Abstract
Craniofacial microsomia (CFM) is one of the most common congenital conditions treated in craniofacial centers worldwide. This condition is variably associated with anomalies of the jaws, ears, facial soft tissue, orbits, and facial nerve function and can be associated with extracranial anomalies. The cause of this condition is unknown, though CFM has been associated withprenatalexposures and genetic abnormalities. Diagnosis, treatment, and outcome assessment in CFM is challenging due to the wide phenotypic spectrum observed in this condition. Surgical treatment requires a coordinated team approach involving multiple specialties, which can include plastic surgery, craniofacial surgery, orthognathic surgery, and microsurgery. A wide variety of surgical options exist, and individual treatment plans should be based on the patient's needs. Although CFM can be challenging to treat, successful outcomes are rewarding. We provide a review of the common craniofacial surgical treatments for individuals with CFM.
Keywords: craniofacial microsomia, hemifacial microsomia, Goldenhar syndrome, OMENS, Phenotypic Assessment Tool-Craniofacial Microsomia (PAT-CFM)
Overview
Craniofacial microsomia (CFM) is estimated to occur in 1:3,000 to 1:5,000 live births1,2; it is the most common congenital disorder of the face after cleft lip and palate.2,3,4 CFM involves an absence or underdevelopment of structures that arise from the first and second pharyngeal arches,5,6 such as the mandible, maxilla, ear, facial soft tissue and muscles, and the facial nerve. These anomalies can result in alterations in the upper airway, facial movement, speech, feeding, eye protection, hearing, and aesthetic appearance. Given the complex nature of this condition, children with CFM are best cared for by a multidisciplinary craniofacial team that can provide specialized, coordinated treatment, which may include feeding therapy, speech therapy, airway management, medical intervention, psychological support, ophthalmologic assessment, audiologic evaluation, orthognathic surgery, plastic surgery, craniofacial surgery, and microsurgery.
Etiology
The cause of CFM remains unknown, but appears to involve a disruption in the development of the first and second pharyngeal arches during the first 6 weeks of gestation. Poswillo produced the CFM phenotype in mice by administering teratogens that caused a hematoma of the stapedial artery and the artery of the second arch and resulted in regional necrosis.7 The wide spectrum of resulting facial anomalies was felt, therefore, to be caused by the extent of tissue injury from this necrosis and its ability to regenerate. More recent studies have demonstrated an association between CFM occurrence and multiple gestation, along with the following maternal risk factors: use of vasoactive medications, second-trimester smoking, diabetes mellitus, and use of assisted reproductive technology.8,9 In addition, autosomal dominant and recessive transmission patterns have been described in families with features of CFM10,11,12 and a positive family history of 50% has been observed in a large series of cases.13,14 A variety of genetic abnormalities have also been described.15,16,17,18 It is possible that etiologic heterogeneity along with variability in penetrance and expression could account for the wide phenotypic spectrum seen in CFM.
Clinical Characteristics
Diagnostic criteria for CFM do not exist. The CFM diagnosis is based on physical exam findings of hypoplasia, aplasia, or malformation of the external ear, mandible, temporal bone, zygoma, middle ear, facial musculature, facial nerve supply, and other adjacent bony and soft tissues. As many as 55% of patients with CFM also have extracranial anomalies, which may include central nervous system (CNS), skeletal, cardiac, lung, gastrointestinal, and kidney defects.19 Interestingly, the presence of these extracranial manifestations may predict a greater phenotypic severity of the facial features. Although isolated branchial remnants are not considered part of the CFM spectrum, whether or not “isolated” microtia represents the mildest form of CFM or a distinct entity continues to be debated3 given the similar risk factors.20,21 Some clinician's prefer the term Goldenhar syndrome for patients with microtia, facial asymmetry, epibulbar dermoids, and cervical spine anomalies22
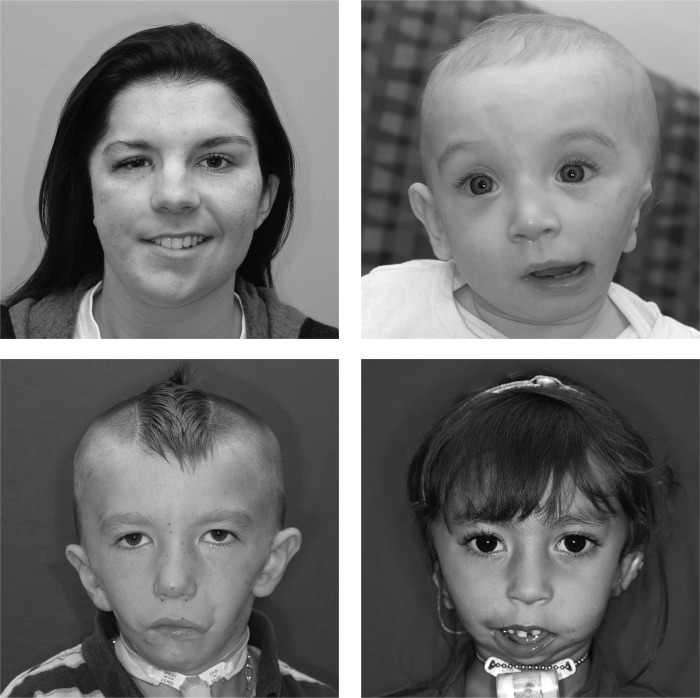
The wide phenotypic presentation associated with CFM creates challenges for studies assessing etiology and treatment outcomes. As seen in Fig. 1, the phenotype may involve the orbit or ear alone, or in combination with jaw malformations. The anomalies may be unilateral or bilateral and range in severity. Treatment plans must be tailored to the individual and will vary significantly among patients, making outcome comparisons challenging. For this reason, classification systems can be used to standardize the phenotypic assessment for clinical care and research.
Figure 1.
The phenotypic spectrum of craniofacial microsomia is displayed in these four patients who are affected unilaterally and bilaterally to varying degrees.
Classification Systems
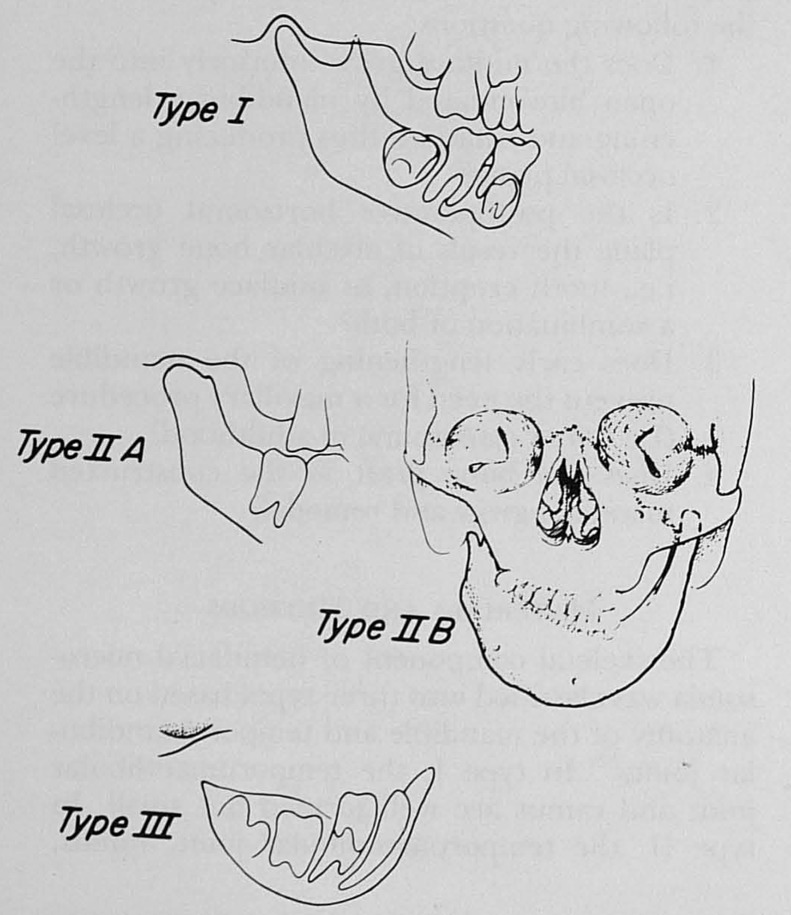
Pruzansky classified the patterns of deformity based on x-rays of the mandible in individuals with CFM (Table 1).23 Grade I mandibles were small, but exhibited growth and the deformity did not progress with time. Grade III mandibles were malformed with atypical growth, and the deformity worsened with time. Kaban et al added a description of the temporomandibular joint and deformity as seen on anteroposterior and lateral cephalogram (Table 2).24,25 The type II mandible was subdivided into two groups depending on the position of the glenoid fossa (Fig. 2). This was an important distinction as they felt the type IIB mandibles required surgical treatment of the TMJ whereas the IIA mandibles did not. These classification systems of the mandibular anomalies associated with CFM are still frequently used, along with newer systems that include features observed on high-resolution three-dimensional computed tomography (CT) scans.26 However, these classification schemata do not include the additional craniofacial malformations associated with CFM.
