Abstract
Lymphatic vessels serve as a route by which interstitial fluid, protein and other macromolecules are returned to the blood circulation and immune cells and antigens gain access to lymph nodes. Lymph flow is an active process promoted by rhythmical contraction–relaxation events occurring in the collecting lymphatic vessels. This lymphatic pumping is an intrinsic property of the lymphatic muscles in the vessel wall and consequent to action potentials. Compromised lymphatic pumping may affect lymph and immune cell transport, an action which could be particularly detrimental during inflammation. Importantly, many inflammatory mediators alter lymphatic pumping. Vasoactive intestinal peptide (VIP) is a neuro- and immuno-modulator thought to be released by nerve terminals and immune cells in close proximity to lymphatic vessels. We demonstrated the presence of the peptide in lymphatic vessels and in the lymph and examined the effects of VIP on mesenteric collecting lymphatic vessels of the guinea pig using pharmacological bioassays, intracellular microelectrode electrophysiology, immunofluorescence and quantitative real-time PCR. We showed that VIP alters lymphatic pumping by decreasing the frequency of lymphatic contractions and hyperpolarizing the lymphatic muscle membrane potential in a concentration-dependent manner. Our data further suggest that these effects are mainly mediated by stimulation of the VIP receptor VPAC2 located on the lymphatic muscle and the downstream involvement of protein kinase A (PKA) and ATP-sensitive K+ (KATP) channels. Inhibition of lymphatic pumping by VIP may compromise lymph drainage, oedema resolution and immune cell trafficking to the draining lymph nodes.
Key points
Lymphatic pumping is characterized by the ability of collecting lymphatic vessels to contract in a phasic manner to propel lymph. This activity is critical for tissue fluid homeostasis and immune cell transport to lymph nodes.
Vasoactive intestinal peptide (VIP) is a neuro-immuno-modulator with anti-inflammatory properties released by peptidergic nerves and by inflammatory cells patrolling the interstitium and lymph.
Here we report that VIP is present in lymphatic vessels as well as in the lymph and that it potently inhibits lymphatic pumping and hyperpolarizes the lymphatic muscle via stimulation of VPAC2 VIP receptors, activation of protein kinase A and opening of ATP-sensitive K+ channels.
These results suggest an important role for VIP in inhibiting lymphatic pumping. This process might become critical during inflammation, where it would lead to decreased lymph drainage, oedema formation and compromised immune cell trafficking.
Introduction
The propulsion of lymph is mediated in part by lymphatic pumping, an intrinsic property of the lymphatic muscle, characterized by a rhythmic constriction–relaxation cycle of the succession of chambers (i.e. lymphangions) that comprise the collecting lymphatic vessels. Lymphangions contract in a heart-like manner, causing the lymph to flow forward into the next lymphangion across a one-way valve. This mechanism allows excess fluid, proteins, cells and debris to be removed from the interstitium, propelled along the lymphatic vessel network and returned back to the blood stream, avoiding swelling and oedema. Studies performed on lymphatic vessels from the guinea pig mesentery have indicated that lymphatic pumping is initiated by a pacemaker mechanism characterized by excitatory electrical events termed spontaneous transient depolarizations (STDs; van Helden, 1993). Large-amplitude STDs, or spatio-temporal summation of sub-threshold events, generate pacemaker potentials, which trigger action potentials and resultant lymphatic muscle contractions (van Helden, 1993; Imtiaz et al. 2007; von der Weid et al. 2008). STDs are generated by a synchronized release of Ca2+, through IP3 receptors in the sarcoplasmic reticulum, causing the opening of Ca2+-activated chloride channels (von der Weid et al. 2008).
Impairment of the lymphatic pump has detrimental consequences leading to profound swelling and oedema, as observed in lymphoedema (see Rockson, 2001). Oedema formation also occurs during inflammation as a result of the action of inflammatory mediators on vascular permeability, leading to an elevation of interstitial fluid pressure at the inflamed site. Lymphatic pumping is very sensitive to increases in interstitial fluid pressure, which would explain the increase in lymph flow observed during oedemagenic episodes (Benoit et al. 1989; Benoit & Zawieja, 1992). However, when lymphatic contractility was examined in inflammatory situations such as experimental ileitis (Wu et al. 2006) or peritonitis (Umarova et al. 2006), where a mounting oedema is also expected, pumping of collecting lymphatics in the mesentery was strongly inhibited and vessel diameter was significantly increased. These alterations are thought to impair lymph drainage, though experimental validation is still required. A possible mechanism by which lymphatic pumping is altered during the inflammatory process is via the action of inflammatory mediators, many of which have vasoactive properties and are present in the lymphatic surroundings and/or in the lymph (reviewed in Johnston, 1987; von der Weid, 2001). Indeed, in the study by Wu et al. (2006), we demonstrated a role for metabolites of the cyclooxygenase pathway in the ileitis-induced inhibition of lymphatic pumping.
