (DMDS) and dimethyltrisulfide (DMTS) in the plant's headspace (Ferry et al. 2007; Soler et al. 2007). Real-time analysis with PTR-MS revealed that methanethiol and dimethylsulfide (DMS), two related sulfur-containing compounds, were also induced in root fly-infested plants, in addition to a specific sulfur-containing marker compound with m/z 60 (Crespo et al. 2012; Danner et al. 2012). The m/z 60 was emitted from the roots when larvae were actively feeding, or directly after artificial damage. In B. nigra plants, methanethiol and sulfide emissions were not enhanced by artificial damage to the roots. Comparisons with pure ITC clearly linked the emission of m/z 60 to the conversion of sinigrin into allylITC, as pure phenylethylITC did result in the production of an m/z 60 signal (Crespo et al. 2012). Because of the close correlation with actively feeding larvae, it was proposed that the emission of m/z 60 may be used as a marker to discriminate between infested and uninfested roots (Crespo et al. 2012). However, there is substantial variation in root glucosinolate profiles within the genus Brassica (Bellostas et al. 2007; van Dam et al. 2009; Kabouw et al. 2010). This implies that not all Brassica species may show m/z 60 emissions in the PTR-MS when damaged artificially or by root herbivores, if allylITC formed after the conversion of sinigrin is indeed the sole source for this marker.
Using PTR-MS we analysed the emissions of sulfur-containing VOCs from damaged roots of six different Brassica species and correlated these to their root glucosinolate composition. We chose six species representing the members of the so-called Brassica U triangle (Nagahara 1935; see Table 1 for species). Based on previous experiments on the same PTR-MS, we focused on the emission of m/z 60 as a tracer for the formation of allylisothiocyanate, as well as the emission of methanethiol (m/z 49), DMS (m/z 63) and DMDS (m/z 95). We tracked the emissions dynamically for several hours after artificial damage or for several days after infestation with D. radicum larvae. By combining the natural and artificial damage-elicited VOC profiles we identified those sulfur-containing compounds that serve as markers for root damage in each plant species. The glucosinolate profiles of the roots were analysed by high-performance liquid chromatography (HPLC) in each species and compared with the PTR-MS data. Based on the results obtained by Crespo et al. (2012) we postulated that artificial damage only induces m/z 60 emissions provided sinigrin is present in the roots but that root feeding by root fly larvae also enhances methanethiol and sulfide emissions independently of the plant's glucosinolate profile.
Table 1.
Names, origin and seed sources of the Brassica species used in the experiments.
| Species name | Details | Source/referencea |
|---|---|---|
| Brassica carinata | var. 007 ‘Utopia’ | M. de Vries, Joordens Zaden, Neer, The Netherlands |
| Brassica juncea | var. Varuna | Mathur et al. (2011) |
| Brassica napus | var. Westar | Borgen et al. (2010) |
| Brassica nigra | Population Wageningen, NL | van Dam et al. (2005) |
| Brassica oleracea | population Winspit, UK | Gols et al. (2008) |
| Brassica rapa | subsp. campestris var. Ys143 | G. Bonnema, Plant Breeding, Wageningen University, Wageningen, The Netherlands |
aPublished references can be found in the reference list.
Materials and methods
Plant and insect rearing
Seeds were obtained from sources shown in Table 1. Seeds were germinated on glass beads and water in 10 × 10 cm plastic containers with a clear lid for 1 week in the greenhouse. Thereafter, selected seedlings were transferred to tall plastic 2.2 L pots (11 × 11 × 21.5 cm) filled with a peat–potting soil mixture (Type ZPV—potting soil for floriculture, Holland Potgrond, Poeldijk, The Netherlands) till ∼5 cm under the rim. The upper 5 cm was filled with fine sand to facilitate the retrieval of the root fly larvae. The pots were transferred to an insect-free greenhouse (16 h daylight, minimum T = 15 °C) supplemented with SON-T high-pressure sodium lamps (Philips, Eindhoven, The Netherlands) when photosynthetically active radiation was <150 µmol m−2 s−1.
Root fly larvae and pupae for rearing were obtained from the Laboratory of Insect Ecology, University of Rennes, France. Essentially, they were reared as described in Neveu et al. (2002) on kohlrabi, turnips or rutabaga, depending on seasonal availability.
PTR-MS
The analysis of sulfur volatile compounds emitted by the roots was performed with a custom-built PTR-MS, described in detail elsewhere (Boamfa et al. 2005). To calibrate the system, a calibration gas mixture was used consisting of acetaldehyde, acetone, isoprene, benzene, toluene, xylene and α-pinene (covering molecular weights from 32 to 136 amu), each in a concentration of 1 ppmv (parts per million volume, ±5 %) (Linde, Dieren, The Netherlands). From this calibration, calibration factors for other compounds could be calculated by considering transmission efficiency factors, collision rate constants and fragmentation ratios (de Gouw and Warneke 2007). In this way, ion intensities in normalized counts per seconds (ncps) were converted to gas mixing ratios (ppbv).