Table 1. Pruzansky Classification System (1969).
| Grade I | Smaller than preserved normal side |
| Grade II | Condyle, ramus, and sigmoid notch identifiable, but grossly distorted in size and shape |
| Grade III | Grossly distorted ramus with loss of landmarks or agenesis |
Pruzansky classification of the mandible deformity in hemifacial microsomia. (From Pruzansky S. Not all dwarfed mandibles are alike. Birth Defects1969; 1:120–129.)
Table 2. A Classification System Modified by Kaban et al to Capture the Craniofacial Microsomia-Related Mandibular Hypoplasia and Temporomandibular Joint (TMJ) Deformity Observed on Cephalograms.
| I | Small mandible |
| IIA | Short mandibular ramus of abnormal shape; glenoid fossa in satisfactory position |
| IIB | TMJ abnormally placed inferiorly, medially and anteriorly |
| III | Absent TMJ |
Kaban's modification of the Pruzansky classification system. (From Kaban LB, Moses MH, Mulliken JB. Surgical correction of hemifacial microsomia in the growing child. Plast Reconstr Surg 1988; 82:9–19.)
Figure 2.
Kaban's modification of the Pruzansky classification system of the mandible in craniofacial microsomia. (Reprinted with permission from Kaban LB, Moses MH, Mulliken JB. Surgical correction of hemifacial microsomia in the growing child. Plast Reconstr Surg, 1988; 82(1):9–19.)
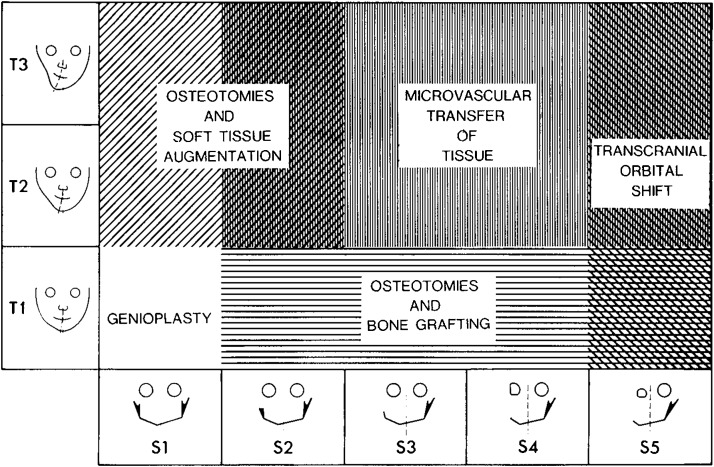
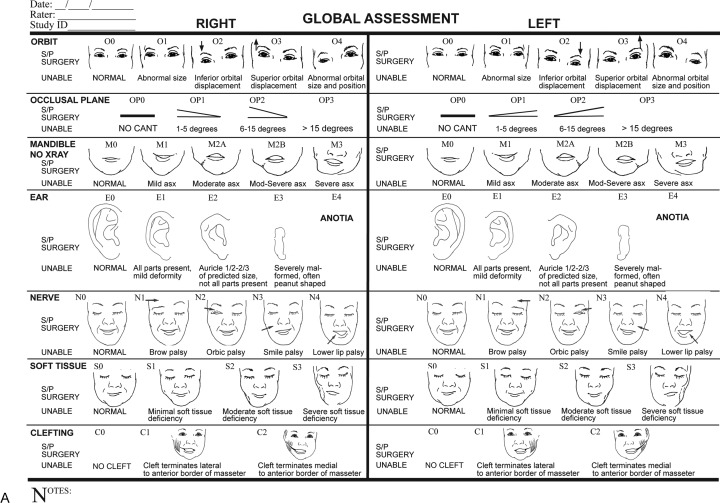
Other classification systems focus on anomalies of the ear and the mandible,27,28,29 and some include additional features such as the zygomatic arch and orbit.30 A grading system similar to the TNM tumor system was created to describe the variety and severity of features seen in CFM (Fig. 3).31 The most widely used system is the OMENS classification scheme,32 later modified to the OMENS+ to include extracranial manifestations.19 The acronym stands for orbit, mandible, ear, nerve, and soft tissue; each feature is assigned a severity score (Table 3). More recently, a pictorial representation of the OMENS system was introduced33 and later modified34 to facilitate ease of use (Figs. 4A, 4B).
Figure 3.
Treatment strategies for varying phenotypic severity in craniofacial microsomia based on the SAT staging system proposed by David et al. (Reprinted with permission from David DJ, Mahatumarat C, Cooter RD. Hemifacial microsomia: a multisystem classification. Plast Reconstr Surg 1987;80(4): 525–535.)
Table 3. A Grading System to Capture the Presence and Severity of the Orbit, Mandible, Ear, Nerve, and Soft Tissue Anomalies Commonly Associated with Craniofacial Microsomia*.
| Orbit | |
| O0 | Normal orbital size and position |
| O1 | Abnormal orbital size |
| O2 | Abnormal orbital position (arrow up or down) |
| O3 | Abnormal orbital size and position |
| Mandible | |
| M0 | Normal mandible |
| M1 | The mandible and glenoid fossa are small. |
| M2A | Short ramus, glenoid fossa is in anatomically acceptable position |
| M2B | Short ramus, TMJ is inferiorly, medially and anteriorly displaced with hypoplastic condyle |
| M3 | Complete absence of ramus, glenoid fossa and TMJ |
| Ear | |
| E0 | Normal ear |
| E1 | Mild hypoplasia & cupping, all structures present |
| E2 | Absence of external auditory canal with hypoplasia of concha |
| E3 | Malpositioned lobule with absent auricle, lobular remnant inferiorly and anteriorly displaced |
| Facial Nerve | |
| N0 | No facial nerve involvement |
| N1 | Upper facial nerve involvement (temporal zygomatic) |
| N2 | Lower facial nerve involvement (buccal, mandibular, cervical) |
| N3 | All branches of facial nerve affected |
| Soft Tissue | |
| S0 | No obvious soft tissue or muscle deficiency |
| S1 | Minimal subcutaneous/muscle deficiency |
| S2 | Moderate–between the two extremes S1 and S3 |
| S3 | Severe soft tissue deficiency due to subcutaneous and muscular hypoplasia |
TMJ, temporomandibular joint
The O.M.E.N.S. Classification System for quantifying the deformity of the orbit, mandible, ear, nerve, and soft tissue in hemifacial microsomia. (From Vento RA, LaBrie RA, Mulliken JB. The O.M.E.N.S. classification of hemifacial microsomia. Cleft Palate-Craniofacial Journal 1991; 28:68–76.)
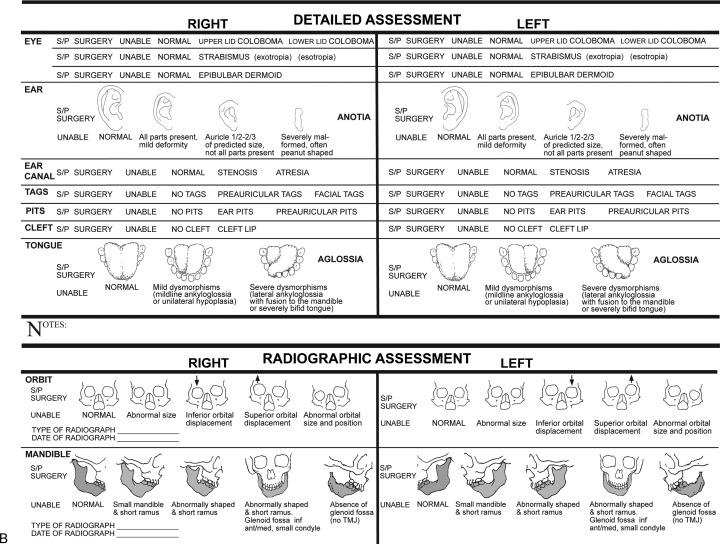
Figure 4.
(A) Global assessment of phenotypic severity as documented using the Phenotypic Assessment Tool-Craniofacial Microsomia (PAT-CFM). (Reprinted with permission from Birgfeld CB et al. A phenotypic assessment tool for craniofacial microsomia. Plast Reconstr Surg 2011;127(1): 313–320.) (B) Detailed assessment of phenotypic severity as documented using the PAT-CFM. (Reprinted with permission from Birgfeld CB et al. A phenotypic assessment tool for craniofacial microsomia. Plast Reconstr Surg 2011; 127(1): 313–320.)