Among the inflammatory mediators, vasoactive intestinal peptide (VIP) represents an interesting candidate as it may exist in the peptidergic innervation of mesenteric lymphatics (Ohhashi et al. 1983; Guarna et al. 1991), as well as be produced and secreted by immune cells (Delgado et al. 2004; Pozo & Delgado, 2004) patrolling around lymphatic vessels and in the circulating lymph. VIP is a conserved basic 28 amino acid peptide with primary sequences identical in most mammals. It has been reported to exert a wide spectrum of biological actions, including vasodilatation, smooth muscle relaxation and neuro- and immuno-modulatory functions for which it is predominantly considered as a potent anti-inflammatory factor (see reviews by Delgado et al. 2004; Pozo & Delgado, 2004; Gonzalez-Rey & Delgado, 2007). VIP has also been shown to strongly inhibit pumping in bovine mesenteric lymphatics (Ohhashi et al. 1983), though its mode of action has not been elucidated.
The importance of lymphatic pumping in tissue fluid homeostasis and immune cell trafficking, the potent vasodilatory effect of VIP and its potential availability to the lymphatic vessels have prompted us to examine the effect of this neuro-immunomodulator on lymphatic contractile activity and the mechanisms by which it acts. Excerpts of this study have been published in abstract form (von der Weid et al. 2009, 2011).
Methods
Ethical approval
The animal handling and experiments were approved by the University of Calgary Animal Care and Ethics Committee and conformed to the guidelines established by the Canadian Council on Animal Care.
Tissue preparation
Guinea pigs (7–15 days of age) were killed by decapitation during deep anaesthesia induced by inhalation of isofluorane. The small intestine with its attached mesentery was rapidly dissected and placed in a physiological saline solution (PSS) of the following composition (mm): CaCl2, 2.5; KCl, 5; MgCl2, 2; NaCl, 120; NaHCO3, 25; NaH2PO4, 1; glucose, 11. The pH was maintained at 7.4 by constant bubbling with 95% O2–5% CO2. Lymphatic tissue was prepared as previously described (von der Weid et al. 1996; Fox & von der Weid, 2002). Briefly, small collecting lymphatic vessels (diameter <230 μm) from the ileal region were dissected together with their associated artery and vein and left intact within the surrounding mesentery, which was used to pin down the tissues on the Sylgard-coated base of a 2 ml organ bath. This preparation was then used for mRNA extraction and PCR, immunofluorescence, ELISA, contractile activity assessment and electrophysiological membrane potential recording, as described below.
Vessel contraction measurements
The bath containing the lymphatic vessel preparation was mounted on the stage of an inverted microscope and continuously superfused at a flow rate of 3 ml min−1 with PSS heated to 36°C. In order to induce a consistent rate of vessel constriction–relaxation cycle, the vessel lumen was perfused with PSS through a fine glass micropipette inserted into a cut opening of the vessel. The cannula was connected to an infusion pump via Teflon tubing allowing the vessel lumen to be perfused in the direction of the valves at a flow rate of 2.5 μl min−1. This flow rate was very reliable in maintaining a regular rhythmical phasic contractile activity in lymphatic vessels, with contraction frequency usually settling at about 80% of the maximum rate and maintained for the duration of the experiment (typically 3–4 h). Lymphatic vessel chambers were observed by video-microscopy, with diameter changes and contraction frequency continuously measured with a video-dimension analyser (Model V94, Living Systems Instrumentation, Burlington, VT, USA). This device, designed to sense the optical dense wall of the vessel, at a chosen scan line seen on the monitor, followed any change in vessel diameter with a rapid (<20 ms) time resolution. Data were then recorded on a computer via an analog-to-digital converter (PowerLab/4SP, ADInstruments, Mountain View, CA, USA). Preparations were allowed a 30 min equilibration period prior to the first drug application.
