Abstract
Objective:
Cervical cancer is the third most common malignancy in women worldwide. Accurate staging of the disease is crucial in planning the optimal treatment strategy. The aim of this study was to evaluate the role of magnetic resonance imaging (MRI) in the assessment of extension and staging of cervical malignancy in correlation with histopathologic examination.
Materials and Methods:
Thirty females with untreated pathologically proven uterine cervical carcinoma were included in this prospective study. The patients were 40 - 65 years of age and their average age was 45 years. All patients were subjected to routine clinical staging workup and underwent MRI for preoperative staging. Preoperative MRI findings were reviewed and compared with the final pathological staging that is the Gold Standard of reference.
Results:
Histopathologic examination established that of the 30 tumors, 22 (73.3%) were squamous cell carcinoma. According to the International Federation of Gynecology and Obstetrics (FIGO) staging criteria, 2/30 patients (6.6%) were stage IB, 12/30 (40.3%) were IIA, 8/30 were IIB (26.6%), and 8/30 (26.6%) were IVA. MRI had a sensitivity of 100% and specificity 85.7% in the detection of parametrial infiltration, and a sensitivity of 100% and specificity of 90% in the detection of vaginal infiltration. It was sensitive (100%) and specific (100%) in detecting tumor extension to the stroma, urinary bladder, and rectum. Pathological examination demonstrated stage IB cervical carcinoma in 2/30 patients (6.6%), stage IIA disease in 10/30 patients (33.3%), stage IIB in 6/30 patients (20%), and stage IV disease in 8/30 patients (26.6%). MRI features demonstrated stage IB in 2/30 patients (6.6%), stage IIA disease in 12/30 patients (40%), stage IIB in 8/30 patients (26.6%), and stage IV disease in 8/30 patients (26.6%). MRI staging of cervical carcinoma was in concordance with histopathologic staging in stages IB and IVA and over-staging in IIA and IIB stages.
Conclusion:
MRI is an optimal non-invasive modality for preoperative staging of uterine cervical malignancy, and crucial in subsequent more accurate treatment planning.
Keywords: Cervical cancer, histopathology, MRI, tumor staging
INTRODUCTION

Carcinoma of the cervix is a major cause of death, especially in Third World countries, where Pap smear screening is often not routinely performed. Important prognostic factors include volume and histological grade of tumor. Accurate staging of the disease is crucial in planning the optimal treatment strategy.[1,2]
The International Federation of Gynecology and Obstetrics (FIGO) recommends a clinical staging system for cervical carcinoma, that includes inspection, palpation (if needed under anesthesia), colposcopy, hysteroscopy, endocervical curettage, cystoscopy, proctoscopy, intravenous urography, and radiographic evaluation of lungs and skeleton. However, there are significant inaccuracies in the FIGO staging system with a 24-39% error rate in gynecologic examinations and it is dependent on the experience of the examining physician.[2–4]
Magnetic resonance imaging (MRI) is widely accepted in the preoperative assessment of patients with cervical carcinoma to optimize the therapeutic strategy. It is optimal for evaluation of important prognostic factors such as lesion volume and metastatic lymph node involvement. MRI obviates the use of invasive procedures such as cystoscopy and proctoscopy. It is an important tool in staging of cervical cancer to distinguish early disease (stage IIA) from advanced disease (stage IIB or greater). MRI has been gaining increasing use for pretreatment staging of uterine cervical carcinoma; however, it is not yet accepted as a Gold Standard.[4–7]
Our aim was to evaluate the role of MRI in assessing extension and staging of uterine cervical malignancy in correlation with histopathologic examination.
MATERIALS AND METHODS
Patients
This study was approved by the local ethical committee of our institution. During the period between February 2009 and August 2010, 30 consecutive females with primary untreated pathologically proven uterine cervical carcinoma were included in this prospective study. Histopathologic diagnosis of the disease was established by means of pretreatment colposcopic biopsy. The patients were 40-65 years of age with the average age being 45 years. All patients were subjected to routine clinical staging workup including physical examination, bimanual pelvic examination, chest radiography, pelvic transvaginal sonography, transabdominal sonography, cystoscopy, excretory urography, and sigmoidoscopy. All patients underwent MRI for preoperative staging. Three patients had to be excluded (one patient was not treated in our institute, one patient had contraindication for MRI, one patient underwent surgery 30 days after MRI examination).
MRI technique
MRI was performed with 1T Closed MR Imager (Gyroscan, Intera, Philips, Holland) using a phased-array coil. Fasting for a minimum of 6 h before the examination was routinely recommended to reduce intestinal motion.