Surgical Treatment
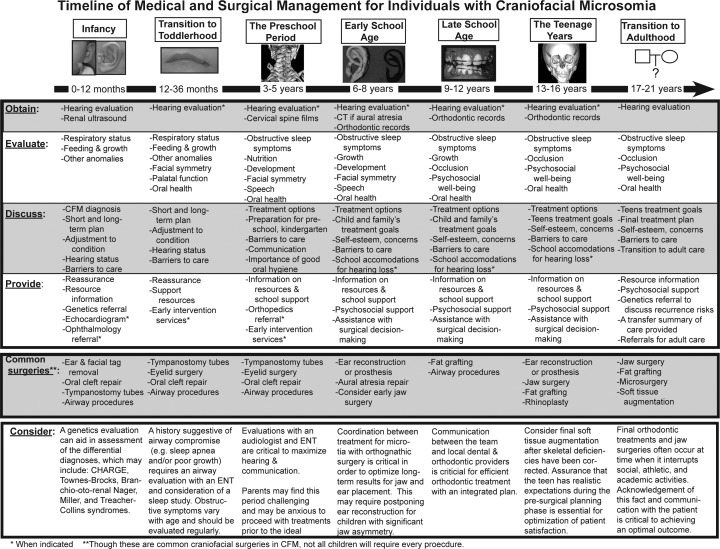
Treatment approaches for individuals with CFM vary widely. The clinical needs of patients with CFM depend entirely on the type and severity of the facial abnormalities, the goals of the patient and family, and the psychosocial support available to the patient. A multidisciplinary team that offers the breadth of specialties required and coordinates the treatment into an optimal timeline for each patient is ideal. We present a proposed timeline (Fig. 5) as a general guideline to aid practitioners in planning their procedures and help patients and families understand the multiple interventions that may be necessary during a child's growth and development. It should be emphasized that each patient requires an individualized treatment plan, tailored to his or her specific needs.
Figure 5.
Timeline for treatment of patients with craniofacial microsomia from birth through adulthood as proposed by members of the Craniofacial Center at Seattle Children's Hospital.
Surgical interventions are designed to restore the patient's craniofacial form and function and must account for the expected facial growth pattern,35 timing of dental eruption, schedules for school and extracurricular activities, along with other psychosocial factors. For example, interventions such as orthognathic surgery are likely most effective if postponed until completion of facial growth. However, infants may require timely treatment for any upper airway obstruction with mandible distraction or tracheostomy. Communication among team members is paramount to coordinate timing of surgical interventions. We describe the common craniofacial surgical procedures below.
Orbit
Procedures for the eye and orbit in CFM typically involve either bony or soft tissue surgery. Infants require stimulation of the visual cortex to avoid amblyopia and may require treatment of epibulbar dermoids36,37 in infancy if the visual axis is disrupted. Eyelid colobomas may require repair to protect the cornea and prevent exposure keratitis and blindness.
Orbital asymmetry in size and/or position (i.e., dystopia) is corrected only if severe and typically is postponed until the orbital growth is complete at around age 3 or 4 years. Orbital repositioning is performed by a circumferential box osteotomy performed through an intracranial approach. The orbit is advanced and lowered or elevated, then fixated into a symmetric position with the contralateral orbit. Orbital repositioning can be coordinated with cranial reconstruction if necessary.
Mandible
Treatment of the mandible remains controversial. Each patient should have a tailored treatment plan based on his or her needs, the morphology of his or her mandible and TMJ, and the skills of the surgeon and orthodontist. Timing of treatment, approach, and surgical technique differ among centers. Some centers offer mandibular distraction in an effort to avoid tracheostomy for infants with CFM and failure to thrive due to a single-level airway obstruction at the mandible. A comprehensive evaluation including monitoring of weight gain, feeding, growth, and airway patency is essential in this population.
Surgical intervention for a type I mandible is often postponed until skeletal growth is complete. In mild cases, the occlusal relationship can be managed with orthodontics. However, the mandibular asymmetry in more severe type I cases can worsen during the growth phase of mixed dentition; orthognathic surgery may be necessary to improve an occlusal cant and facial symmetry. Cephalometrics and occlusal casts can be used to assess the dentofacial relationship and determine whether the patient needs unilateral or bilateral mandibular advancement, or if bimaxillary surgery is warranted.
Costochondral Rib Graft
Treatment recommendations for the type II mandible differ based on distinction between the IIA and IIB subgroups as described by Kaban.24 The IIA subgroup requires vertical lengthening of the mandible, typically with an osteotomy and interposed bone graft, performed after skeletal maturity. The IIB subgroup is classically treated with costochondral bone graft of the ramus and condyle38 with reconstruction of the glenoid fossa.
Treatment of the type III mandible is similar to that of the IIB mandible and involves reconstruction of the ramus and condyle using a costochondral rib graft and glenoid fossa reconstruction to create a functioning temporomandibular joint. This operation is undertaken when the patient begins to show an occlusal cant to the maxilla, which generally correlates with dental eruption in children ages 2 to 5 years.24
Although this approach is common, inadequacies of the reconstruction and complications with the costochondral rib graft and neo-TMJ have been well described. A costochondral graft can have unpredictable growth and resorption.39,40,41 Lack of regional soft tissue and decreased vascularity likely contribute to the resorption of these grafts.42 Therefore, microsurgical techniques using fibula osteocutaneous free flaps for the treatment of the type III mandible have been introduced.43,44 Costochondral reconstruction has also been associated with TMJ ankylosis.24 However, this technique does not address the soft tissue deficiency that is common in CFM. Distraction osteogenesis of the mandible was introduced in an attempt to address these deficiencies.
Mandibular Distraction Osteogenesis
Distraction osteogenesis was initially described by Ilizarov,45,46 then applied to the craniofacial skeleton by Snyder47 and popularized by McCarthy.48,49 Mandibular distraction osteogenesis (MDO) has some distinct advantages over costochondral grafting. MDO increases the vertical length of the mandible,50 produces greater bone stock,51,52 improves soft tissue asymmetry,53,54 and has less relapse.55,56 Other benefits include shorter operative times, less blood loss,55 greater vector control of advancement,57,58,59 and the ability to lengthen the mandible at a younger age as bone grafts are not always necessary.55 MDO can be used to treat type IIa and IIb mandibles, and combined with a bone graft for treatment of type III mandibles. The MDO procedure requires selection of (1) a vector orientation, (2) the type of device, and (3) an internal or external approach.
The vector of advancement should be based on the mandibular shape.60 A vertical vector is often adequate for the short ramus associated with the IIa mandible, while the IIb mandible often requires a more obliquely oriented vector to treat the vertical and horizontal ramal deficiency. The surgeon first assesses the TMJ,61 then plans a vector orientation that will lengthen the ramus, upright the condyle,62 and create a gonial angle.59,63
Both single and multivector external devices and semiburied internal devices are available (Fig. 6); each has unique benefits and deficiencies. External devices allow greater freedom to mold the regenerate64 by changing the vector of distraction after the osteotomy is made. Additionally, pin placement requires little bone stock, which allows for accurate placement of the devices in hypoplastic mandibles and with minimal disruption of the periosteum. However, the external devices create unsightly scars, dislodge easily, significantly alter the patient's appearance during MDO, and are prone to pin site infections (Fig. 7). Internal devices can be multivector, are less visible, create less scarring, and are less prone to trauma and infection.65 Greater preoperative planning is necessary, however, because the vector cannot be altered once the device is positioned. They also require an additional surgery to remove unless a resorbable system is selected.66,67
Figure 6.

Example of a semiburied internal distractor device.
Figure 7.
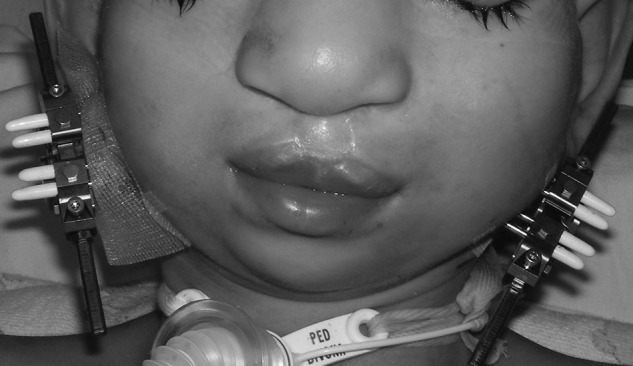
Example of external multivector distraction devices in situ.