Treatments were only performed on vessels with a consistent contraction frequency of at least 5 contractions min−1 during the minimal 30 min equilibrium period. A 5 min control period of contractile activity was recorded prior to a 1 min application of VIP and effects on vessel contraction frequency were assessed during the four subsequent minutes. Decreases in vessel diameter greater than 50% of the diastolic diameter were counted as contractions. Concentration–response curves were obtained by applying increasing concentrations of VIP with a washout period between each application of at least 15 min or the time necessary for the vessel to recover from the previous application. When the effect of inhibitors was examined, the concentration–response curve was constructed with the inhibitor present in the superfusion solution 15 min before application of the first VIP concentration and for the duration of the experiment. Data were expressed as percentage of the mean of the 5 min control period immediately preceding the agonist application. In experiments in which the effects of VIP receptor antagonists were investigated, VIP was tested at a concentration giving 60–70% of maximal effect, first as a control and a second time 30 min later with the antagonist present in the superfusion solution for at least 15 min. This period was considered sufficient, as two successive applications 30 min apart gave responses to the same concentration of agonist that were not significantly different (P = 0.263, n = 9: see Fig. 3D). Time course–frequency histograms were constructed by expressing the number of contractions occurring every minute as a percentage of the mean of the 5 min preceding the treatment.
Figure 4. Pharmacological characterization of VIP receptors involved in the VIP-induced hyperpolarization.
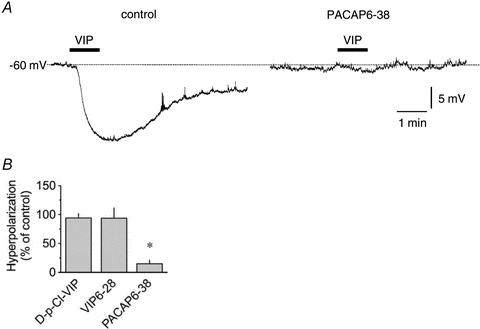
A, original intracellular microelectrode recordings displaying hyperpolarizing response to 100 nm VIP applied for 1 min (horizontal bars) in control conditions and in the presence of 1 μm PACAP6-38. B, summary graph of the effect of 1 μm[D-p-Cl-Phe6,Leu17]-VIP (D-p-Cl-VIP, n = 4), 1 μm VIP-28 (n = 6) and 1 μm PACAP6-38 (n = 5) on hyperpolarization caused by 100 nm VIP. Columns are mean ± SEM expressed as percentage of control VIP hyperpolarization obtained during the same impalement. *P < 0.05 versus control.
Lysis of endothelium
The lymphatic endothelium was damaged in vitro by passing a small stream of air through the lumen of the vessels at a rate of 3.5 μl min−1 as previously described (Gao et al. 1999; Fox & von der Weid, 2002; Chan et al. 2004). The success of the endothelial destruction was confirmed first by the abolition of the endothelium-dependent pumping inhibition to 10 μm acetylcholine and second, at the end of the experiment, by the extent of the vessel lumen staining by a 4% BSA–0.5% Evans blue PBS solution (Moitra et al. 2007). Only experiments where endothelial destruction was proved successful were considered (∼50% of the vessels).
Electrophysiology
Mesenteric preparations were mounted in a small organ bath (volume 100 μl), placed on the stage of an inverted microscope and continuously superfused with PSS heated to 36°C at a flow rate of 3 ml min−1, causing a change-over time of <7 s, as previously described (von der Weid et al. 2001; Fox & von der Weid, 2002). Impalements of lymphatic muscle cells were obtained from the adventitial side of a lymphatic vessel using conventional glass intracellular microelectrodes filled with 0.5 m KCl (resistance 150–250 MΩ). Electrodes were connected to an amplifier (Intra 767, World Precision Instruments, Sarasota, FL, USA) through an Ag–AgCl half-cell. Resting membrane potential was monitored on a digital oscilloscope (VC6525, Hitachi) and simultaneously recorded on a computer via an analog–digital converter (PowerLab/4SP, ADInstruments).
Lymphatic muscle impalements were characterized by a sharp drop in potential that settled after 10–15 s to a value typically more negative than −45 mV. Impalements were maintained for more than 5 min in >90% of the cases and up to 3 h optimally. In experiments where the effects of VIP were studied in the presence of antagonists or inhibitors, VIP was applied first as a control and then, at least 20 min later, in the presence of the antagonist that had been superfused for at least 10 min. This protocol was usually performed during the same impalement. However, in some instances when the impalement was lost, successive impalements were obtained from neighbouring cells in the same segment. In preliminary recordings, no significant difference in the responses was found during successive applications (20 min intervals) of VIP, at the same concentration.
Immunofluorescence
To preserve the shape of the lymphatic vessels, a solution of 2% agarose in RPMI was perfused via the intralumenal cannula at 37°C and the preparation chilled at 4°C until the agarose hardened. Vessels were dissected out, carefully cleaned of fat and connective tissue and fixed for 20 min in formalin (10% in PBS) at 37°C. They were then permeabilized for 5 min in TWEEN-20 (5% in PBS) at 37°C and incubated for 10 min in donkey serum (3% in PBS) at room temperature. The vessels were incubated overnight at 4°C with a primary antibody against VPAC1 or VPAC2 (1:500 in 3% donkey serum–PBS; Santacruz Biotechnology, Santacruz, CA, USA) and then for 1 h at room temperature in fluorophore-coupled secondary antibodies (Alexa488 and Alexa594, respectively, Invitrogen, Burlington, ON, Canada). Vessels were gently placed into observation chambers and imaged using ×20 (air), ×40 (oil immersion) or ×60 (oil immersion) objectives on a confocal microscope (Olympus FV1000; Imaging Core Facility, Snyder Institute, University of Calgary). Image frames were collected sequentially.