The following pulse sequences and scan plans were obtained: Axial T2W FSE MRI from the renal hilum to the symphysis pubis or beyond [TR range/effective TE range, 3500/90–110; echotrain length, 13–15; slice thickness, 5–7 mm; gap, 1–2 mm; field of view, 24–38 cm; excitations (NSA), 3; and matrix, 304 × 512]; sagittal T2W fast FSE MRI [TR range/effective TE range, 3500/90–110; echotrain length, 8; slice thickness, 4–6 mm; gap, 1–2 mm; field of view, 24–38 cm; excitations (NSA), 3; and matrix, 304 × 512] from one femoral head to the other; coronal T2W FSE MRI [TR range/effective TE range, 3500/90–110; echotrain length, 8; slice thickness, 4-6 mm; gap, 1-2 mm; field of view, 24-38 cm; excitations (NSA), 3; and matrix, 304 × 512] of the pelvis from the aortic bifurcation to the symphysis; and axial T1W spin echo MRI [TR range/TE range, 400–640/10–14; slice thickness, 5–8 mm; gap, 1–2 mm; field of view, 24–38 cm; excitations (NSA), 1–2; matrix, 256 × 256; and respiratory compensation] from the renal hilum to the symphysis pubis or beyond to cover larger masses. Anterior saturation bands were routinely placed over the anterior abdominal wall to reduce artifacts due to respiratory motion. Intravenous Gadolinium (IV Gad) was given to all patients (OMNISCAN Gd-DTPA: Gadolinium-di-ethylene-triamine-penta-acetic acid), in a dose of about 1 ml/5 kg with a maximum dose of about 10 ml and a minimum dose of 3 ml.
Image analysis
MR images were evaluated in one session by three radiologists who were unaware of the pathological results and clinical details. Decisions on the findings were reached by a consensus. MR images were reviewed for the following characteristics of the cervical tumor: morphological characteristics, including origin, greatest diameters, margin (smooth or irregular), tumor shape (round, ovoid, or lobular), signal intensity, growth pattern, and internal appearance (homogeneous or heterogeneous); the presence or absence of tumor invasion into the parametrium, vagina, and adjacent organs as well as the presence or absence of lymphadenopathy. Tumor origin from endocervical canal or from the cervical stroma was noted. Cervical carcinoma appeared isointense to cervical stroma on T1-weighted image (T1WI) and had a high signal intensity mass surrounded by low signal intensity cervical stroma on T2-weighted image (T2WI). T2WI was the most useful sequence in tumor depictions; if cervical cancer was confined to the stroma, there was a low signal intensity stromal ring surrounded by a high signal intensity tumor. In cases of full thickness stromal invasion, the low signal intensity stroma was completely replaced by high signal intensity tumor. Vaginal invasion was detected when the normal low signal intensity of the vaginal wall was replaced by the high signal intensity tumor. Parametrial invasion was detected when there was disruption of the stromal ring with nodular or irregular tumor signal intensity extending to the parametrium. A smooth tumor–parametrial interface excluded parametrial invasion. Bladder and rectal invasions were diagnosed when there was disruption of their normal hypointense walls on T2WI, with or without a mass protruding into the lumen. Pelvic side wall invasion was determined to be present if the tumor high signal intensity extended to the internal obturator, piriform, or levator ani muscles, with or without hydroureter. Lymph nodes greater than 1 cm were considered pathological and the presence of intranodal necrosis was considered a confirmatory finding.
Histopathology assessment
Sixteen patients underwent hysterectomy and lymphadenectomy (modified radical hysterectomy was performed in 2/16 patients and radical hysterectomy in 14/16 patients). All of the remaining 14 patients underwent colposcopic vaginal biopsy and laparoscopic staging with pelvic lymph node sampling. Six patients underwent cystoscopic biopsy and two patients underwent colonoscopic biopsy. All specimens obtained were analyzed by a single pathologist without reference to the MR images.
Statistical analysis
Preoperative MRI findings were reviewed and compared with the final pathological diagnosis as the standard of reference. All data entry and analyses were done with IBM compatible computer using software SPSS for Windows, version 13. Graphics were done using Excel. McNemar's test was used for statistical comparison of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
RESULTS
This study included 30 female patients with primary untreated pathologically proven uterine cervical carcinoma (age range: 40–65 years, mean age = 45 years). According to the FIGO staging criteria, 2/30 patients were Stage IB (6.6%), 12/30 (40%) were IIA, 8/30 (26.6%) were IIB, and 8/30 patients (26.6%) were IVA. Histopathologic diagnosis was squamous cell carcinoma in 22/30 patients (73.3%), adenocarinoma in 6/30 (20%), and anaplastic carcinoma in 2/30 (6.6%). Twenty-six patients (86.6%) had presented with irregular uterine bleeding. Data are summarized in Table 1.
Table 1.