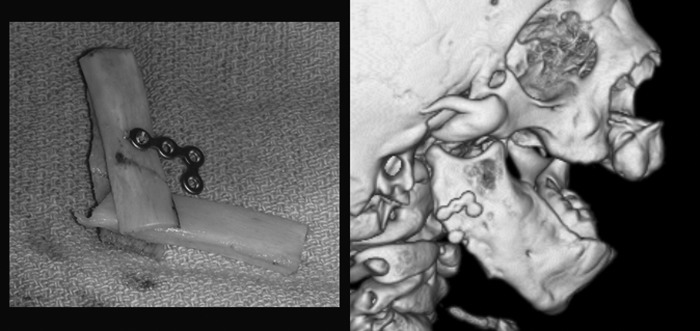
In the type III mandible, a bone graft is first required to create sufficient ramal bone stock for distraction (Fig. 8). Whether a fibula free flap, a costochondral graft or a costal graft is used, the bone is first fixated to the remnant angle or body, then once healed, the distraction can be performed. This may allow preservation of the molar tooth bud, although some surgeons prefer to sacrifice this tooth bud prior to distraction to prevent cyst formation or ankylosis.68 Although the graft can be distracted,44,69,70 it is our preference to distract the native mandible whenever possible and use the grafted area to secure the footplates of the distraction device.
Figure 8.
Clinical example of bone graft augmentation of deficient mandible angle in preparation for distraction osteogenesis in patient with Pruzansky type 3 mandible.
Coordination between the surgeon and the orthodontist is critical. Distraction techniques create an open bite once the mandible is lengthened. To prevent relapse, this open bite must be maintained until the maxillary dentition can be brought down to create a stable occlusion. This occurs quickly in children age 4 to 6 years and may require little management. During mixed dentition, however, the open bite is often managed through occlusal splints or tooth borne or bone-anchored orthodontics. The maxilla may require concomitant movement with the mandible using bimaxillary distraction after skeletal maturity.71
Ear
The ear anomalies associated with CFM can be categorized into external ear malformations (e.g., microtia), middle ear malformations and atresia, and the presence of branchial remnants and sinus tracts. Although the presence of branchial remnants in isolation is generally not considered part of the CFM spectrum, the existence of isolated microtia is often considered a component of CFM3,6 as the risk factors and affected tissues are similar.20,21 The severity of the external ear deformity may predict the degree of middle ear involvement.20,29 Multiple classification systems have been developed to characterize the external ear anomalies,72,73,74,75,76,77,78,79 and the OMENS classification system incorporates the system of Marx and Meurman.32
Surgical treatment of the E1 ear (i.e., mild hypoplasia and cupping with all structures present) involves reshaping existing cartilage. Recreation of the normal folds of the upper ear can be accomplished through Stenstrom rasps, scoring or weakening the cartilage with a burr with or without suture stabilization. A contralateral otoplasty may also be necessary.80 In the E2 ear (i.e., absence of the external auditory canal with variable hypoplasia of the concha) and the E3 ear (i.e., malpositioned lobule with absent auricle) the remnant cartilage is often discarded and a new framework is made of alloplast or autograft.
Various materials have been used for alloplastic ear reconstruction with mixed results.81,82,83 Porous polyethylene's inert nature and pore size provides the best safety profile and allows for some tissue ingrowth. It is available in two prefabricated constructs of various sizes, which can be matched to the contralateral ear, secured, and covered with a temporoparietal fascia flap followed by a skin graft. Benefits include rigidity of construct, lack of donor site morbidity, and the ability to reconstruct younger, smaller patients. Criticisms include extrusion83 and infection, though some report none of these problems.84
Autologous reconstruction requires a multistaged approach to (1) harvest the costal cartilage grafts and carve a framework, (2) place the graft, (3) transpose the lobule, and (4) create a postauricular sulcus.73,74,75,76,77,78,85,86,87,88,89 Burt Brent described a four-stage approach,72 which has been modified to three stages using costal cartilage grafts from the synchondrosis of ribs 6 and 7 and the cartilage of rib 8.85 Stage I is performed after age 6 years when the ear has reached 85% of its adult size90 and adequate cartilage is available. A template is traced from the contralateral ear and a pocket is dissected to place the framework in harmony with facial features and symmetrically with the opposite ear. Remnant cartilage is discarded. Stage 2 involves lobule transposition and the surgeon creates a postauricular sulcus with scalp advancement and use of a skin graft in Stage 3.
The Nagata technique involves a two-stage approach.75,76,77,78,91 Patients are not generally treated until age 10 years and chest circumference of at least 60 cm to ensure adequate cartilage is present for an adult-sized construct. Cartilage from ribs 6,7,8, and 9 are harvested in the subperichondrial plane to allow regrowth and minimize donor-site deformity (Fig. 9).In Stage 1, a three-dimensional construct is carved, placed, and the lobule is then transposed (Fig. 10). In stage 2, the construct is elevated using a cartilage graft wrapped in temporoparietal fascia (Fig. 11). This is generally covered with a split skin graft taken from the scalp.
Figure 9.
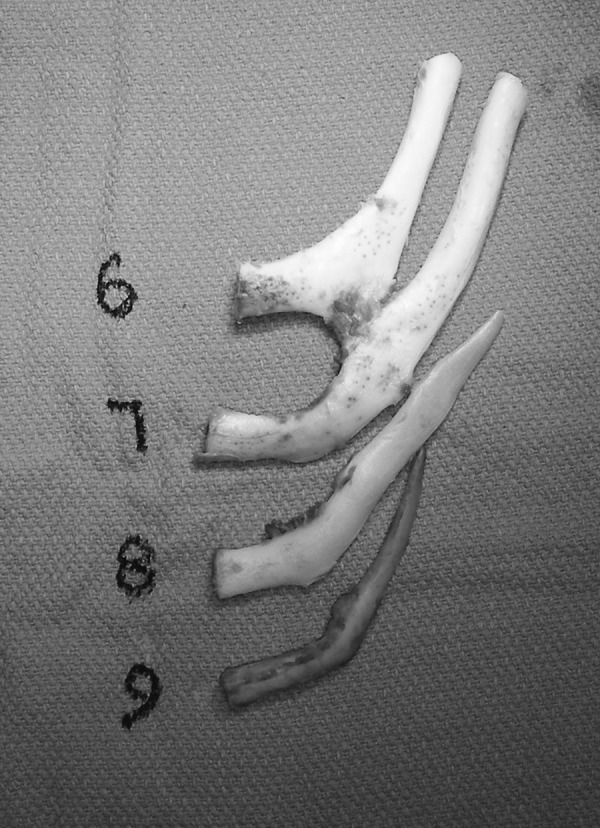
Clinical example of costal cartilage harvested for autologous external ear reconstruction using the Nagata technique.
Figure 10.
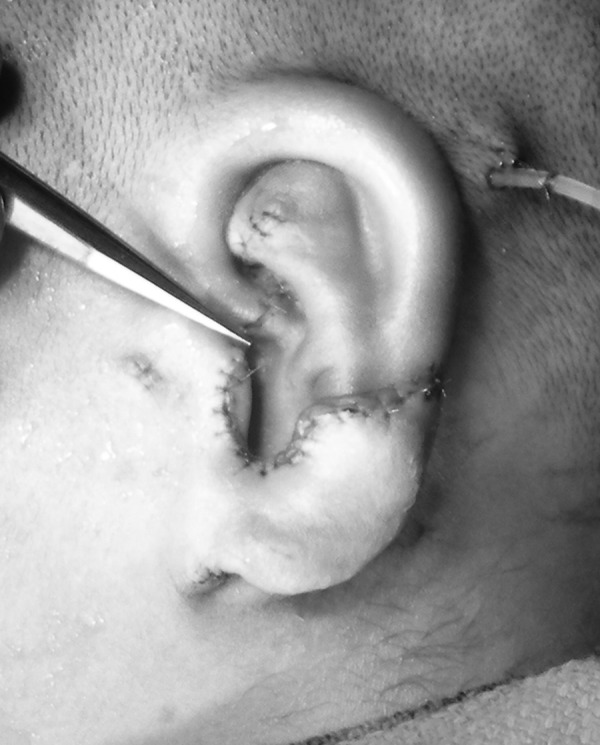
Clinical example of autologous external ear reconstruction in type 3 microtia using the Nagata technique.
Figure 11.
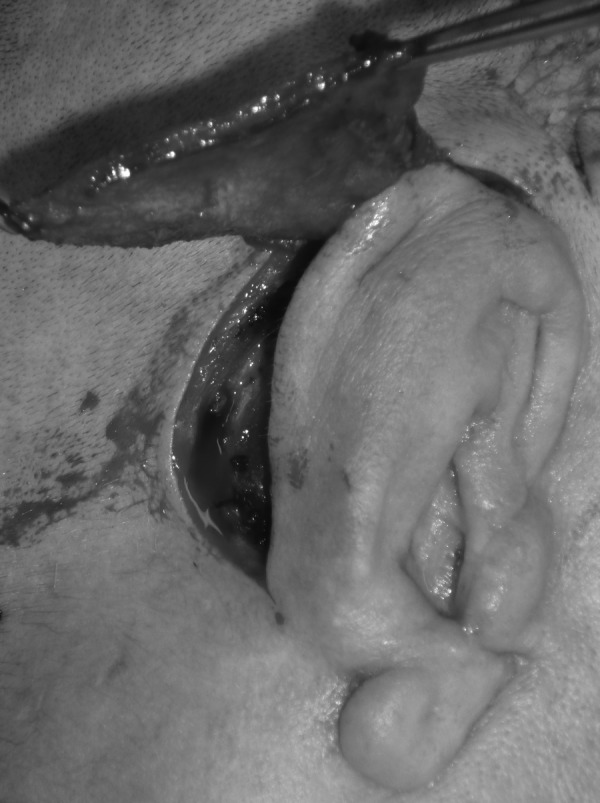
Clinical example of temporoparietal fascia flap for elevation of construct and creation of post-auricular sulcus in second stage autologous external ear reconstruction using the Nagata technique.