Quantitative real-time PCR
Mesenteric lymphatic vessels, arteries and ileum were quickly microdissected out, flushed with PBS to remove luminal content, cleaned of surrounding tissue and immediately immersed into RNase- and DNase-free collection tubes containing the RNA stabilization reagent, RNAlater (Qiagen, Mississauga, ON, CAN). The RNA-stabilized, microdissected tissue was homogenized and disrupted using sonication QIAshredder (Qiagen), and underwent RNA extraction using the Qiagen Micro RNeasy kit (Qiagen). cDNA was subsequently synthesized using Superscript II reverse transcriptase enzyme (Invitrogen) with oligo (dT) primers. Real-time amplification of target genes was performed using Quanti Tect Syber Green (Qiagen) in an iCycler iQ real-time PCR instrument (Bio-Rad Laboratories, Hercules, CA, USA). Guinea pig real-time primer sequences for VPAC1, VPAC2, SUR2B and Kir6.1 were designed using the first-release guinea pig genome sequence published on Ensembl (http://www.ensembl.org) using Primer 3 software (http://fokker.wi.mit.edu/primer3). β-actin cDNA expression was used to normalize expression of the receptors. Real-time primer sequences, as well as the associated Ensembl accession numbers are listed in Table 1. cDNA amplification for all genes was performed using an annealing temperature of 55°C and 50 cycles. Real-time PCR results were analysed using the ΔΔCT method. PCR products sequences were verified by the University of Calgary Core DNA service.
Table 1.
Real-time primer sequences (all 5′ to 3′)
| Gene | Ensembl Accession no. | Forward | Reverse | Size (bp) |
|---|---|---|---|---|
| β-actin | ENSCPOG00000013467 | AGGGTGTAACGCAGCAAAGT | ACTCTCCACCTTCCAGCAGA | 118 |
| VPAC1 | ENSCPOG00000024209 | ACTGCACCCGGAACTACATC | CAACTCCTCGCTGTGAAACA | 104 |
| VPAC2 | ENSCPOG00000006143 | TCCACCTGAACCTGTTCCTC | GAATACCTGGCTGAGCTTGC | 143 |
| SUR2B | ENSRNOG00000036960 | AGAAATTGCCCAGCTGAAGA | CCAGGCAAAAGAGCTGTCTC | 116 |
| Kir6.1 | ENSRNOG00000013463 | AATGGCAAGCTCTGCTTCAT | TGAATAGGCACCACCTCTCC | 112 |
ELISA
The concentration of VIP was determined with an enzyme-linked immunosorbent assay (ELISA) kit (Phoenix Pharmaceuticals, Belmont, CA, USA) according to the manufacturer's instructions. Briefly, microdissected lymphatic vessels and ileum were homogenized separately in Tris buffer (pH 7.4) (1 ml (g tissue weight)−1) and centrifuged to collect supernatant. Supernatant from both lymphatic vessel and ileal samples were run on the plate, along with proper controls. Optical density was measured at 450 nm using an ELISA plate reader (Bio-Rad).
Chemicals and drugs
VIP and PACAP6-38 were purchased from American Peptide Co. (Sunnyvale, CA, USA); [D-p-Cl-Phe6,Leu17]-VIP, VIP6-28, glibenclamide, indomethacin and NG-nitro-l-arginine (l-NNA) from Sigma-Aldrich; H89 and ODQ from Alexis (San Diego, CA, USA) and [Ala11,22,28]VIP from Tocris Cookson (Ellisville, MO, USA). Stock solutions of the peptides were prepared in distilled water, l-NNA in 0.1 m HCl, indomethacin in ethanol and glibenclamide H89 and ODQ in DMSO. The compounds were then diluted in PSS to achieve the appropriate concentration. The final concentration of each vehicle was always ≤0.1% (v/v), a concentration that had no effect on lymphatic contractile and electrical activities.
Data and statistical analysis
Data are expressed as means ± one standard error of the mean (SEM). Concentration–response curves were built and the negative log of the concentration of the agonist giving half-maximal response (pEC50) to VIP was estimated by computerized non-linear regression analysis (Prism, GraphPad software, Inc.). Statistical significance was assessed using a two-tailed Student's paired t test (unless specified in the text), with P < 0.05 being considered significant.