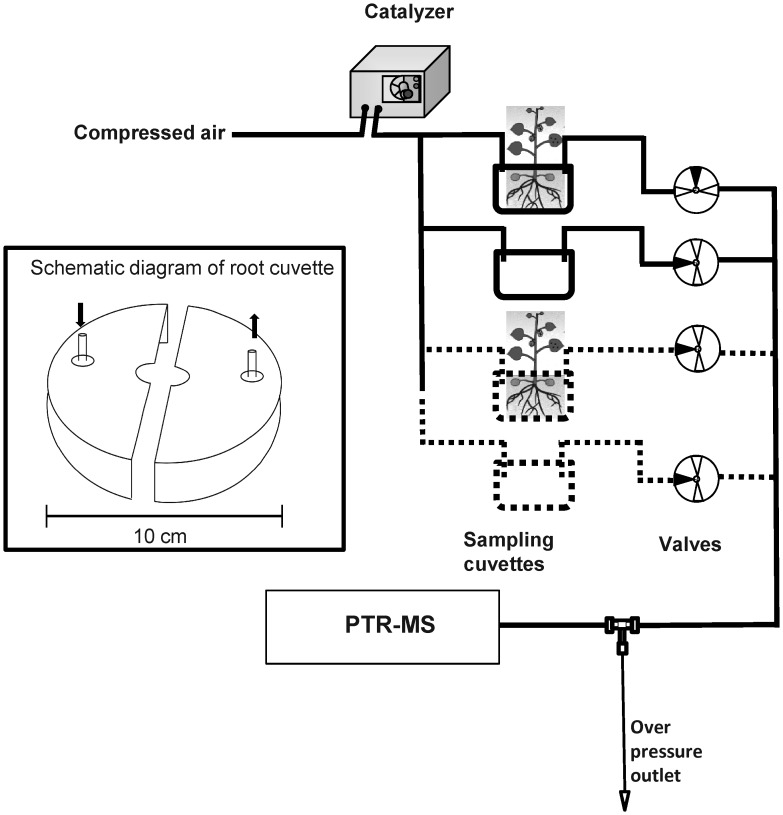
Two types of experiment were performed. In the first experiment, the sulfur VOCs emitted during root damage after D. radicum larval infestation were measured. In the second experiment, the roots of different plants of the same Brassica species were mechanically damaged with a scalpel and the sulfur-containing VOCs were monitored. To collect the emitted gasses, the sampling cuvette was placed around the stem on top of the soil. This cuvette, custom made from two halves of a 9-cm (diameter) glass Petri dish (see Fig. 1, inset), was placed around the base of the stem and sealed with a synthetic rubber-based sealant (Terostat IX). Each cuvette was fitted with one gas inlet and outlet port. A constant gas inlet flow of 2 L h−1 with hydrocarbon-free air was regulated by mass flow controllers (Brooks Instrument, Ede, The Netherlands) to flush the headspace of the roots and act as a carrier gas. The outlet of the cuvette was connected via an automated valve system to the PTR-MS instrument where VOCs were measured online alternately for 30 min for each cuvette (see Fig. 1). The sampling line from the cuvette to the PTR-MS instrument was heated to 55 °C to prevent the condensation of compounds and to minimize memory effects. In this study, four plants were used per experiment, from which two were infested with five (third instar) to 10 (second instar) D. radicum larvae and two were left undamaged (control). The D. radicum larvae were added to the plants 2–3 h before the experiment started. The VOC measurements were performed continuously over 2 days (45–50 h) at a constant temperature of 21 °C and 16 h photoperiod provided by sodium lamps (225 µmol m−2 s−1).
Fig. 1.
Schematic representation of the set-up for measuring volatiles emitting from roots of Brassica plants. A constant inlet flow of 2 L h−1 hydrocarbon-free air was used to flush the headspace of plant root cuvettes. An automated valve system was used to switch between cuvettes. The pressure in the drift tube chamber of the PTR-MS was regulated by an overpressure outlet. Inset: schematic diagram of the root cuvette, which was made from two halves of a 9-cm glass Petri dish and placed on the sand on both sides of the stem. The gap between the halves was closed with Terostat IX, a synthetic rubber-based sealant. Arrows indicate the inlet and outlet ports.
In a separate experiment, the plant roots were artificially damaged with a scalpel to compare the VOC emissions with those from the larval damage experiments. The VOC emissions were measured for 1–2 h after a single bout of artificial damage. In total, three replicates for each damage treatment (artificial or Delia infestation) were analysed. The data were converted to ppbv by using the calibration factors previously obtained. After converting the values into ppbv (= nL L−1), the compound emission rates were calculated as nL h−1 by using the inlet flow value (2 L h−1), and averaged per three replicates.
Glucosinolate analysis
For glucosinolate analyses, the upper 2 cm of the main root, where root fly larvae are typically feeding, were collected and frozen at −20 °C. The root pieces were lyophilized and ground to a fine powder using a Retsch mill (Retch GmbH & Co., Haan, Germany). For each sample, 50.0 mg of ground and dried root material were extracted and analysed on an HPLC equipped with a photodiode array. Sinigrin (sinigrin monohydrate, ACROS, NJ, USA) was used as an external standard. We used the response factors at 229 nm from Buchner (1987) and Brown et al. (2003) to calculate the concentrations of the other glucosinolates. The desulfoglucosinolate peaks were identified by comparison of retention times and UV spectra with a certified rapeseed standard (Community Bureau of Reference, Brussels, code BCR-367R) and authentic standards (progoitrin, gluconapin, glucoiberin, glucobrassicanapin, glucotropeaolin, gluconasturtiin, glucoraphanin, glucoerucin, glucobrassicin, sinalbin; Phytoplan, Heidelberg, Germany).