Each technique has its strengths and weaknesses. The surgeon and the family must determine the approach that will provide the most natural appearing, symmetric, problem-free, long-term result for the patient.
Nerve
The facial nerve can be affected in CFM to varying degrees. The OMENS classification categorizes loss of nerve function into upper (N71), lower (N72), and total (N73). The hypoglossal (N12) and trigeminal (N5) nerves can also be affected. When the physician identifies facial nerve palsy, she must first determine whether the patient can protect and lubricate his cornea. If not, eye drops, lubricant, or a surgical procedure such as a tarsorrhaphy or gold weight with eyelid tendon sling should be considered. Exposure keratitis of the cornea can lead to permanent blindness.
A facial reanimation procedure should be considered for a patient who cannot move his or her mouth due to deficiencies of the buccal and marginal mandibular branches. Nerve transfer procedures are not effective because there are no motor endplates to reinnervate. Rather, a functioning muscle must be transferred. The temporalis muscle can be detached from the coronoid and advanced to the commissure92 or flipped over with a fascial extension to provide movement of the lateral mouth (Fig. 12).93 Benefits of this approach include ease of surgery, ease of recovery, and reliability of establishing movement. Criticisms include weak strength of pull, limitations on vector of pull, and the need to activate cranial nerve V to stimulate a smile.
Figure 12.
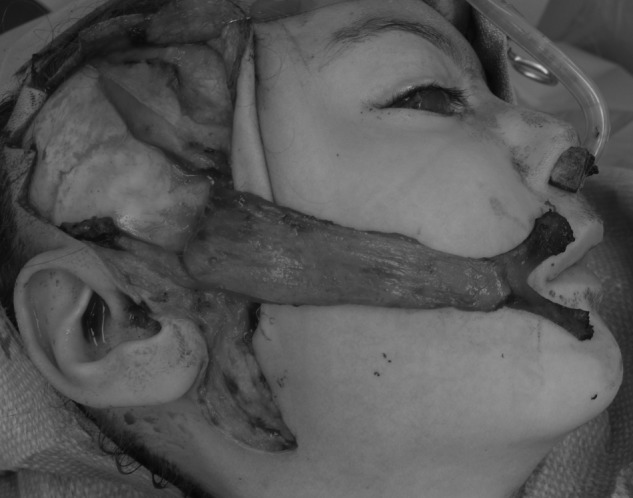
Clinical example of minitemporalis flap for facial reanimation.
Facial reanimation can also be accomplished using a two-stage approach with a cross-face nerve graft and muscle free flap. In the first stage, a sural nerve graft is harvested and attached to the cut end of a redundant buccal branch on the functioning side. The graft is then passed to the upper lip where it sits until axonal ingrowth has occurred (~6 months at a rate of 1 mm/d). In stage 2, the nerve is biopsied to confirm the presence of myelin; then a muscle free flap is harvested and brought to the face (Fig. 13). The muscle is attached to the (1) commissure, (2) upper lip, (3) lower lip, and (4) the zygomatic arch in a direction that mimics the smile vector on the contralateral side (Fig. 14). Microscopic anastomosis of the vein, artery, and nerve are then performed.94 This approach allows for creation of a spontaneous smile in a vector more closely resembling the unaffected side. Drawbacks include length of surgery, recovery time, and need for microsurgical skills.
Figure 13.

Example of a partial gracilis muscle free flap harvested in preparation for transfer to the face in a facial reanimation procedure.
Figure 14.
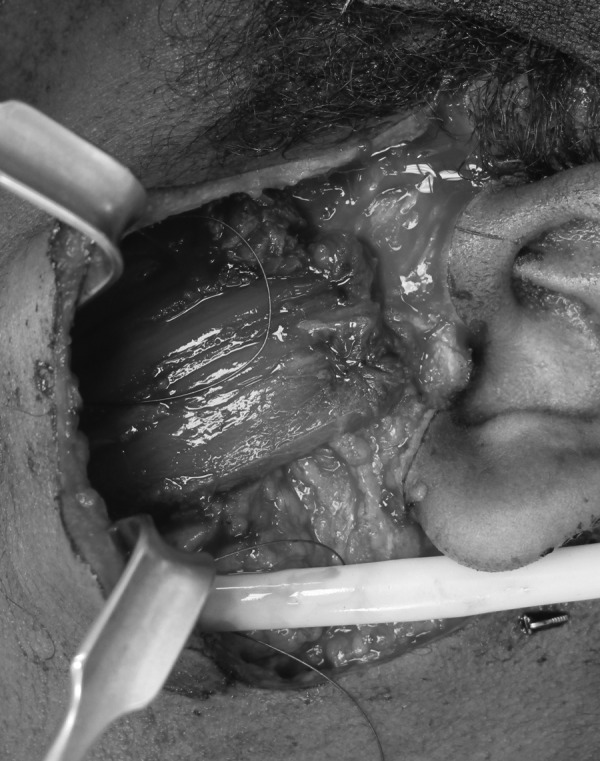
Example of gracilis muscle free flap in situ demonstrating vector of pull and resting tension applied once muscle flap is inset.
The timing of reanimation surgery must be based on the patient's needs and planned around other surgical interventions. It is preferable to perform microtia reconstruction and major craniofacial and/or orthognathic surgery prior to undertaking facial reanimation surgery.
Soft Tissue
Soft tissue deficiency in CFM can be associated with orofacial clefting, deficiency of musculature, and/or a lack of subcutaneous fat and skin. Clefts of the lip and/or commissure (Tessier VII clefts) are typically repaired in infancy to increase feeding efficiency. Other soft tissue deficiencies may become apparent with growth of the maxilla, mandible, and masticatory muscles. The reconstructive techniques described below can be used for a patient with a soft tissue deficiency in isolation or with underlying bony asymmetry.
Free Flap
An adipofascial free flap is the best way to provide a large amount of soft tissue in a single surgical procedure for patients with severe deficiencies.95,96 Free flap selection includes scapula, parascapular,97 groin,98,99 omentum,100 anterolateral thigh (ALT),101 and deep inferior epigastric perforator (DIEP) among others. Because adipofascial free flap transfer can provide such augmentation, it may be necessary to follow this with a debulking procedure.102 Other drawbacks include donor-site morbidity and scarring, length of procedure, and the need for microsurgical skills. This approach is generally performed after the skeletal anomalies of CFM have been corrected.
Dermal Fat Graft
Soft tissue augmentation with dermal fat grafts is another, well proven technique. Dermal fat grafts can provide adequate bulk in moderate and mild deformities, but are prone to some degree of resorption and patients may require additional augmentations.102 Donor site morbidity and scarring are also risks, and the selected donor site should not be constrained by vascular anatomy or angiosomes to make it easier to conceal scars.
Structural Fat Graft
Structural fat grafts have revolutionized the way many conditions are treated, including CFM. This technique requires (1) fat harvest from the abdomen, flanks, thighs, or buttocks; (2) purification, and then (3) injection of small aliquots (<0.1cc) in multiple planes within the areas of facial deficiency. The benefits of a microfat injection are precision of delivery, minimal scarring, and minimal donor-site morbidity. Additionally, the small aliquots do not disrupt the connecting ligaments of the face, so the fat is less likely to droop or disrupt normal facial movement. Some report improvement in the texture and appearance of the overlying skin. The downside of this technique is resorption. One can expect 30 to 80% of the injected fat to resorb depending on location. This necessitates multiple fat graft sessions. Our preference is to coordinate these treatments with other procedures throughout childhood to minimize recovery and provide improvement in facial symmetry during the developmental years of school age and adolescence.
Conclusion
Craniofacial microsomia includes a wide spectrum of anomalies that involve structures of the head and face. Individual treatment plans are based on the patients' needs with consideration to airway, feeding, growth, hearing, speech, development, and quality of life. Communication and care coordination are required to provide patients with CFM timely care with optimal long-term results. The multidisciplinary craniofacial team is uniquely qualified to provide such care.