Results
Effect of VIP on mesenteric lymphatic vessel rhythmical phasic contractions
Intraluminal perfusion of mesenteric lymphatic vessels helped to sustain a regular rhythmical contraction–relaxation cycle (lymphatic pumping) with a mean frequency of 11 ± 0.6 min−1 (n = 58). Administration of VIP for 1 min induced a concentration-dependent decrease in lymphatic pumping (Fig. 1). Noticeable responses were observed between 1 and 3 nm, with significance achieved at the latter peptide concentration (83 ± 2% of control, P = 0.004, Student's paired t test). Maximum effect was reached with a concentration of 100 nm (11.2 ± 5.7% of control), for a pEC50 of 8.1 ± 0.1 (n = 14).
Figure 1. Effect of VIP on pumping of lymphatic vessels from the guinea pig mesentery.
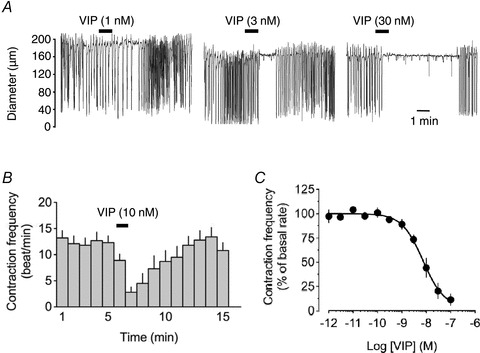
A, original traces of vessel diameter changes (downward deflections represent contractions) in an actively contracting chamber in response to 1, 3 and 30 nm VIP, applied for 1 min (horizontal bars). B, time-course histogram of the lymphatic response to 10 nm VIP. Columns represent contractions per minute (mean ± SEM, n = 14). C, concentration–response relationship of the VIP-induced pumping inhibition (n = 9–14).
Effects of VIP on lymphatic muscle membrane potential
Microelectrode recordings obtained from lymphatic muscles in short vessel segments (length 125–300 μm) revealed a mean resting potential value of −57 ± 1 mV (n = 93). Superfusion of these preparations with VIP for 1 min caused concentration-dependent hyperpolari-zations reaching a maximum at concentrations equal or higher than 30 nm (pEC50 8.1 ± 0.3, n = 6–10; Fig. 2). At VIP concentrations over 100 nm, hyperpolarizations ranged from 8 to 24 mV, with the less polarized segments usually generating the largest hyperpolarization amplitudes, to consistently reach a membrane potential around −70 mV (−69 ± 2 mV, n = 22).
Figure 2. Effect of VIP on membrane potential of guinea pig mesenteric lymphatic muscle.
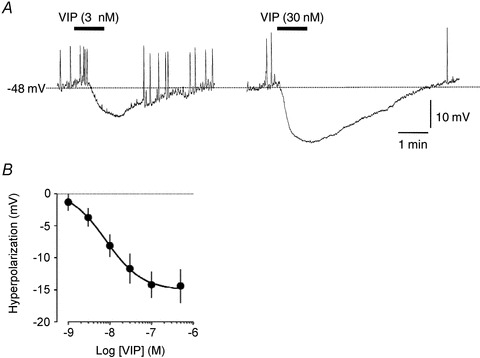
A, original intracellular microelectrode recordings displaying hyperpolarizing responses to 3 and 30 nm VIP applied for 1 min (horizontal bars). B, concentration–response relationship of the VIP-induced hyperpolarization (n = 3–10).
Pharmacological assessment of the receptors involved in lymphatic vessel responses to VIP
To identify the receptor(s) mediating VIP-induced pumping inhibition and hyperpolarization, we assessed responses to the peptide in the presence of the VPAC1/VPAC2 receptor antagonist, VIP6-28, the VPAC1 receptor antagonist [D-p-Cl-Phe6,Leu17]-VIP and the VPAC2/PAC1 receptor antagonist, PACAP6-38. Decrease in lymphatic contraction frequency in response to 10 nm VIP was significantly inhibited by 1 μm PACAP6-38 (Fig. 3A), as was the hyperpolarization caused by 100 nm VIP (n = 5, Fig. 4A and B). Pumping inhibition to 10 nm VIP was also significantly inhibited by 1 μm VIP6-28 (Fig. 3B).
Figure 3. Pharmacological characterization of VIP receptors involved in the VIP-induced inhibition of lymphatic pumping.