Patients main symptoms, pathological type and clinical FIGO staging
MRI features of the 30 examined uterine cervical tumor confirmed 4/30 (13.3%) were endocervical, 26 (86.6%) were exophytic, 28 (93.3%) were located in combined anterior and posterior cervical walls, 2/30 (6.6%) were located in posterior wall, while no tumor could be detected in anterior wall. MRI was able to show cervical tumor extension into intrauterine or extrauterine location. Intrauterine extension was detected in 2/30 patients (6.6%), and extrauterine in 28/30 (93.3%) [upper two-thirds of the vagina in 12/30 patients (40%), parametria in 8/30 (26.6%) patients], urinary bladder in 6/30 patients (20%), rectum in 2/30 patients (6.6%), and no extension to the pelvic side wall was detected. MRI detected pelvic lymph node enlargement in 10/30 patients (33.3%); they proved pathologically to be metastatic in six patients and reactive hyperplasia in four patients. MRI features are listed in Table 2 [Figures 1–3].
Table 2.
Growth pattern, location, extension of cervical carcinoma, and location of enlarged lymph nodes as detected by MRI
Figure 1.
Stage IB cervical carcinoma in a 42-year-old woman. (a) Axial, (b) sagittal T2-weighted images show a well-defined hyperintense mass in the uterine cervix (short arrows). The lesion is located almost within the cervical canal. The tumor is completely surrounded by hypointense cervical stroma (long arrows). This proved to be moderately differentiated squamous cell carcinoma.
Figure 3.
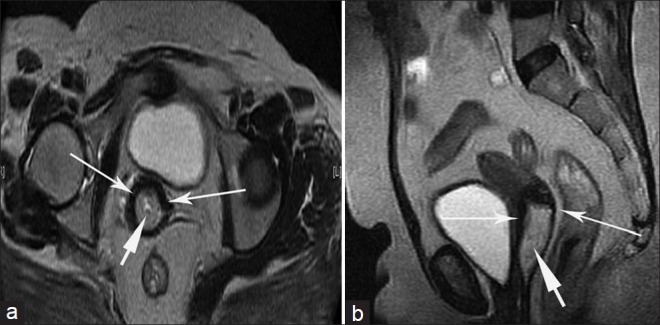
Stage IVA cervical carcinoma in a 60-year-old woman. (a) Sagittal and (b) coronal T2-weighted images show a large hyperintense mass that involves the entire circumference of the uterine cervix with involvement of the entire stromal thickness; parametrial infiltration is evidenced by irregular myometrial/ parametrial interface, and involvement of the vaginal fornices is present as evidenced by the loss of T2W signal intensity (short arrows). The tumor extends to invade the posterior wall of the urinary bladder with intraluminal tumor extension through the disrupted bladder wall (arrow heads). This proved to be anaplastic carcinoma of the cervix.
Figure 2.
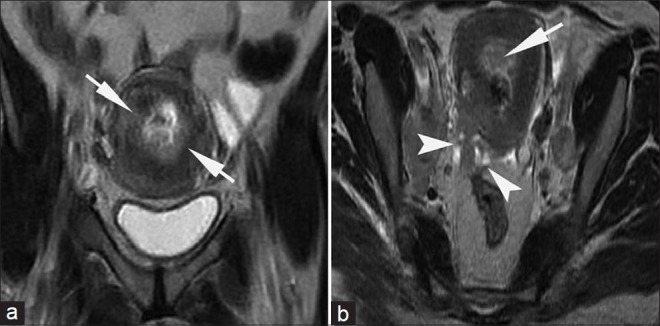
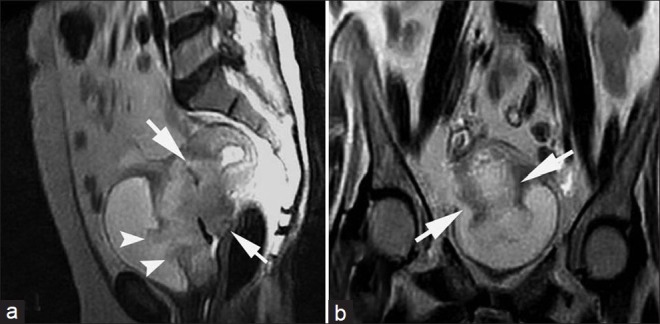
Stage IIB cervical carcinoma in a 45-year-old woman. (a) Coronal and (b) axial T2-weighted images show that the cervix is almost entirely replaced by a hyperintense mass that involves its anterior and posterior walls with involvement of the entire stromal thickness (short arrows). The tumor protrudes into the parametrium (arrow heads in b). This proved to be moderately differentiated squamous cell carcinoma.