Results
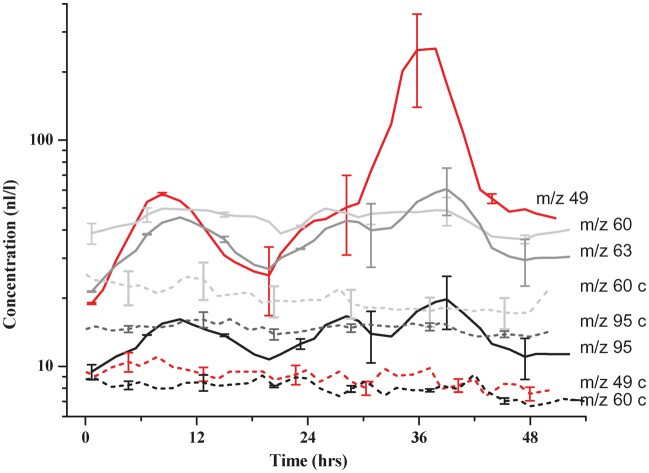
In control plants, the overall emission of the VOCs of interest (m/z 49—methanethiol, m/z 60—ITC, m/z 63—DMS, m/z 95—DMDS) remained low and constant between 5 and 30 nl L−1 (see Fig. 2 for an example of infested and control Brassica carinata plants—note the logarithmic scale). For reasons of clarity, the control values were therefore omitted from consecutive graphs.
Fig. 2.
Temporal dynamics of sulfur VOC emissions from roots of B. carinata plants after D. radicum larval infestation (solid lines) compared with non-infested plant root (dash lines, m/z marked with ‘c’). Vertical bars indicate the standard mean error (n = 3). m/z 49—methanethiol, m/z 60—ITC marker, m/z 63—DMS and m/z 95—DMDS. On the x-axis t = 0 indicates the time of experiment starts. Delia larvae were added to plants 2–3 h before the experiment starts.
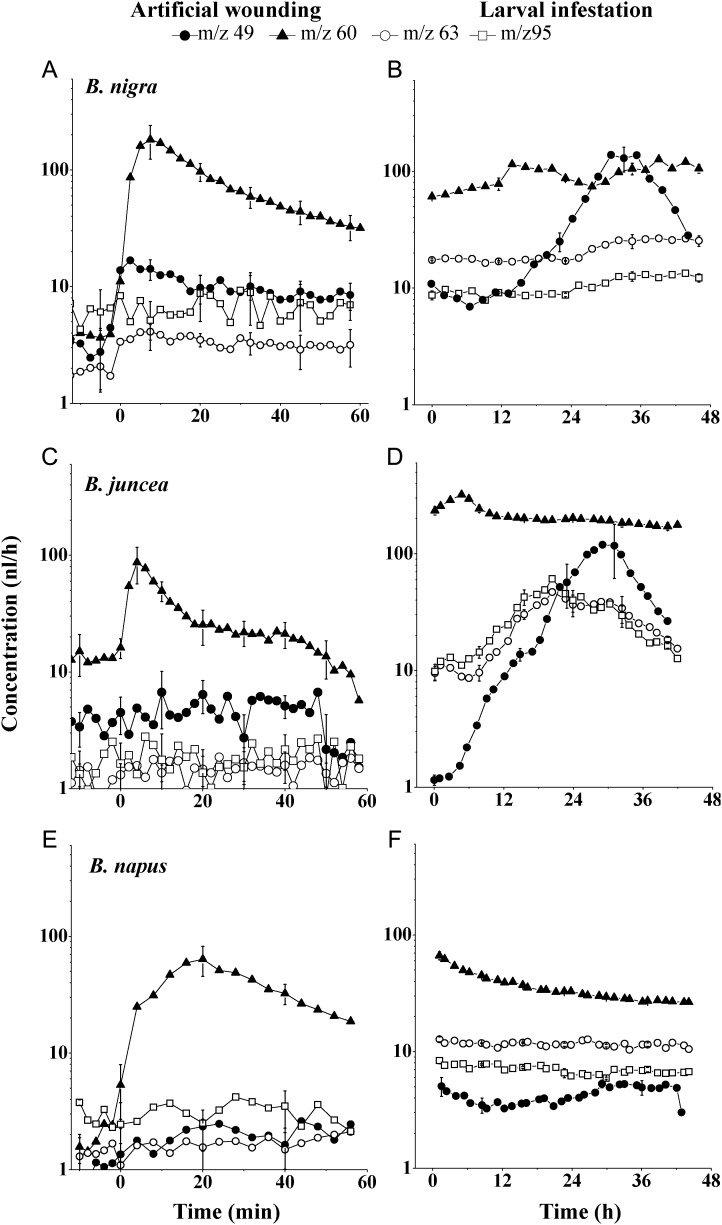
Artificial wounding with a scalpel as well as damage with D. radicum larvae resulted in two types of primary response among the six plant species. Three out of six Brassica plant species, namely B. nigra, B. juncea and B. napus, primarily emitted m/z 60 immediately after artificial root damage (Fig. 3A, C and E). With the exception of m/z 49, which showed a minor increase in B. nigra, sulfide emissions did not increase after artificial damage. The emission of m/z 60 was also increased in these three plant species when they were infested with root fly larvae (Fig. 3B, D and F). Interestingly, in B. nigra and B. juncea, methanethiol (m/z 49), DMS (m/z 63) and DMDS (m/z 95) emissions were also enhanced, 6–12 h after infestation with root fly larvae (Fig. 3B and D).
Fig. 3.
Emission of sulfur-containing volatile compounds after root damage by artificial wounding with a scalpel (left panels) or larval infestation (right panels) in B. nigra (A, B), B. juncea (C, D) and B. napus (E, F). m/z 60—ITC marker, m/z 49—methanethiol, m/z 63—DMS and m/z 95—DMDS. Vertical bars indicate the standard error of the mean (n= 3). In (A), (C) and (E), t = 0 indicates the time at which the root was damaged with a scalpel. In (B), (D) and (F), t = 0 is the starting time of PTR-MS analysis; Delia larvae were added to plants 2–3 h before analysis.