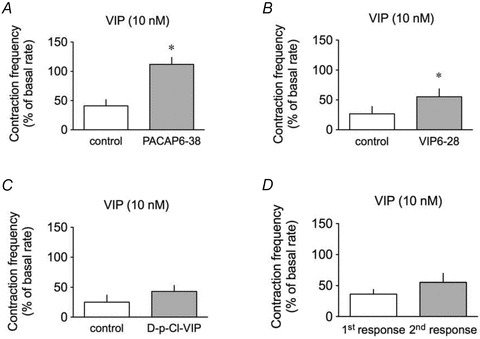
Effect of 10 nm VIP on lymphatic pumping in the absence (control, open columns) and in the presence (grey columns) of 1 μm PACAP6-38 (n = 5, A), 1 μm VIP6-28 (n = 6, B) and 1 μm[D-p-Cl-Phe6,Leu17]-VIP (D-p-Cl-VIP, n = 4, C), or without antagonist (n = 9, D). Columns are mean ± SEM.*P < 0.05 versus control.
In contrast, VIP-induced pumping inhibition was not affected by 1 μm[D-p-Cl-Phe6,Leu17]-VIP (Fig. 3C), and neither VIP6-28 nor [D-p-Cl-Phe6,Leu17]-VIP had significant effect on the hyperpolarization induced by 10 nm (n = 2, data not shown) or 100 nm VIP (n = 4, Fig. 4B), respectively. Moreover, in the presence of the selective VPAC1 agonist [Ala11,22,28]-VIP (100 nm), contraction frequency was not inhibited (n = 3, data not shown) and membrane potential not hyperpolarized (−60 ± 2 mV maintained throughout, n = 4).
Expression of VIP and VIP receptors in guinea pig mesenteric lymphatic vessels
In order to assess whether the main players (VIP and its receptors) were present in lymphatic vessels, we first measured tissue level of the peptide by ELISA. VIP could be detected in mesenteric lymphatics (Fig. 5A), however, at a level much lower than the ileum, used as positive control. The lymphatic vessels used for these experiments had been previously flushed of their luminal content to remove potential contamination from cells present in the lymph. When the procedure was repeated with lymphatic vessel samples, which were not cleared of lymph, the amount of VIP detected was markedly increased (P < 0.05).
Figure 5. Expression of VIP, VPAC1 and VPAC2 mRNA in guinea pig mesenteric lymphatic vessels.
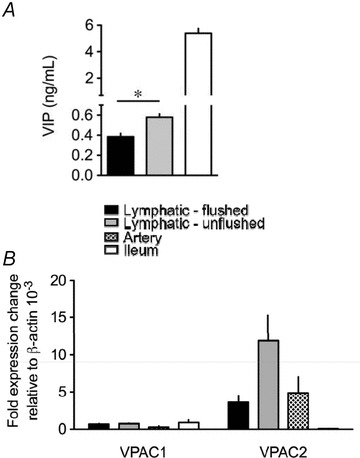
A, level of VIP detected by ELISA in flushed and unflushed lymphatic vessels and in ileum. Data are mean ± SEM of 6 animals. B, quantitative real-time PCR analysis of VPAC1 and VPAC2 mRNA expression in lymphatic vessels, arteries and in ileum. Data are mean ± SEM of 3 animals, expressed relative to the number of copies of β-actin. *P < 0.05 versus control. Key applies to both panels.
Expression of VPAC1 and VPAC2 mRNAs in lymphatic vessels was assessed by quantitative real-time PCR and compared to the receptor mRNAs expressed in mesenteric arteries and ileum. As illustrated in Fig. 5B, lymphatic vessels expressed mRNA for both receptors. Expression of VPAC2 always predominated and was comparable to that of mesenteric artery. Comparing mRNA expression with that obtained from unflushed vessels revealed similar VPAC1 expression level; however, VPAC2 expression was increased 3-fold in the unflushed vessels (Fig. 5B).
Confocal immunofluorescence experiments revealed immunoreactivity for VPAC1 and VPAC2 in mesenteric lymphatic vessels. Staining for VPAC1 was diffuse with most of the fluorescence emitted at the level of the endothelium. A faint staining was sometimes also observed at the level of the muscles, but co-localization with smooth muscle α-actin was difficult to confirm (n = 5, near surface, Fig. 6A). Staining for VPAC2 was stronger and well localized in lymphatic endothelial and muscle cells (n = 5, Fig. 6B).
Figure 6. Immunolocalization of VPAC1 and VPAC2 in guinea pig mesenteric lymphatic vessels.