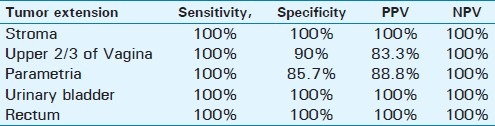
MRI was sensitive (100%) and specific (100%) in detecting tumor extension to the stroma, urinary bladder, and rectum. It had a sensitivity of 100% and specificity of 85.7% in detection of parametrial infiltration, and sensitivity of 100% and specificity of 90% in detection of vaginal infiltration. The estimated sensitivity, specificity, and the predictive values of MRI in detecting tumor extension are presented in Table 3.
Table 3.
Sensitivity specificity, PPV and NPV of MRI in detection of cervical tumor extension
Histopathologic examination demonstrated Stage IB cervical carcinoma in 2/30 patients (6.6%), Stage IIA disease in 10/30 patients (33.3%), Stage IIB in 6/30 patients (20%), and Stage IV disease in 8/30 patients (26.6%). MRI features demonstrated Stage IB in 2/30 patients (6.6%), Stage IIA disease in 12/30 patients (40%), Stage IIB in 8/30 patients (26.6%), and Stage IV disease in 8/30 patients (26.6%). MRI staging of cervical carcinoma was in concordance with histopathologic staging in Stage IB and IVA and over-staging in IIA and IIB stages. Comparison between MRI and pathological staging of cervical carcinoma is shown in Figures 4 and 5.
Figure 4.
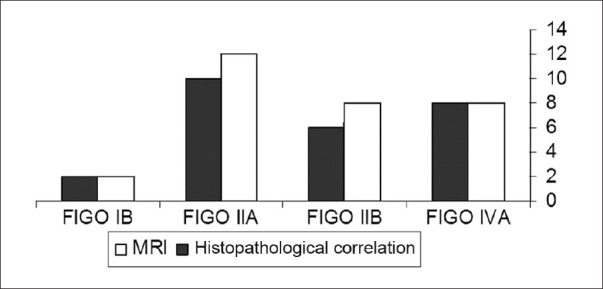
Histogram demonstrates comparison between MRI staging of uterine cervical carcinoma and pathological staging of cervical carcinoma.
Figure 5.

Histogram demonstrates cervical tumor growth pattern, location, extension, and lymph pelvic node involvement, as detected by MRI, histopathology, and surgery.
DISCUSSION
Cervical cancer is the leading cause of death among women in the developing world. Over 80% of women with newly diagnosed cervical cancer live in developing countries; most are diagnosed with advanced disease.[1,2]
Collettini (2011), Stenstedt (2011), and Zand (2007) had addressed the impact of MRI in the preoperative staging and management of uterine cervical cancer. They had proposed that MRI should be considered the most reliable modality for preoperative staging and treatment workup of cervical cancer. It provides a “one stop” shop for local disease assessment and should be performed as part of the pre-treatment evaluation of the tumor larger than 2 cm and in obese or pregnant patients.[2,3,6]
In this study, the most common pathological type of the examined cervical mass was the squamous cell carcinoma representing 73.3% of the total studied cervical malignancy. This incidence was nearly compatible with that of Collettini (2011) and Sahdev (2007), who reported that about 80–90% of cervical carcinomas are squamous cell carcinoma.[2,7]
Our study showed that most of cervical masses were exophytic (86.6%) and involved both the anterior and posterior cervical walls (93.3%). This growth pattern was in agreement with Okamoto (2003) who reported that most cervical squamous cell carcinomas grow at the squamo-columnar junction (SCJ) which is located outside the external uterine ostium uteri (os) in younger women, and the tumor tends to grow outward (exophytic growth pattern).[4]
Cervical cancer was identified in our study as a mass of intermediate to hyperintense on T2W signal that disrupts the low signal intensity stroma. Okamoto (2003) also reported that cervical cancers appear as hyperintense masses on T2-weighted images regardless of histopathologic type.[4]
In our study, MRI was highly sensitive (100%) and specific (100%) in determining tumor extension to the stroma, urinary bladder, and rectum. Morimura (2000) reported that MRI showed very high specificity (99.2%) and high sensitivity (88.5%) in detecting cervical stromal invasion.[8]
For staging purposes, vaginal invasion is categorized into upper two-thirds and lower-one third invasions. Invasion of the lower one-third of the vagina increases the stage from IIA to IIIA, which affects the strategy of radiation therapy. In our study, the upper two-thirds of the vagina was the most common site for extrauterine tumor invasion, with an incidence of 40%; this incidence was nearly similar to that of Chen (1984) who reported that the overall incidence of vaginal invasion by cervical carcinoma was 34.2%.[9] In the current study, MRI was highly sensitive (100%) in detecting vaginal invasion with a specificity of 90%; this was in agreement with Zand (2007) who described that MRI is highly sensitive (86–93%) in depiction of vaginal infiltration.