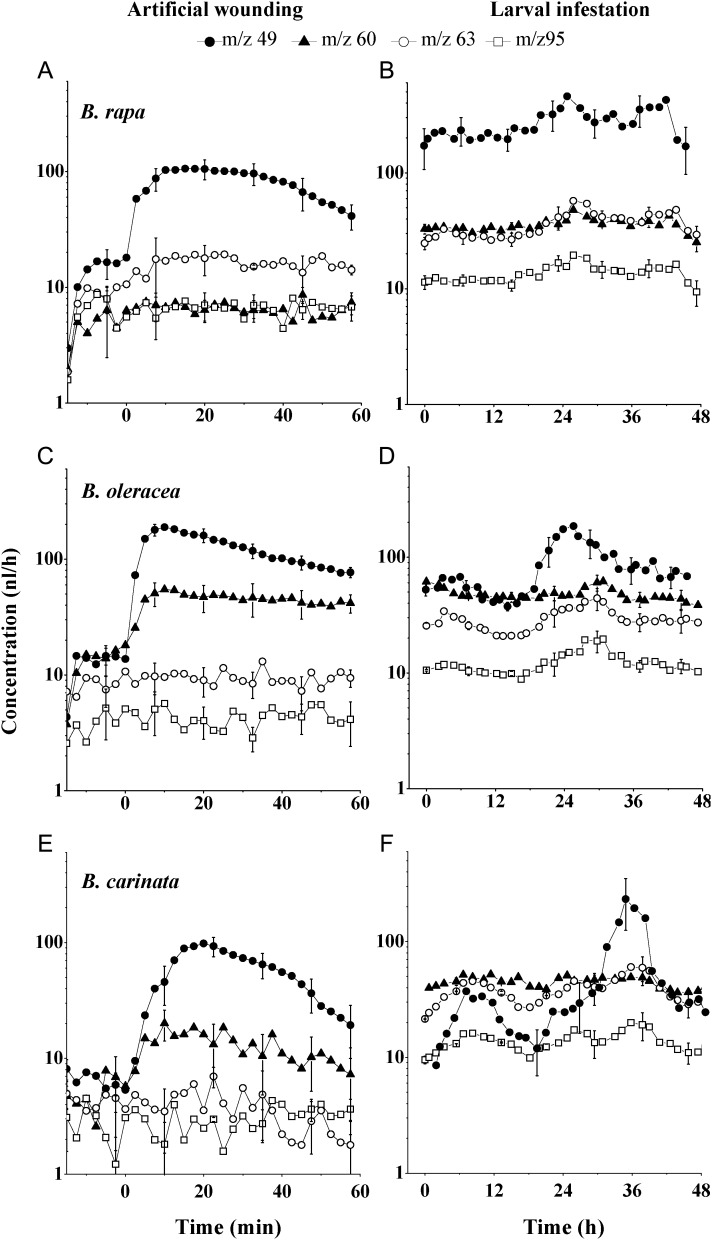
The three other species, B. rapa, B. carinata and B. oleracea, emitted methanethiol (m/z 49) as the primary compound immediately after artificial root damage (Fig. 4A, C and E). Even though methanethiol was the primary compound emitted after root damage, m/z 60 emissions were also enhanced in artificially damaged B. oleracea and B. carinata. When plants were infested with D. radicum larvae, all three species emitted methanethiol (m/z 49) more prominently than m/z 60 (Fig. 4B, D and F). In B. oleracea and B. carinata, the DMS (m/z 63) and DMDS (m/z 95) emissions were also enhanced above base levels (Figs. 2, 4D and 4F).
Fig. 4.
Emission of sulfur-containing volatile compounds after root damage by artificial wounding with a scalpel (left panels) or larval infestation (right panels) in B. rapa (A, B), B. oleracea (C, D) and B. carinata (E,F). m/z 60—ITC marker, m/z 49—methanethiol, m/z 63—DMS and m/z 95—DMDS. Vertical bars indicate the standard error of the mean (n= 3). In (A), (C) and (E), t = 0 indicates the time at which the root was damaged with a scalpel. In (B), (D) and (F), t = 0 is the starting time of PTR-MS analysis; Delia larvae were added to plants 2–3 h before analysis.
Root glucosinolate profiles
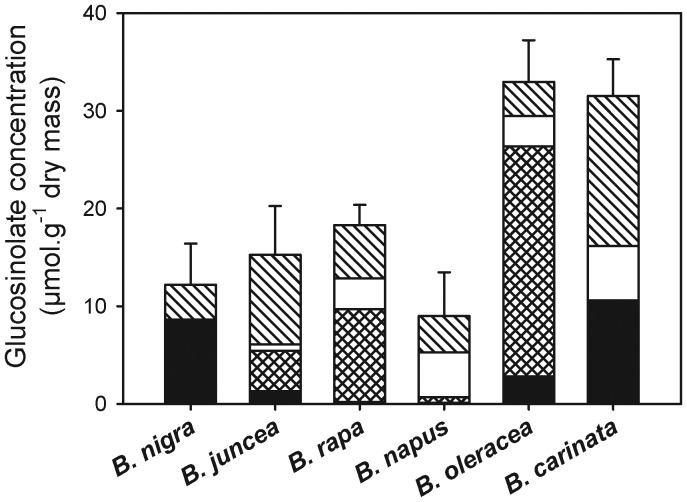
Overall, glucosinolate profiles and concentrations in the roots were similar to those reported for mature roots of B. nigra, B. juncea, B. rapa and B. napus (Bellostas et al. 2007). Among the six species of Brassica under study, B. nigra, B. juncea, B. oleracea and B. carinata showed the presence of sinigrin in their roots, whereas B. napus and B. rapa did not contain sinigrin (Fig. 5). On the other hand, gluconasturtiin was present in all six species. Substantial levels of aliphatic glucosinolates other than sinigrin, as well as indole glucosinolates, were found in all species except B. nigra, which almost exclusively contained sinigrin and gluconasturtiin. The root glucosinolate profiles of these six Brassica species were compared with VOC emissions as measured in the PTR-MS to test the hypothesis that the conversion of sinigrin is required for the emission of m/z 60. Apparently, this was not the case, as B. napus roots, which lack sinigrin, clearly emitted m/z 60 after artificial damage. On the other hand, the emissions of m/z 60 in B. carinata were relatively low despite the fact that these roots had the highest sinigrin levels of all species.