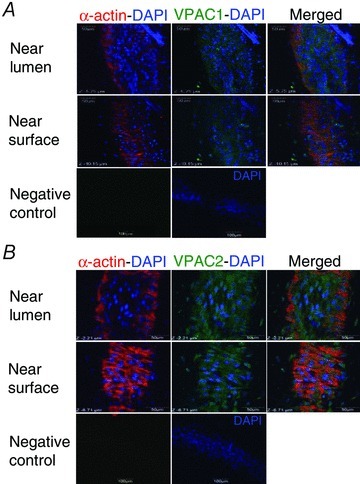
Confocal photomicrographs showing immunoreactivity for VPAC1 (A) and VPAC2 (B) in collecting lymphatic vessels scanned near the lumen to visualize the endothelium and near the surface in the muscle layer. VPAC1 immunoreactivity is mainly observed associated with endothelial cells, which have their DAPI-stained nucleus oriented along the vessel length (top images in A). A faint VPAC1 immunoreactivity is also seen closer to the vessel surface, but co-localization with lymphatic muscle visualized by α-actin immunoreactivity is weak (middle images in A). VPAC2 immunoreactivity more clearly delineates lymphatic muscle (middle images in B) and is also strongly associated with the endothelium (top images in B). Negative controls are merged with DAPI staining to show vessel orientation (bottom right images). Images are representative of 5 experiments for each receptor antibody.
Role of the endothelium in lymphatic vessel contractile and electrical responses to VIP
Studies reporting a role for the endothelium in VIP-induced actions on blood vessels (see for example: Hattori et al. 1992; Jovanovic et al. 1998) and previous demonstration that the lymphatic endothelium can release nitric oxide (NO) and cyclooxygenase products (von der Weid et al. 1996; Mizuno et al. 1998; Gao et al. 1999; Chan et al. 2004), prompted us to examine whether VIP-induced lymphatic pumping inhibition and hyperpolarization was due to the release of endothelial factors. We first examined the possible involvement of NO in the responses to VIP using the NO synthase inhibitor l-NNA. l-NNA (100 μm) did not change basal lymphatic contraction frequency and did not significantly alter VIP-induced inhibition of lymphatic pumping (pEC50 8.2 ± 0.1, P = 0.660 versus control, n = 4; Fig. 7A). l-NNA depolarized lymphatic muscle membrane potential from a control value of −58 ± 2 mV to −54 ± 2 mV (P < 0.05; n = 9), confirming the inhibition of a basal endogenous release of NO (see von der Weid et al. 1996, 2001). However, it did not affect VIP-induced hyperpolarization Fig. 8C). Consistently, the guanylyl cyclase inhibitor ODQ (10 μm) did not alter VIP-induced pumping inhibition (pEC50 8.6 ± 0.2, P = 0.075 versus control, n = 5; Fig. 7A).
Figure 7. Role of the endothelium and endothelium-derived factors in the VIP-induced responses.
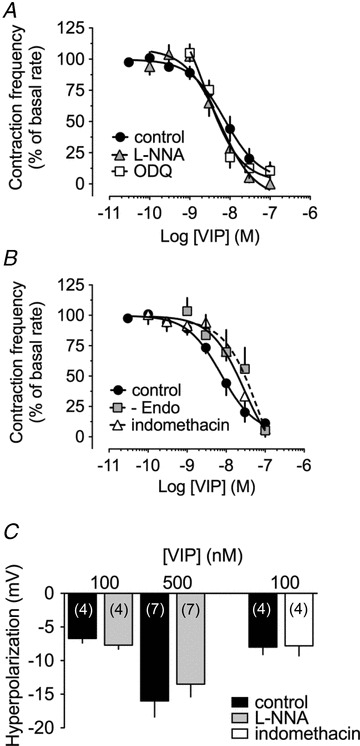
A, effect of 100 μm l-NNA (n = 4) and 10 μm ODQ (n = 4) and B, of 10 μm indomethacin (n = 4) and endothelial lysis (n = 5) on the concentration-dependent pumping inhibition induced by VIP. C, effect of 100 μm l-NNA and 10 μm indomethacin on VIP-induced hyperpolarizations. Columns are mean ± SEM expressed as percentage of control VIP hyperpolarization obtained during the same impalements.
Figure 8. Role of KATP channels in VIP-induced responses, and mRNA expression level of the channels subunits.