[6] In our study, MRI overestimated vaginal invasion in only 2 out of 12 patients identified by MRI to have vaginal invasion, and this was due to large exophytic tumor; pathologically this tumor showed no vaginal invasion. MRI overestimation of vaginal invasion was previously reported by Zand (2007), Nicolet (2000), and Sheu (2001) and was thought that large, exophytic tumors may expand the vaginal fornix and stretch the vaginal wall without invading it. The stretched and thin vaginal fornix may be difficult to recognize as intact, even though it is not disrupted by tumor.[6,10,11] Nicolet (2000) reported that vaginal extension of cervical carcinoma is well evaluated clinically, and therefore the role of MRI is not crucial in this regard.[10]
Invasion of the parametrium is an important factor in the preoperative evaluation of cancer cervix that significantly influences staging and treatment.[12–14] In the present study, MRI was highly sensitive (100%) in detecting parametrial invasion, with a specificity of 85.7%. This was in agreement with previous studies.[15,16] The estimated Negative Predictive Value (NPV) was 100% which was in agreement with Yu (1998).[15] The importance of high NPV was addressed by Nicolet (2000) and Jena (2005), who reported that preservation of a hypointense fibrous stromal ring surrounding the cervical tumor in high-resolution axial T2-weighted MR images has a high NPV for parametrial invasion which is generally the accepted indicator for absence of parametrial invasion.[10,14] In our study, one false-positive parametrial invasion was in a patient with large exophytic tumor; pathologically this tumor showed full thickness stromal invasion with peritumoral edema, with no parametrial invasion. This explanation was comparable to what was previously reported by Nicolet (2000), who postulated that peritumoral inflammatory tissue may be falsely interpreted as parametrial infiltration, and by Sala (2007) and Kaur (2003), who reported that stromal edema caused by tumor compression or inflammation may lead to a higher rate of false-positive assessment of parametrial invasion in patients with large tumors.[10,17,18]
Scheidler (1998) and Kinkle (2006) reported that contrast material-enhanced T1-weighted imaging has not proved to be more accurate than T2-weighted imaging in diagnosing parametrial invasion. Likewise, we noted that contrast material-enhanced T1-weighted imaging did not increase MRI sensitivity and specificity for detection of parametrial invasion.[19,20]
Bladder and rectal invasion was detected in only eight of our patients; this was comparable with Rockall (2006) who reported that invasion of the bladder or rectum is rare in cervical carcinoma. The rarity of rectal invasion can be explained by the presence of the pouch of Douglas which separates the posterior fornix from the rectum that makes the direct invasion of the rectum uncommon.[21] The uterosacral ligaments provide the preferred route for rectal invasion.[6] Kim and Han (1997) reported a sensitivity, specificity, and NPV of 83%, 100%, and 100%, respectively, in detecting bladder invasion.[22] They stated that the false-positive findings of MRI in detecting bladder invasion may be due to the edematous response that can mimic tumor infiltration on T2-weighted images and false-negative findings were due to microscopic tumor invasion. In our study, a 100% NPV in detection of bladder and rectal invasion was similar to that reported by Rockall (2006) who postulated that the absence of bladder or rectal invasion can be diagnosed with sufficient confidence using MRI (NPV = 100%). This obviates the need for invasive cystoscopic or endoscopic staging in the majority of patients with cervical cancer.[21]
Lymph node metastasis does not change the clinical FIGO staging of cervical cancer.[23] However, the presence of a metastatic lymph node radically modifies the prognosis and treatment of cervical cancer patients.[24] Nodal metastasis indicates a poorer prognosis within each stage with a marked decrease in survival rates, especially in cases of paraaortic node involvement.[10,25,26] Pelvic lymph node metastases are considered to be equivalent to pelvic side wall tumor extension (stage IIIB), and paraaortic or inguinal lymph node metastases are considered extrapelvic tumor spread and are classified as Stage IVB.[4] We detected pelvic lymph node enlargement in 10 patients and no paraaortic or inguinal lymph node enlargement was detected; we used a short-axis diameter of 10 mm or more to predict metastatic lymph node. This size criterion has been widely accepted.[27–31] Choi (2006) postulated that size and type of margin (spiculated or lobulated) were the useful criteria for predicting lymph node metastasis from cervical cancer.[24]