References
- 1.Poswillo D. The aetiology and pathogenesis of craniofacial deformity. Development. 1988;103(Suppl):207–212. doi: 10.1242/dev.103.Supplement.207. [DOI] [PubMed] [Google Scholar]
- 2.Grabb W C. The first and second branchial arch syndrome. Plast Reconstr Surg. 1965;36(5):485–508. doi: 10.1097/00006534-196511000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bennun R D, Mulliken J B, Kaban L B, Murray J E. Microtia: a microform of hemifacial microsomia. Plast Reconstr Surg. 1985;76(6):859–865. [PubMed] [Google Scholar]
- 4.Rollnick B R, Kaye C I. Hemifacial microsomia and variants: pedigree data. Am J Med Genet. 1983;15(2):233–253. doi: 10.1002/ajmg.1320150207. [DOI] [PubMed] [Google Scholar]
- 5.Stark R B, Saunders D E. The first branchial syndrome. The oral-mandibular-auricular syndrome. Plast Reconstr Surg Transplant Bull. 1962;29:229–239. doi: 10.1097/00006534-196203000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Converse J M, Coccaro P J, Becker M, Wood-Smith D. On hemifacial microsomia. The first and second branchial arch syndrome. Plast Reconstr Surg. 1973;51(3):268–279. [PubMed] [Google Scholar]
- 7.Poswillo D. The pathogenesis of the first and second branchial arch syndrome. Oral Surg Oral Med Oral Pathol. 1973;35(3):302–328. doi: 10.1016/0030-4220(73)90070-4. [DOI] [PubMed] [Google Scholar]
- 8.Werler M M, Sheehan J E, Hayes C, Mitchell A A, Mulliken J B. Vasoactive exposures, vascular events, and hemifacial microsomia. Birth Defects Res A Clin Mol Teratol. 2004;70(6):389–395. doi: 10.1002/bdra.20022. [DOI] [PubMed] [Google Scholar]
- 9.Werler M M, Sheehan J E, Hayes C, Padwa B L, Mitchell A A, Mulliken J B. Demographic and reproductive factors associated with hemifacial microsomia. Cleft Palate Craniofac J. 2004;41(5):494–50. doi: 10.1597/03-110.1. [DOI] [PubMed] [Google Scholar]
- 10.Taysi K, Marsh J L, Wise D M. Familial hemifacial microsomia. Cleft Palate J. 1983;20(1):47–53. [PubMed] [Google Scholar]
- 11.Juriloff D M, Harris M J, Froster-Iskenius U. Hemifacial deficiency induced by a shift in dominance of the mouse mutation far: a possible genetic model for hemifacial microsomia. J Craniofac Genet Dev Biol. 1987;7(1):27–44. [PubMed] [Google Scholar]
- 12.Poonawalla H H, Kaye C I, Rosenthal I M, Pruzansky S. Hemifacial microsomia in a patient with Klinefelter syndrome. Cleft Palate J. 1980;17(3):194–196. [PubMed] [Google Scholar]
- 13.Kaye C I, Rollnick B R, Pruzansky S. Malformations of the auricle: isolated and in syndromes. IV. Cumulative pedigree data. Birth Defects Orig Artic Ser. 1979;15(5C):163–169. [PubMed] [Google Scholar]
- 14.Smahel Z. Craniofacial changes in hemifacial microsomia. J Craniofac Genet Dev Biol. 1986;6(2):151–170. [PubMed] [Google Scholar]
- 15.Padwa B L, Bruneteau R J, Mulliken J B. Association between “plagiocephaly” and hemifacial microsomia. Am J Med Genet. 1993;47(8):1202–1207. doi: 10.1002/ajmg.1320470815. [DOI] [PubMed] [Google Scholar]
- 16.Fryns J P, Lemaire J, Timmermans J, Soekarman D, den Berghe H Van. The association of hemifacial microsomia, homolateral micro/anophthalmos, hemihypotrophy, dental anomalies, submucous cleft palate, CNS malformations and hypopigmented skin lesions following Blaschko's lines in two unrelated female patients. Further evidence for a lethal mutation surviving in mosaic form in “hypomelanosis of Ito”. Genet Couns. 1993;4(1):63–67. [PubMed] [Google Scholar]
- 17.Beals R K, Robbins J R, Rolfe B. Anomalies associated with vertebral malformations. Spine. 1993;18(10):1329–1332. doi: 10.1097/00007632-199308000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Duncan P A, Shapiro L R. Interrelationships of the hemifacial microsomia-VATER, VATER, and sirenomelia phenotypes. Am J Med Genet. 1993;47(1):75–84. doi: 10.1002/ajmg.1320470116. [DOI] [PubMed] [Google Scholar]
- 19.Horgan J E, Padwa B L, LaBrie R A, Mulliken J B. OMENS-Plus: analysis of craniofacial and extracraniofacial anomalies in hemifacial microsomia. Cleft Palate Craniofac J. 1995;32(5):405–412. doi: 10.1597/1545-1569_1995_032_0405_opaoca_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 20.Caldarelli D D, Hutchinson J C, Gould H J. Hemifacial microsomia: priorities and sequence of comprehensive otologic management. Cleft Palate J. 1980;17(2):111–115. [PubMed] [Google Scholar]
- 21.Dellon A L, Claybaugh G J, Hoopes J E. Hemipalatal palsy and microtia. Ann Plast Surg. 1983;10(6):475–479. doi: 10.1097/00000637-198306000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Feingold M, Baum J. Goldenhar's syndrome. Am J Dis Child. 1978;132(2):136–138. doi: 10.1001/archpedi.1978.02120270034006. [DOI] [PubMed] [Google Scholar]
- 23.Pruzansky S. Not all dwarfed mandibles are alike. Birth Defects. 1969;1:120–129. [Google Scholar]
- 24.Kaban L B, Moses M H, Mulliken J B. Surgical correction of hemifacial microsomia in the growing child. Plast Reconstr Surg. 1988;82(1):9–19. [PubMed] [Google Scholar]
- 25.Kaban L B, Mulliken J B, Murray J E. Three-dimensional approach to analysis and treatment of hemifacial microsomia. Cleft Palate J. 1981;18(2):90–99. [PubMed] [Google Scholar]
- 26.Steinbacher D M, Gougoutas A, Bartlett S P. An analysis of mandibular volume in hemifacial microsomia. Plast Reconstr Surg. 2011;127(6):2407–2412. doi: 10.1097/PRS.0b013e3182131cc8. [DOI] [PubMed] [Google Scholar]
- 27.Converse J M, Wood-Smith D, McCarthy J G, Coccaro P J, Becker M H. Bilateral facial microsomia. Diagnosis, classification, treatment. Plast Reconstr Surg. 1974;54(4):413–423. doi: 10.1097/00006534-197410000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Longacre J J. The surgical management of the first and second branchial arch syndrome. Br J Plast Surg. 1965;18:243–253. doi: 10.1016/s0007-1226(65)80044-3. [DOI] [PubMed] [Google Scholar]
- 29.Figueroa A A, Pruzansky S. The external ear, mandible and other components of hemifacial microsomia. J Maxillofac Surg. 1982;10(4):200–211. doi: 10.1016/s0301-0503(82)80042-8. [DOI] [PubMed] [Google Scholar]
- 30.Lauritzen C, Munro I R, Ross R B. Classification and treatment of hemifacial microsomia. Scand J Plast Reconstr Surg. 1985;19(1):33–39. doi: 10.3109/02844318509052863. [DOI] [PubMed] [Google Scholar]
- 31.David D J, Mahatumarat C, Cooter R D. Hemifacial microsomia: a multisystem classification. Plast Reconstr Surg. 1987;80(4):525–535. doi: 10.1097/00006534-198710000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Vento A R LaBrie R A Mulliken J B The O.M.E.N.S. classification of hemifacial microsomia Cleft Palate Craniofac J 199128168–76., discussion 77 [DOI] [PubMed] [Google Scholar]
- 33.Gougoutas A J, Singh D J, Low D W, Bartlett S P. Hemifacial microsomia: clinical features and pictographic representations of the OMENS classification system. Plast Reconstr Surg. 2007;120(7):112e–120e. doi: 10.1097/01.prs.0000287383.35963.5e. [DOI] [PubMed] [Google Scholar]
- 34.Birgfeld C B, Luquetti D V, Gougoutas A J. et al. A phenotypic assessment tool for craniofacial microsomia. Plast Reconstr Surg. 2011;127(1):313–320. doi: 10.1097/PRS.0b013e3181f95d15. [DOI] [PubMed] [Google Scholar]
- 35.Moss M L, Rankow R M. The role of the functional matrix in mandibular growth. Angle Orthod. 1968;38(2):95–103. doi: 10.1043/0003-3219(1968)038<0095:TROTFM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Hertle R W, Quinn G E, Katowitz J A. Ocular and adnexal findings in patients with facial microsomias. Ophthalmology. 1992;99(1):114–119. doi: 10.1016/s0161-6420(92)32029-9. [DOI] [PubMed] [Google Scholar]