Fig. 5.
Glucosinolate concentrations and profiles in roots of six different Brassica species. Error bars indicate standard error of the mean of the total glucosinolate concentration (n = 5–7 per species). Black bars: sinigrin (allyl glucosinolate); crossed bars: aliphatic glucosinolates other than sinigrin; white bars: indole glucosinolates; striped bars: 2-phenylethylglucosinolate.
Discussion
This study shows that root damage to Brassica plants, either by root-feeding insects or by artificial damage using a scalpel, resulted in the emission of various sulfur-containing VOCs. Two types of primary response were observed; three species mainly emitted m/z 60, whereas the other three primarily emitted methanethiol (m/z 49) after larval infestation and artificial damage. Overall, the primary compound that was emitted by each species was consistent for artificial and natural root damage. The type of sulfur-containing compounds that were emitted after damage thus depends mainly on plant species and not on the type of damage that is inflicted.
Our results also challenge the hypothesis that the presence of sinigrin is the main factor for m/z 60 emissions; plants lacking sinigrin in their roots also showed enhanced emissions of m/z 60 after damage. Previously, it was established experimentally that the emission of m/z 60 as an immediate response after artificial damage to B. nigra was related to the glucosinolate–myrosinase system that is constitutively present in Brassica plants (Halkier and Gershenzon 2006; Crespo et al. 2012). Isotope-ratio correlations and analysis of pure compounds suggested that m/z 60 is a sulfur-containing fragment of a glucosinolate breakdown product (Crespo et al. 2012). Most notably, allylITC, the product formed after the reaction of sinigrin with myrosinase, was found to yield m/z 60. 2-PhenylethylITC, the product of the reaction of myrosinase with gluconasturtiin—the other main root compound in B. nigra—did not result in m/z 60. However, our current study shows that there may be other sources for the m/z 60 signal than sinigrin alone. Based on the literature, there appear to be several possibilities. First, Crespo et al. (2012) showed that pure thiocyanic acid (HCNS, Mw 59) also gives a m/z 60 signal in the PTR-MS. Thiocyanic acid may therefore arise after the reaction of indole glucosinolates with myrosinase. The ITC formed from indole glucosinolates are unstable in the presence of water and rapidly form carbinols under the release of thiocyanate ions (CNS−) (Attieh et al. 2000; Bones and Rossiter 2006). Thiocyanate ions may easily take up an H+, thus forming thiocyanic acid. It was found by Crespo et al. (2012) that pure ethylITC gives a signal at m/z60 on the PTR-MS. Even though ethylglucosinolate, which would be the direct precursor for ethylITC production, was not detected in the roots of the species analysed in this experiment, it shows that ITC emerging from aliphatic glucosinolates other than sinigrin may also give rise to m/z 60. Indeed, most species lacking sinigrin possessed other aliphatic glucosinolates that may be additional sources of m/z 60 (Fig. 5). This hypothesis, however, now needs testing by analysing the various ITC that may arise from the reactions of these glucosinolates with myrosinase in the PTR-MS.
Other than the production of ITC and related products from glucosinolates, the emission of methanethiol and sulfides is not exclusively linked to members of the Brassicaceae. Volatile sulfides may be produced by a wide range of plant species and other organisms, including algae, bacteria and fungi (Kai et al. 2010). The above-ground parts of broccoli and other cultivated cabbages have been found to contain several enzymes, mainly transferases and lyases that convert the amino acids methionine, cystine or cysteine into methanethiol and sulfides (Chin and Lindsay 1994; Derbali and Makhlouf 1998). When the activity of these enzymes was chemically inhibited, the emission of sulfur-containing VOCs was reduced by 95 %, showing the involvement of these plant enzymes in the production of these volatiles (Derbali and Makhlouf 1998).
However, in Brassica species there is also a more specific plant-based source of sulfides that is related to the formation of glucosinolate conversion products. High levels of thiol methyltransferase (TMT) activities have been found in shoots and roots of various Brassica species (Saini et al. 1995; Attieh et al. 2002). This enzyme is thought to have a function in detoxifying the phytotoxic sulfur-containing (by)products of glucosinolate conversion, such as cyanides and HS− ions (Attieh et al. 2000). Thiol methyltransferase methylates ITC and related sulfur-containing reaction products from glucosinolate conversions, thereby producing methylsulfides (Attieh et al. 2000). Active interactions with herbivores or micro-organisms is not required as sulfides are also produced after artificial damage when Brassica species are ploughed into the soil for biofumigation purposes (Wang et al. 2009) and under sterile conditions (Derbali and Makhlouf 1998).