In the present study, MRI correctly identified metastatic pelvic lymph node in 6 (60%) of 10 patients, proved pathologically to have metastases; the other 4 patients were falsely diagnosed to have metastatic pelvic lymph nodes and pathology proved them to have reactive hyperplasia. This was in agreement with Zand (2007) who reported that that CT and MRI fail to differentiate reactive enlargement (in bulky necrotic tumors) from malignant infiltration, and more importantly, they lack the resolution to detect micrometastases in normal-sized nodes.[6] The accuracy and sensitivity rates of MRI for detecting nodal metastases are reported to be between 76% and 100% and between 36% and 89.5%, respectively.[29–31] The low sensitivity of MRI results from its inability to identify metastasis in normal-sized lymph nodes.[17]
The use of lymph node-specific MRI contrast agents, such as ultra-small superparamagnetic iron oxide (USPIO) particles, has been shown to improve the sensitivity without loss of its high specificity of detection of lymph node metastases in patients with cervical cancer.[32] Positron emission tomography (PET)/CT fusion imaging is valuable for lymph node staging with a sensitivity and specificity of 100% and 99.7%, respectively, for lymph nodes larger than 5 mm in diameter.[33]
The most important prognostic factors for cervical cancer are tumor stage, size, and nodal metastases.[34] Other prognostic factors include depth of invasion, tumor grade, and lymphatic vascular space invasion.[10] In our study, MRI staging of cancer cervix ranged from stage IB to stage IVA; earlier stage (stage IA) could not be detected by MRI. This was in agreement with Zand (2007) who reported that T2-weighted image may be normal in microinvasive (Stage IA) carcinoma.[6] Once abnormal increased signal is observed on the axial scans, the tumor is usually at least at Stage IB, which is clinically invasive tumor confined to the cervix without invasion of the vagina or the parametrium. Stromal invasion more than 5 mm is always detected on T2WI; visible tumor indicates Stage IB or higher.
According to FIGO staging, MRI staging of cancer cervix is comparable with histopathologic staging in Stages IB and IVB; however, in the current study, MRI over staging was encountered in one patient in Stage IIA and one patient in Stage IIB. This was due to overestimation of upper two-thirds of the vaginal invasion and parametrial invasion, respectively. Sala (2007) reported that an important pitfall of MRI staging accuracy is overestimation of parametrial invasion.[17] Sheu (2001) also reported that pitfalls leading to staging errors include difficulties in differentiating cancer foci from the surrounding tissue edema and excluding vaginal invasion in the presence of large cervical cancer.[11] In our study, the accuracy of MRI in staging of cancer cervix was 92.2%. This is consistent with previous results.[11,35,36] Sheu (2001) had postulated that MRI had an accuracy of 83.8 and 96.7% in determining the stage of cervical carcinoma and differentiating operable (< or = stage IIA) from advanced disease (> or = stage IIB).[11] Our results are also similar to a recent study done by Nilu (2012), which stated that the overall accuracy of MRI in staging carcinoma of cervix was 89.3%.[37]
To summarize, MRI is highly accurate in detection, characterization, and staging of cancer cervix. So, preoperative MRI is recommended to determine the treatment strategy which is largely dependent on and tailored according to the tumor stage.
CONCLUSION
MRI is a non-invasive optimal modality for preoperative evaluation of patients with suspected uterine cervical malignancy, not only for detecting and characterizing the tumor itself, but also for efficient assessment of the tumor invasion and abdominal and pelvic lymphadenopathy, with subsequent more accurate tumor staging and treatment planning.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/42/99175