- 37.Nevares R L, Mulliken J B, Robb R M. Ocular dermoids. Plast Reconstr Surg. 1988;82(6):959–964. doi: 10.1097/00006534-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Ross R B. Costochondral grafts replacing the mandibular condyle. Cleft Palate Craniofac J. 1999;36(4):334–339. doi: 10.1597/1545-1569_1999_036_0334_cgrtmc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 39.Guyuron B Lasa C I Unpredictable growth pattern of costochondral graft Plast Reconstr Surg 1992905880–886., discussion 887–889 [PubMed] [Google Scholar]
- 40.Figueroa A A, Gans B J, Pruzansky S. Long-term follow-up of a mandibular costochondral graft. Oral Surg Oral Med Oral Pathol. 1984;58(3):257–268. doi: 10.1016/0030-4220(84)90050-1. [DOI] [PubMed] [Google Scholar]
- 41.Perrott D H, Umeda H, Kaban L B. Costochondral graft construction/reconstruction of the ramus/condyle unit: long-term follow-up. Int J Oral Maxillofac Surg. 1994;23(6 Pt 1):321–328. doi: 10.1016/s0901-5027(05)80046-3. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Blake F, Heiland M, Schmelzle R, Pohlenz P. Long-term evaluation after mandibular reconstruction with fibular grafts versus microsurgical fibular flaps. J Oral Maxillofac Surg. 2007;65(2):281–286. doi: 10.1016/j.joms.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Warren S M, Borud L J, Brecht L E, Longaker M T, Siebert J W. Microvascular reconstruction of the pediatric mandible. Plast Reconstr Surg. 2007;119(2):649–661. doi: 10.1097/01.prs.0000246482.36624.bd. [DOI] [PubMed] [Google Scholar]
- 44.Santamaría E, Morales C, Taylor J A, Hay A, Ortiz-Monasterio F. Mandibular microsurgical reconstruction in patients with hemifacial microsomia. Plast Reconstr Surg. 2008;122(6):1839–1849. doi: 10.1097/PRS.0b013e31818cc349. [DOI] [PubMed] [Google Scholar]
- 45.Ilizarov G A, Devyatov A A, Kamerin V K. Plastic reconstruction of longitudinal bone defects by means of compression and subsequent distraction. Acta Chir Plast. 1980;22(1):32–41. [PubMed] [Google Scholar]
- 46.Ilizarov G. Kurgan USSR: Kurgan Regional Scientific Medical Society; 1954. A new principle of osteosynthesis with the use of crossing pins and rings. In: Collection of Scientific Works of the Kurgan Regional Scientific Medical Society; pp. 145–160. [Google Scholar]
- 47.Snyder C C, Levine G A, Swanson H M, Browne E Z. Mandibular lengthening by gradual distraction. Preliminary report. Plast Reconstr Surg. 1973;51(5):506–508. doi: 10.1097/00006534-197305000-00003. [DOI] [PubMed] [Google Scholar]
- 48.McCarthy J G Schreiber J Karp N Thorne C H Grayson B H Lengthening the human mandible by gradual distraction Plast Reconstr Surg 19928911–8., discussion 9–10 [PubMed] [Google Scholar]
- 49.Karp N S, Thorne C H, McCarthy J G, Sissons H A. Bone lengthening in the craniofacial skeleton. Ann Plast Surg. 1990;24(3):231–237. doi: 10.1097/00000637-199003000-00007. [DOI] [PubMed] [Google Scholar]
- 50.McCarthy J G. The role of distraction osteogenesis in the reconstruction of the mandible in unilateral craniofacial microsomia. Clin Plast Surg. 1994;21(4):625–631. [PubMed] [Google Scholar]
- 51.Cakir-Ozkan N, Eyibilen A, Ozkan F, Ozyurt B, Aslan H. Stereologic analysis of bone produced by distraction osteogenesis or autogenous bone grafting in mandible. J Craniofac Surg. 2010;21(3):735–740. doi: 10.1097/SCS.0b013e3181d7a49c. [DOI] [PubMed] [Google Scholar]
- 52.Roth D A Gosain A K McCarthy J G Stracher M A Lefton D R Grayson B H A CT scan technique for quantitative volumetric assessment of the mandible after distraction osteogenesis Plast Reconstr Surg 19979951237–1247., discussion 1248–1250 [DOI] [PubMed] [Google Scholar]
- 53.Fisher E, Staffenberg D A, McCarthy J G, Miller D C, Zeng J. Histopathologic and biochemical changes in the muscles affected by distraction osteogenesis of the mandible. Plast Reconstr Surg. 1997;99(2):366–371. doi: 10.1097/00006534-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Mackool R J, Hopper R A, Grayson B H, Holliday R, McCarthy J G. Volumetric change of the medial pterygoid following distraction osteogenesis of the mandible: an example of the associated soft-tissue changes. Plast Reconstr Surg. 2003;111(6):1804–1807. doi: 10.1097/01.PRS.0000055431.19215.0A. [DOI] [PubMed] [Google Scholar]
- 55.McCarthy J G, Katzen J T, Hopper R, Grayson B H. The first decade of mandibular distraction: lessons we have learned. Plast Reconstr Surg. 2002;110(7):1704–1713. doi: 10.1097/01.PRS.0000036260.60746.1B. [DOI] [PubMed] [Google Scholar]
- 56.Strijen P J van, Breuning K H, Becking A G, Tuinzing D B. Stability after distraction osteogenesis to lengthen the mandible: results in 50 patients. J Oral Maxillofac Surg. 2004;62(3):304–307. doi: 10.1016/j.joms.2003.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Hollier L H, Rowe N M, Mackool R J. et al. Controlled multiplanar distraction of the mandible. Part III: Laboratory studies of sagittal (anteroposterior) and horizontal (mediolateral) movements. J Craniofac Surg. 2000;11(2):83–95. doi: 10.1097/00001665-200011020-00004. [DOI] [PubMed] [Google Scholar]
- 58.Williams J K, Rowe N M, Mackool R J. et al. Controlled multiplanar distraction of the mandible, Part II: Laboratory studies of sagittal (anteroposterior) and vertical (superoinferior) movements. J Craniofac Surg. 1998;9(6):504–513. doi: 10.1097/00001665-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Singh D J, Glick P H, Bartlett S P. Mandibular deformities: single-vector distraction techniques for a multivector problem. J Craniofac Surg. 2009;20(5):1468–1472. doi: 10.1097/SCS.0b013e3181b09ab2. [DOI] [PubMed] [Google Scholar]
- 60.Grayson B H McCormick S Santiago P E McCarthy J G Vector of device placement and trajectory of mandibular distraction J Craniofac Surg 199786473–480., discussion 481–482 [DOI] [PubMed] [Google Scholar]
- 61.Kitai N, Murakami S, Takashima M, Furukawa S, Kreiborg S, Takada K. Evaluation of temporomandibular joint in patients with hemifacial microsomia. Cleft Palate Craniofac J. 2004;41(2):157–162. doi: 10.1597/02-108. [DOI] [PubMed] [Google Scholar]
- 62.Padwa B L Zaragoza S M Sonis A L Proximal segment displacement in mandibular distraction osteogenesis J Craniofac Surg 2002132293–296., discussion 297 [DOI] [PubMed] [Google Scholar]
- 63.Molina F Ortiz Monasterio F Mandibular elongation and remodeling by distraction: a farewell to major osteotomies Plast Reconstr Surg 1995964825–840., discussion 841–842 [PubMed] [Google Scholar]
- 64.McCarthy J G, Hopper R A, Hollier L H, Peltomaki T, Katzen T, Grayson B H. Molding of the regenerate in mandibular distraction: clinical experience. Plast Reconstr Surg. 2003;112(5):1239–1246. doi: 10.1097/01.PRS.0000080726.50460.3E. [DOI] [PubMed] [Google Scholar]
- 65.Rachmiel A, Manor R, Peled M, Laufer D. Intraoral distraction osteogenesis of the mandible in hemifacial microsomia. J Oral Maxillofac Surg. 2001;59(7):728–733. doi: 10.1053/joms.2001.24280. [DOI] [PubMed] [Google Scholar]
- 66.Burstein F D. Resorbable distraction of the mandible: technical evolution and clinical experience. J Craniofac Surg. 2008;19(3):637–643. doi: 10.1097/SCS.0b013e31816b6c8f. [DOI] [PubMed] [Google Scholar]
- 67.Burstein F D, Williams J K, Hudgins R. et al. Single-stage craniofacial distraction using resorbable devices. J Craniofac Surg. 2002;13(6):776–782. doi: 10.1097/00001665-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 68.Murray D J, Chong D K, Sándor G K, Forrest C R. Dentigerous cyst after distraction osteogenesis of the mandible. J Craniofac Surg. 2007;18(6):1349–1352. doi: 10.1097/scs.0b013e3181506699. [DOI] [PubMed] [Google Scholar]