Attieh and colleagues (1995) found a range of medium to very high TMT activities in shoots of B. napus (100–250 nmol day−1 g−1 fresh mass), B. juncea (250–500), B. rapa (500–1000) and B. oleracea (>2000). Interestingly, the latter two species, which had the highest TMT activities in their leaves, were also found primarily to emit methanethiol after artificial and natural damage to their roots. Brassica juncea, which was classified primarily as an m/z 60 emitter under artificial damage, also emits substantial levels of m/z 49 when infested with root fly larvae, athough at later time points than the m/z 60 emission. This suggests that the level of TMT activity may play an important role in the production of sulfides versus ITC and co-determines which sulfur compound is primarily formed after artificial and natural root damage. Unfortunately, B. nigra and B. carinata were not analysed for their TMT activity by Attieh et al. (1995). Based on their emission patterns, it can be hypothesized that B. carinata should have TMT activity levels close to those of B. oleracea, whereas B. nigra TMT activities would be close to those of B. juncea. Further analyses of TMT activities in these species, especially in the roots, should be performed to confirm this hypothesis.
In D. radicum-infested plants, there is another potential source contributing to the formation of sulfides and methanethiol. It was found that the midgut of D. radicum larvae contains a wide range of (symbiotic) bacteria which are essential to digest the tough root tissue that the larvae feed on (Lukwinski et al. 2006). The bacterial gut community included several Serratia species, some of which emit DMDS, DMTS and methanethiol when grown on artificial medium (Lukwinski et al. 2006; Kai et al. 2010). The slower evolving emissions of methanethiol in D. radicum-infested B. nigra and B. juncea—which did not occur in artificially damaged plants of these species—may therefore come from the growing population of gut bacteria in the digested root materials. However, the immediate emission of methanethiol after artificial damage in B. rapa, B. oleracea and B. carinata suggests that exposure to gut bacteria is not an absolute requisite for the production of these volatiles. Further research is needed to determine the relative roles of plant enzymes such as TMT and bacterial gut communities in the formation of these volatile compounds.
Conclusions and forward look
Here we show that real-time PTR-MS analysis is a powerful tool for the analysis of sulfur-containing VOCs emitted from plants infested with cryptically feeding root herbivores. In all cases, undamaged plants could be clearly distinguished from damaged plants on the basis of one or two marker VOCs. The type of sulfur-containing VOC emitted is species specific and independent of sinigrin being present in the roots. Before PTR-MS can be implemented for detecting root fly-infested Brassica plants or vegetables, further tests are required to investigate which compound can serve as a marker for each species or variety. Artificial damage experiments can be used to identify the most prominent marker for each species, though it must be considered that, at later time points, additional VOCs may be produced under larval infestation.
Our results show that within the genus Brassica, the combination of various enzymes and compounds is a versatile platform for the launch of defence operations such as the generation of toxic and noxious sulfur-containing VOCs (Rausch and Wachter 2005). To understand the mechanisms leading to the formation of the various sulfur-containing VOCs in different species better, the role of enzymes involved in the catabolism of glucosinolate breakdown products will need to be studied in more detail. The outcome will improve our understanding of the formation of off-flavours in brassicaceous vegetables. To better understand the role of sulfur-containing VOCs in attracting natural enemies and predators, the relative roles of sulfides versus ITC must be addressed. Both sulfides and ITC can be perceived by parasitoids and predators, and are used by these biocontrol agents to locate the most suitable herbivorous hosts (Reddy et al. 2002; Ferry et al. 2007; Soler et al. 2007). Interestingly, our results also show that VOC emission patterns of ITC and sulfides are very dynamic in time and differ considerably between plant species. It will be vital to assess how and when natural enemies deal with this variation in time to find their preferred hosts in different host plant species.
Sources of funding
This work was supported by base funding (N.M.v.D.) by the Institute of Water and Wetland Research (IWWR) and the Institute of Molecules and Materials (D.S., F.H. and S.C.) at Radboud University Nijmegen, The Netherlands.
Contributions by the authors
N.M.v.D.: conceptual design of the experiments, HPLC and statistical analyses of glucosinolates, data interpretation and lead author; D.S.: PTR-MS analyses and data processing, glucosinolate extraction, design Fig. 1, co-author; F.J.M.H.: supervision of PTR-MS analyses, co-author; and S.M.C.: direct supervision of PTR-MS analyses, graphic representation of PTR-MS data, corresponding author.
Conflict of interest statement
None declared.
Acknowledgements
We thank Cor Sikkens and Peter Claus for technical assistance on the PTR-MS and Holger Danner for the schematic drawing of the root cuvette. Thom van Duinhoven is thanked for helping rearing plants and larvae. Rieta Gols (Entomology, Wageningen University, The Netherlands), Guusje Bonnema (Plant Breeding, Wageningen University, The Netherlands) and Michiel de Vries (Joordens Seeds, The Netherlands) generously provided seeds.