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Collettini F, Hamm B. Uterine cervical cancer: Preoperative staging with magnetic resonance imaging. Radiologe. 2011l;51:589–95. doi: 10.1007/s00117-010-2119-1. [DOI] [PubMed] [Google Scholar]
- 3.Stenstedt K, Hellströöm AC, Fridsten S, Blomqvist L. Impact of MRI in the management and staging of cancer of the uterine cervix. Acta Oncologica. 2011;50:420–6. doi: 10.3109/0284186X.2010.541932. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto Y, Tanaka YO, Nishida M, Tsunoda H, Yoshikawa H, Itai Y. MR imaging of the uterine cervix: Imaging-pathologic correlation. Radiographics. 2003;23:425–45. doi: 10.1148/rg.232025065. [DOI] [PubMed] [Google Scholar]
- 5.Testa AC, Ludovisi M, Manfredi R, Zannonis G, Basso D, DI Legge A, et al. Transvaginal ultrasonography and magnetic resonance imaging for assessment of presence, size and extent of invasive cervical cancer. Ultrasound Obstet Gynecol. 2009;34:335–44. doi: 10.1002/uog.7325. [DOI] [PubMed] [Google Scholar]
- 6.Zand KR, Reinhold C, Abe H, Maheshwari S, Mohamed A, Upegui D. Magnetic resonance imaging of the cervix. Cancer Imaging. 2007;7:69–76. doi: 10.1102/1470-7330.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahdev A, Sohaib SA, Wenaden AE, Shepherd JH, Reznek RH. The performance of magnetic resonance imaging in early cervical carcinoma: A long-term experience. Int J Gynecol Cancer. 2007;17:629–36. doi: 10.1111/j.1525-1438.2007.00829.x. [DOI] [PubMed] [Google Scholar]
- 8.Morimura Y, Soeda S, Hashimoto T, Takano Y, Ohwada M, Yamada H, et al. The value of pre-operative diagnostic procedures for cervical involvement in uterine corpus carcinoma. Fukushima J Med Sci. 2000;46:1–11. doi: 10.5387/fms.46.1. [DOI] [PubMed] [Google Scholar]
- 9.Chen NJ. Vagina invasion by cervical carcinoma. Acta Med Okayama. 1984;38:305–13. doi: 10.18926/AMO/30348. [DOI] [PubMed] [Google Scholar]
- 10.Nicolet V, Carignan L, Bourdon F, Prosmanne O. MR imaging of cervical carcinoma: A practical staging approach. Radiographics. 2000;20:1539–49. doi: 10.1148/radiographics.20.6.g00nv111539. [DOI] [PubMed] [Google Scholar]
- 11.Sheu M, Chang C, Wang J, Yen M. MR staging of clinical stage I and IIa cervical carcinoma: A reappraisal of efficacy and pitfalls. Eur J Radiol. 2001;38:225–31. doi: 10.1016/s0720-048x(00)00278-3. [DOI] [PubMed] [Google Scholar]
- 12.Greco A, Mason A, Leung AW, Dische S, McIndoe AJ, Anderson MC. Staging of carcinoma of the uterine cervix: MRI surgical correlation. Clin Radiol. 1989;40:401–5. doi: 10.1016/s0009-9260(89)80136-9. [DOI] [PubMed] [Google Scholar]
- 13.Togashi K, Nishimura K, Sagoh T, Minami S, Noma S, Fujisawa I. Carcinoma of the cervix: Staging with MR imaging. Radiology. 1989;171:245–51. doi: 10.1148/radiology.171.1.2928532. [DOI] [PubMed] [Google Scholar]
- 14.Jena A, Oberoi R, Rawal S, Das SK, Pandey KK. Parametrial invasion in carcinoma of cervix: Role of MRI measured tumour volume. Br J Radiol. 2005;78:1075–7. doi: 10.1259/bjr/36116150. [DOI] [PubMed] [Google Scholar]
- 15.Yu KK, Hricak H, Subak LL, Zaloudek CJ, Powell CB. Preoperative staging of cervical carcinoma: Phased array coil fast spin-echo versus body coil spin-echo T2-weighted MR imaging. AJR Am J Roentgenol. 1998;171:707–11. doi: 10.2214/ajr.171.3.9725301. [DOI] [PubMed] [Google Scholar]
- 16.Sironi S, Belloni C, Taccagni G, DelMaschio A. Carcinoma of the cervix: Value of MR imaging in detecting parametrial involvement. AJR Am J Roentgenol. 1991;156:753–6. doi: 10.2214/ajr.156.4.2003441. [DOI] [PubMed] [Google Scholar]
- 17.Sala E, Wakely S, Senior E, Lomas D. MRI of malignant neoplasms of the uterine corpus and cervix. AJR Am J Roentgenol. 2007;188:1577–87. doi: 10.2214/AJR.06.1196. [DOI] [PubMed] [Google Scholar]
- 18.Kaur H, Silverman PM, Iyer RB, Verschraegen CF, Eifel PJ, Charnsangavej C. Diagnosis, staging, and surveillance of cervical carcinoma. AJR Am J Roentgenol. 2003;180:1621–31. doi: 10.2214/ajr.180.6.1801621. [DOI] [PubMed] [Google Scholar]
- 19.Scheidler J, Heuck AF, Steinborn M, Kimmig R, Reiser MF. Parametrial invasion in cervical carcinoma: Evaluation of detection at MR imaging with fat suppression. Radiology. 1998;206:125–9. doi: 10.1148/radiology.206.1.9423661. [DOI] [PubMed] [Google Scholar]
- 20.Kinkel K. Pitfalls in staging uterine neoplasm with imaging: A review. Abdom Imaging. 2006;31:164–73. doi: 10.1007/s00261-005-0383-8. [DOI] [PubMed] [Google Scholar]