- 69.Stelnicki E J Hollier L Lee C Lin W Y Grayson B McCarthy J G Distraction osteogenesis of costochondral bone grafts in the mandible Plast Reconstr Surg 20021093925–933., discussion 934–935 [DOI] [PubMed] [Google Scholar]
- 70.Eski M, Turegun M, Deveci M, Gokce H S, Sengezer M. Vertical distraction osteogenesis of fibular bone flap in reconstructed mandible. Ann Plast Surg. 2006;57(6):631–636. doi: 10.1097/01.sap.0000235452.87390.d6. [DOI] [PubMed] [Google Scholar]
- 71.Ortiz Monasterio F, Molina F, Andrade L, Rodriguez C, Sainz Arregui J. Simultaneous mandibular and maxillary distraction in hemifacial microsomia in adults: avoiding occlusal disasters. Plast Reconstr Surg. 1997;100(4):852–861. doi: 10.1097/00006534-199709001-00005. [DOI] [PubMed] [Google Scholar]
- 72.Brent B. The correction of microtia with autogenous cartilage grafts: I. The classic deformity? Plast Reconstr Surg. 1980;66(1):1–12. doi: 10.1097/00006534-198007000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Brent B. The correction of microtia with autogenous cartilage grafts: II. Atypical and complex deformities. Plast Reconstr Surg. 1980;66(1):13–21. doi: 10.1097/00006534-198007000-00002. [DOI] [PubMed] [Google Scholar]
- 74.Brent B Auricular repair with autogenous rib cartilage grafts: two decades of experience with 600 cases Plast Reconstr Surg 1992903355–374., discussion 375–376 [PubMed] [Google Scholar]
- 75.Nagata S Modification of the stages in total reconstruction of the auricle: Part IV. Ear elevation for the constructed auricle Plast Reconstr Surg 1994932254–266., discussion 267–268 [PubMed] [Google Scholar]
- 76.Nagata S Modification of the stages in total reconstruction of the auricle: Part III. Grafting the three-dimensional costal cartilage framework for small concha-type microtia Plast Reconstr Surg 1994932243–253., discussion 267–268 [PubMed] [Google Scholar]
- 77.Nagata S Modification of the stages in total reconstruction of the auricle: Part II. Grafting the three-dimensional costal cartilage framework for concha-type microtia Plast Reconstr Surg 1994932231–242., discussion 267–268 [PubMed] [Google Scholar]
- 78.Nagata S Modification of the stages in total reconstruction of the auricle: Part I. Grafting the three-dimensional costal cartilage framework for lobule-type microtia Plast Reconstr Surg 1994932221–230., discussion 267–268 [PubMed] [Google Scholar]
- 79.Meurman Y. Congenital microtia and meatal atresia; observations and aspects of treatment. AMA Arch Otolaryngol. 1957;66(4):443–463. doi: 10.1001/archotol.1957.03830280073008. [DOI] [PubMed] [Google Scholar]
- 80.Bauer B S. Reconstruction of the microtic ear. J Pediatr Surg. 1984;19(4):440–445. doi: 10.1016/s0022-3468(84)80271-7. [DOI] [PubMed] [Google Scholar]
- 81.Lynch J B, Pousti A, Doyle J E, Lewis S R. Our experiences with silastic ear implants. Plast Reconstr Surg. 1972;49(3):283–285. doi: 10.1097/00006534-197203000-00007. [DOI] [PubMed] [Google Scholar]
- 82.Reinisch J F, Lewin S. Ear reconstruction using a porous polyethylene framework and temporoparietal fascia flap. Facial Plast Surg. 2009;25(3):181–189. doi: 10.1055/s-0029-1239448. [DOI] [PubMed] [Google Scholar]
- 83.Wellisz T. Clinical experience with the Medpor porous polyethylene implant. Aesthetic Plast Surg. 1993;17(4):339–344. doi: 10.1007/BF00437109. [DOI] [PubMed] [Google Scholar]
- 84.Yang S L, Zheng J H, Ding Z, Liu Q Y, Mao G Y, Jin Y P. Combined fascial flap and expanded skin flap for enveloping Medpor framework in microtia reconstruction. Aesthetic Plast Surg. 2009;33(4):518–522. doi: 10.1007/s00266-008-9249-0. [DOI] [PubMed] [Google Scholar]
- 85.Brent B Microtia repair with rib cartilage grafts: a review of personal experience with 1000 cases Clin Plast Surg 2002292257–271., vii vii. [DOI] [PubMed] [Google Scholar]
- 86.Cho B C, Kim J Y, Byun J S. Two-stage reconstruction of the auricle in congenital microtia using autogenous costal cartilage. J Plast Reconstr Aesthet Surg. 2007;60(9):998–1006. doi: 10.1016/j.bjps.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 87.Cho B C, Lee S H. Surgical results of two-stage reconstruction of the auricle in congenital microtia using an autogenous costal cartilage alone or combined with canaloplasty. Plast Reconstr Surg. 2006;117(3):936–947. doi: 10.1097/01.prs.0000200612.62079.59. [DOI] [PubMed] [Google Scholar]
- 88.Pan B, Jiang H, Guo D, Huang C, Hu S, Zhuang H. Microtia: ear reconstruction using tissue expander and autogenous costal cartilage. J Plast Reconstr Aesthet Surg. 2008;61 01:S98–S103. doi: 10.1016/j.bjps.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 89.Firmin F. [Auricular reconstruction in cases of microtia. Principles, methods and classification] Ann Chir Plast Esthet. 2001;46(5):447–466. doi: 10.1016/s0294-1260(01)00056-5. [DOI] [PubMed] [Google Scholar]
- 90.Farkas L G. Anthropometry of normal and anomalous ears. Clin Plast Surg. 1978;5(3):401–412. [PubMed] [Google Scholar]
- 91.Nagata S. A new method of total reconstruction of the auricle for microtia. Plast Reconstr Surg. 1993;92(2):187–201. doi: 10.1097/00006534-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 92.Sidle D M, Fishman A J. Modification of the orthodromic temporalis tendon transfer technique for reanimation of the paralyzed face. Otolaryngol Head Neck Surg. 2011;145(1):18–23. doi: 10.1177/0194599811403895. [DOI] [PubMed] [Google Scholar]
- 93.May M Drucker C Temporalis muscle for facial reanimation. A 13-year experience with 224 procedures Arch Otolaryngol Head Neck Surg 19931194378–382., discussion 383–384 [DOI] [PubMed] [Google Scholar]
- 94.Birgfeld C, Neligan P N. Surgical approaches to facial nerve deficits. Skull Base. 2011;21(3):177–184. doi: 10.1055/s-0031-1275252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Longaker M T, Siebert J W. Microsurgical correction of facial contour in congenital craniofacial malformations: the marriage of hard and soft tissue. Plast Reconstr Surg. 1996;98(6):942–950. doi: 10.1097/00006534-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 96.Siebert J W, Anson G, Longaker M T. Microsurgical correction of facial asymmetry in 60 consecutive cases. Plast Reconstr Surg. 1996;97(2):354–363. doi: 10.1097/00006534-199602000-00013. [DOI] [PubMed] [Google Scholar]
- 97.Siebert J W, Longaker M T, Angrigiani C. The inframammary extended circumflex scapular flap: an aesthetic improvement of the parascapular flap. Plast Reconstr Surg. 1997;99(1):70–77. doi: 10.1097/00006534-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 98.Anderl H. Free vascularized groin fat flap in hypoplasia and hemiatrophy of the face (a three years observation) J Maxillofac Surg. 1979;7(4):327–332. doi: 10.1016/s0301-0503(79)80059-4. [DOI] [PubMed] [Google Scholar]
- 99.Cooper T M, Lewis N, Baldwin M A. Free groin flap revisited. Plast Reconstr Surg. 1999;103(3):918–924. doi: 10.1097/00006534-199903000-00022. [DOI] [PubMed] [Google Scholar]
- 100.Jurkiewicz M J, Nahai F. The omentum: its use as a free vascularized graft for reconstruction of the head and neck. Ann Surg. 1982;195(6):756–765. doi: 10.1097/00000658-198206000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tuncali D, Baser N T, Terzioglu A, Aslan G. Romberg's disease associated with Horner's syndrome: contour restoration by a free anterolateral thigh perforator flap and ancillary procedures. Plast Reconstr Surg. 2007;120(5):67e–72e. doi: 10.1097/01.prs.0000279325.32286.7a. [DOI] [PubMed] [Google Scholar]
- 102.Mordick T G, Larossa D, Whitaker L. Soft-tissue reconstruction of the face: a comparison of dermal-fat grafting and vascularized tissue transfer. Ann Plast Surg. 1992;29(5):390–396. doi: 10.1097/00000637-199211000-00002. [DOI] [PubMed] [Google Scholar]