References
- Ahuja I, Rohloff J, Bones AM. Defence mechanisms of Brassicaceae: implications for plant-insect interactions and potential for integrated pest management. A review. Agronomy for Sustainable Development. 2010;30:311–348. [Google Scholar]
- Attieh JM, Hanson AD, Saini HS. Purification and characterization of a novel methyltransferase responsible for biosynthesis of halomethanes and methanethiol in Brassica oleracea. Journal of Biological Chemistry. 1995;270:9250–9257. doi: 10.1074/jbc.270.16.9250. [DOI] [PubMed] [Google Scholar]
- Attieh J, Kleppinger-Sparace KF, Nunes C, Sparace SA, Saini HS. Evidence implicating a novel thiol methyltransferase in the detoxification of glucosinolate hydrolysis products in Brassica oleracea L. Plant, Cell and Environment. 2000;23:165–174. [Google Scholar]
- Attieh J, Djiana R, Koonjul P, Etienne C, Sparace SA, Saini HS. Cloning and functional expression of two plant thiol methyltransferases: a new class of enzymes involved in the biosynthesis of sulfur volatiles. Plant Molecular Biology. 2002;50:511–521. doi: 10.1023/a:1019865829534. [DOI] [PubMed] [Google Scholar]
- Bellostas N, Sorensen JC, Sorensen H. Profiling glucosinolates in vegetative and reproductive tissues of four Brassica species of the U-triangle for their biofumigation potential. Journal of the Science of Food and Agriculture. 2007;87:1586–1594. [Google Scholar]
- Biasioli F, Yeretzian C, Gasperi F, Mark TD. PTR-MS monitoring of VOCs and BVOCs in food science and technology. Trac-Trends in Analytical Chemistry. 2011;30:968–977. [Google Scholar]
- Boamfa EI, Steeghs MML, Cristescu SM, Harren FJM. Trace gas detection from fermentation process in apples; an intercomparision study between proton transfer reaction mass spectrometry and laser photoacoustics. International Journal of Mass Spectrometry. 2005;239:193–201. [Google Scholar]
- Bones AM, Rossiter JT. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry. 2006;67:1053–1067. doi: 10.1016/j.phytochem.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Borgen BH, Thangstad OP, Ahuja I, Rossiter JT, Bones AM. Removing the mustard oil bomb from seeds: transgenic ablation of myrosin cells in oilseed rape (Brassica napus) produces MINELESS seeds. Journal of Experimental Botany. 2010;61:1683–1697. doi: 10.1093/jxb/erq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Muller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction ‘time-of-flight’ mass spectrometry (PTR-TOF) Plos One. 2011;6:e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry. 2003;62:471–481. doi: 10.1016/s0031-9422(02)00549-6. [DOI] [PubMed] [Google Scholar]
- Buchner R. Approach to determination of HPLC response factors for glucosinolates. In: Wathelet JP, editor. Glucosinolates in rapeseed. Dordrecht, The Netherlands: Martinus Nijhoff; 1987. [Google Scholar]
- Chin HW, Lindsay RC. Mechanisms of formation of volatile sulfur-compounds following the action of cysteine sulfoxide lyases. Journal of Agricultural and Food Chemistry. 1994;42:1529–1536. [Google Scholar]
- Crespo E, Hordijk CA, De Graaf R, Cristescu SM, Harren FJM, van Dam NM. Phytochemistry. 2012. On-line detection of root-induced volatiles in Brassica nigra plants infested by Delia radicum L. root fly larvae. in press. [DOI] [PubMed] [Google Scholar]
- Cristescu SM, Gietema HA, Blanchet L, Kruitwagen CLJJ, Munnik P, van Klaveren RJ, Lammers JWJ, Buydens L, Harren FJM, Zanen P. Screening for emphysema via exhaled volatile organic compounds. Journal of Breath Research. 2011;5:046009. doi: 10.1088/1752-7155/5/4/046009. [DOI] [PubMed] [Google Scholar]
- Danner H, Samudrala D, Cristescu SM, van Dam NM. Tracing hidden herbivores: time resolved, non invasive analyses of belowground volatiles by proton transfer reaction mass spectrometry (PTR-MS) Journal of Chemical Ecology. 2012;38:785–794. doi: 10.1007/s10886-012-0129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gouw J, Warneke C. Measurements of volatile organic compounds in the earth's atmosphere using proton transfer reaction mass spectrometry. Mass Spectrometry. 2007;26:223–257. doi: 10.1002/mas.20119. [DOI] [PubMed] [Google Scholar]
- de Gouw J, Warneke C, Karl T, Eerdekens G, van der Veen C, Fall R. Sensitivity and specificity of atmospheric trace gas detection by proton transfer reaction mass spectrometry. International Journal of Mass Spectrometry. 2003;223:193–201. [Google Scholar]
- Derbali E, Makhlouf J. Biosynthesis of sulfur volatile compounds in broccoli seedlings stored under anaerobic conditions. Postharvest Biology and Technology. 1998;13:191–204. [Google Scholar]
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help. Trends in Plant Science. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Ferry A, Dugravot S, Delattre T, Christides JP, Auger J, Bagneres AG, Poinsot D, Cortesero AM. Identification of a widespread monomolecular odor differentially attractive to several Delia radicum ground-dwelling predators in the field. Journal of Chemical Ecology. 2007;33:2064–2077. doi: 10.1007/s10886-007-9373-3. [DOI] [PubMed] [Google Scholar]
- Geervliet JBF, Posthumus MA, Vet LEM, Dicke M. Comparative analysis of headspace volatiles from different caterpillar-infested or uninfested food plants of Pieris species. Journal of Chemical Ecology. 1997;23:2935–2954. [Google Scholar]
- Gols R, Wagenaar R, Bukovinszky T, van Dam NM, Dicke M, Bullock JM, Harvey JA. Genetic variation in defense chemistry in wild cabbages affects herbivores and their endoparasitoids. Ecology. 2008;89:1616–1626. doi: 10.1890/07-0873.1. [DOI] [PubMed] [Google Scholar]
- Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annual Review of Plant Biology. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- Hansel A, Jordan A, Holzinger R, Prazeller P, Vogel W, Lindinger W. Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. International Journal of Mass Spectrometry and Ion Processes. 1995;149/150:609–619. [Google Scholar]
- Holopainen JK. Multiple functions of inducible plant volatiles. Trends in Plant Science. 2004;9:529–533. doi: 10.1016/j.tplants.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kabouw P, van der Putten WH, van Dam NM, Biere A. Effects of intraspecific variation in white cabbage (Brassica oleracea var. capitata) on soil organisms. Plant and Soil. 2010;336:509–518. [Google Scholar]
- Kai M, Crespo E, Cristescu SM, Harren FJM, Francke W, Piechulla B. Serratia odorifera: analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Applied Microbiology and Biotechnology. 2010;88:965–976. doi: 10.1007/s00253-010-2810-1. [DOI] [PubMed] [Google Scholar]
- Kissen R, Rossiter JT, Bones AM. The ‘mustard oil bomb’: not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochemistry Reviews. 2009;8:69–86. [Google Scholar]
- Lindinger W, Hansel A, Jordan A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. International Journal of Mass Spectrometry and Ion Processes. 1998;173:191–241. [Google Scholar]
- Lukwinski AT, Hill JE, Khachatourians GG, Hemmingsen SM, Hegedus DD. Biochemical and taxonomic characterization of bacteria associated with the crucifer root maggot (Delia radicum) Canadian Journal of Microbiology. 2006;52:197–208. doi: 10.1139/w05-123. [DOI] [PubMed] [Google Scholar]
- Mathur V, Ganta S, Raaijmakers CE, Reddy AS, Vet LEM, van Dam NM. Temporal dynamics of herbivore-induced responses in Brassica juncea and their effect on generalist and specialist herbivores. Entomologia Experimentalis et Applicata. 2011;139:215–225. [Google Scholar]
- McCully ME, Miller C, Sprague SJ, Huang CX, Kirkegaard JA. Distribution of glucosinolates and sulfur-rich cells in roots of field-grown canola (Brassica napus) New Phytologist. 2008;180:193–205. doi: 10.1111/j.1469-8137.2008.02520.x. [DOI] [PubMed] [Google Scholar]
- Neveu N, Grandgirard J, Nenon JP, Cortesero AM. Systemic release of herbivore-induced plant volatiles by turnips infested by concealed root-feeding larvae Delia radicum L. Journal of Chemical Ecology. 2002;28:1717–1732. doi: 10.1023/a:1020500915728. [DOI] [PubMed] [Google Scholar]
- Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434:732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- Rausch T, Wachter A. Sulfur metabolism: a versatile platform for launching defence operations. Trends in Plant Science. 2005;10:503–509. doi: 10.1016/j.tplants.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Reddy GVP, Holopainen JK, Guerrero A. Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. Journal of Chemical Ecology. 2002;28:131–143. doi: 10.1023/a:1013519003944. [DOI] [PubMed] [Google Scholar]
- Ruuskanen TM, Müller M, Schnitzhofer R, Karl T, Graus M, Bamberger I, Hörtnagl L, Brilli F, Wohlfahrt G, Hansel A. Eddy covariance VOC emission and deposition fluxes above grassland using PTR-TOF. Atmospheric Chemistry and Physics. 2011;11:611–625. doi: 10.5194/acp-11-611-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini HS, Attieh JM, Hanson AD. Biosynthesis of halomethanes and methanethiol by higher-plants via a novel methyltransferase reaction. Plant, Cell and Environment. 1995;18:1027–1033. [Google Scholar]
- Schaub A, Blande JD, Graus M, Oksanen E, Holopainen JK, Hansel A. Real-time monitoring of herbivore induced volatile emissions in the field. Physiologia Plantarum. 2010;138:123–133. doi: 10.1111/j.1399-3054.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- Soler R, Harvey JA, Kamp AFD, Vet LEM, Van der Putten WH, Van Dam NM, Stuefer JF, Gols R, Hordijk CA, Bezemer TM. Root herbivores influence the behaviour of an aboveground parasitoid through changes in plant-volatile signals. Oikos. 2007;116:367–376. [Google Scholar]
- Steeghs M, Bais HP, de Gouw J, Goldan P, Kuster W, Northway M, Fall R, Vivanco JM. Proton-transfer-reaction mass spectrometry as a new tool for real time analysis of root-secreted volatile organic compounds in arabidopsis. Plant Physiology. 2004;135:47–58. doi: 10.1104/pp.104.038703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara U. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Japanese Journal of Botany. 1935;7:389–452. [Google Scholar]
- van Dam NM. Belowground herbivory and plant defenses. Annual Review of Ecology, Evolution, and Systematics. 2009;40:373–391. [Google Scholar]
- van Dam NM, Raaijmakers CE, Van der Putten WH. Root herbivory reduces growth and survival of the shoot feeding specialist Pieris rapae on Brassica nigra. Entomologia Experimentalis et Applicata. 2005;115:161–170. [Google Scholar]
- van Dam NM, Tytgat TOG, Kirkegaard JA. Root and shoot glucosinolates: a comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochemistry Reviews. 2009;8:171–186. [Google Scholar]
- van Tol RWHM, van der Sommen ATC, Boff MIC, van Bezooijen J, Sabelis MW, Smits PH. Plants protect their roots by alerting the enemies of grubs. Ecology Letters. 2001;4:292–294. [Google Scholar]
- Wang D, Rosen C, Kinkel L, Cao A, Tharayil N, Gerik J. Production of methyl sulfide and dimethyl disulfide from soil-incorporated plant materials and implications for controlling soilborne pathogens. Plant and Soil. 2009;324:185–197. [Google Scholar]
- Wittstock U, Halkier BA. Glucosinolate research in the Arabidopsis era. Trends in Plant Science. 2002;7:263–270. doi: 10.1016/s1360-1385(02)02273-2. [DOI] [PubMed] [Google Scholar]