- 21.Rockall AG, Ghosh S, Alexander-Sefre F, Babar S, Younis MT, Naz S, Jacobs IJ, Reznek RH. Can MRI rule out bladder and rectal invasion in cervical cancer to help select patients for limited EUA? Gynecol Oncol. 2006;101:244–9. doi: 10.1016/j.ygyno.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Han MC. Invasion of the urinary bladder by uterine cervical carcinoma: Evaluation with MR imaging. AJR Am J Roentgenol. 1997;168:393–7. doi: 10.2214/ajr.168.2.9016214. [DOI] [PubMed] [Google Scholar]
- 23.Kamura T, Tsukamoto N, Tsuruchi N, Saito T, Matsuyama T, Akazawa K, et al. Multivariate analysis of the histopathologic prognostic factors of cervical cancer in patients undergoing radical hysterectomy. Cancer. 1992;69:181–6. doi: 10.1002/1097-0142(19920101)69:1<181::aid-cncr2820690130>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Choi HJ, Kim SH, Seo SS, Kang S, Lee S, Kim JY, et al. MRI for pretreatment lymph node staging in uterine cervical cancer. AJR Am J Roentgenol. 2006;187:538–43. doi: 10.2214/AJR.05.0263. [DOI] [PubMed] [Google Scholar]
- 25.Inoue T, Morita K. The prognostic significance of number of positive nodes in cervical carcinoma stages IB, IIA, and IIB. Cancer. 1990;65:1923–7. doi: 10.1002/1097-0142(19900501)65:9<1923::aid-cncr2820650909>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.Takeshima N, Yanoh K, Tabata T, Nagai K, Hirai Y, Hasumi K. Assessment of the revised International Federation of Gynecology and Obstetrics staging for early invasive squamous cervical cancer. Gynecol Oncol. 1999;74:165–9. doi: 10.1006/gyno.1999.5473. [DOI] [PubMed] [Google Scholar]
- 27.Roy C, Le Bras Y, Mangold L, Saussine C, Tuchmann C, Pfleger D, et al. Small pelvic lymph node metastases: Evaluation with MR imaging. Clin Radiol. 1997;52:437–40. doi: 10.1016/s0009-9260(97)80004-9. [DOI] [PubMed] [Google Scholar]
- 28.Scheidler J, Hricak H, Yu KK, Subak L, Segal MR. Radiological evaluation of lymph node metastases in patients with cervical cancer: A metaanalysis. JAMA. 1997;278:1096–101. [PubMed] [Google Scholar]
- 29.Kim SH, Kim SC, Choi BI, Han MC. Uterine cervical carcinoma: Evaluation of pelvic lymph node metastasis with MR imaging. Radiology. 1994;190:807–11. doi: 10.1148/radiology.190.3.8115631. [DOI] [PubMed] [Google Scholar]
- 30.Choi SH, Kim SH, Choi HJ, Park BK, Lee HJ. Preoperative magnetic resonance imaging staging of uterine cervical carcinoma: Results of prospective study. J Comput Assist Tomogr. 2004;28:620–7. doi: 10.1097/01.rct.0000138007.77725.0a. [DOI] [PubMed] [Google Scholar]
- 31.Yang WT, Lam WW, Yu MY, Cheung TH, Metreweli C. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am J Roentgenol. 2000;175:759–66. doi: 10.2214/ajr.175.3.1750759. [DOI] [PubMed] [Google Scholar]
- 32.Rockall AG, Sohaib SA, Harisinghani MG, Babar SA, Singh N, Jeyarajah AR, et al. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol. 2005;23:2813–21. doi: 10.1200/JCO.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 33.Sironi S, Buda A, Picchio M, Perego P, Moreni R, Pellegrino A, et al. Lymph node metastasis in patients with clinical early-stage cervical cancer: Detection with integrated FDG PET/CT. Radiology. 2006;238:272–9. doi: 10.1148/radiol.2381041799. [DOI] [PubMed] [Google Scholar]
- 34.Engin G, Küçücük S, Ölmez H, Haşıloğlu ZI, Dişçi R, Aslay I. Correlation of clinical and MRI staging in cervical carcinoma treated with radiation therapy: A single-center experience. Diagn Interv Radiol. 2011;17:44–51. doi: 10.4261/1305-3825.DIR.3114-09.1. [DOI] [PubMed] [Google Scholar]
- 35.Bipat S, Glas AS, van der Velden J, Zwinderman AH, Bossuyt PM, Stoker J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: A systematic review. Gynecol Oncol. 2003;91:59–66. doi: 10.1016/s0090-8258(03)00409-8. [DOI] [PubMed] [Google Scholar]
- 36.Subak LL, Hricak H, Powell CB, Azizi L, Stern JL. Cervical carcinoma: Computed tomography and magnetic resonance imaging for preoperative staging. Obstet Gynecol. 1995;86:43–50. doi: 10.1016/0029-7844(95)00109-5. [DOI] [PubMed] [Google Scholar]
- 37.Nilu MD, Vinay K, Atul S, Amal CK, Debabrata B, Utpal B. Evaluation of carcinoma cervix using magnetic resonance imaging: Correlation with clinical FIGO staging and impact on management. J Med Imaging Radiat Oncol. 2012;56:58–65. doi: 10.1111/j.1754-9485.2011.02333.x. [DOI] [PubMed] [Google Scholar]