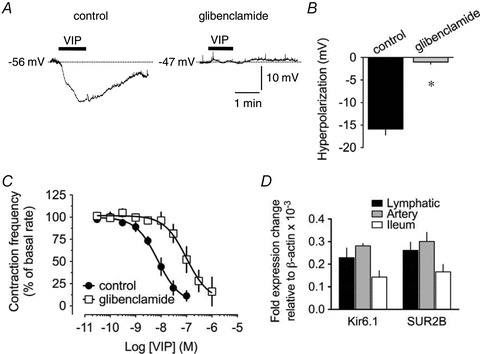
A, original intracellular microelectrode recordings displaying the inhibitory effect of 10 μm glibenclamide on the hyperpolarizing response to 500 nm VIP applied for 1 min (horizontal bars). B, summary graph of the effect of glibenclamide on VIP-induced hyperpolarization (n = 7). Columns are mean ± SEM expressed as percentage of control VIP hyperpolarization obtained during the same impalement. *P < 0.05 versus control. C, effect of 1 μm glibenclamide (n = 4) on the concentration-dependent pumping inhibition induced by VIP. D, quantitative real-time PCR analysis of KATP subunits Kir6.1 and SUR2B in guinea pig mesenteric lymphatic vessels, arteries and ileum. Data are mean ± SEM of 4 animals, expressed relative to the number of copies of β-actin.
We then assessed whether cyclooxygenase products were involved in the VIP-induced responses. In the presence of the non-selective cyclooxygenase inhibitor indomethacin (10 μm), VIP-induced pumping inhibition was reduced compared to control and the concentration–response curve slightly, but significantly, shifted to the right (pEC50 7.5 ± 0.2, P < 0.05 versus VIP control, n = 4; Fig. 7B). At the maximal VIP concentration tested (100 nm), the response in the presence of indomethacin was not changed. This was similar to membrane potential measurements where hyperpolarization induced by 100 nm VIP was not affected by indomethacin (96 ± 6% of control, P = 0.704, n = 5; Fig. 7C). Indomethacin did not modify resting lymphatic muscle membrane potential (−60 ± 1 mV throughout, n = 5). We further investigated the effect of VIP on de-endothelized lymphatics and, as in the presence of indomethacin, noticed a shift of the concentration–response curve to VIP (pEC50 7.1 ± 0.5, P < 0.05 versus control, n = 5; Fig. 7B). We finally also examined whether membrane potential of the lymphatic endothelium was affected by VIP, but did not observe any induced changes to a 1 min application of 100 nm VIP (mean membrane potential −72 ± 1 mV maintained throughout, n = 6; data not shown; see also von der Weid & van Helden, 1997).
Role of KATP channels in guinea pig mesenteric lymphatic vessel responses to VIP
ATP-sensitive K+ (KATP) channel opening has been consistently suggested to be responsible for agonist-induced hyperpolarizations and inhibition of contractile activity in lymphatic vessel preparations (von der Weid, 1998; Chan & von der Weid, 2003; Rehal et al. 2009) and involved in mediating VIP-induced responses in some vascular beds (Yang et al. 2008). VIP-induced hyperpolarization and inhibition of contraction frequency were examined in the presence of the KATP channels blocker glibenclamide. As reported in previous studies (von der Weid, 1998; von der Weid et al. 2001; Chan & von der Weid, 2003; Hosaka et al. 2006), glibenclamide significantly depolarized lymphatic muscle membrane potential from a control value of −58 ± 2 mV to −49 ± 1 mV (n = 11). Hyperpolarizations induced by 100 and 500 nm VIP were decreased by glibenclamide (1 and 10 μm, respectively) to 21 ± 7% (n = 4) and 8 ± 4% of control (n = 7; Fig. 8A and B). The VIP-induced decrease in lymphatic pumping was also reversed in the presence of 1 μm glibenclamide, and the concentration-dependent relationship significantly shifted to the right (pEC50 7.0 ± 0.2, P < 0.05, n = 6; Fig. 8C). Molecular expression of the KATP channel subunits Kir6.1 and SUR2B in lymphatic vessels was assessed at the mRNA level by quantitative real-time PCR and compared to expression in mesenteric arteries and ileum. As illustrated in Fig. 8D, both subunits were expressed at similar mRNA levels in lymphatic vessel and these levels were comparable to those measured in mesenteric artery. The Kir6.2 subunit was, however, not detected (n = 4; data not shown).
Involvement of protein kinase A in guinea pig mesenteric lymphatic vessel responses to VIP
Both VPAC1 and VPAC2 have been demonstrated to be coupled to Gs G-proteins with stimulation of these receptors leading to increased production of cAMP and activation of protein kinase A (PKA) (Laburthe & Couvineau, 2002). In order to investigate a role for PKA in VIP-induced responses, experiments were performed in the presence of H89, a PKA inhibitor. H89 (10 μm) significantly reduced the hyperpolarization induced by 500 nm VIP to 8 ± 4% of control (n = 4; Fig. 9A and B) and VIP-induced decrease in lymphatic contraction frequency (pEC50 7.5 ± 0.2, P < 0.05, n = 5; Fig. 9C).
Figure 9. Role of protein kinase A in VIP-induced responses.
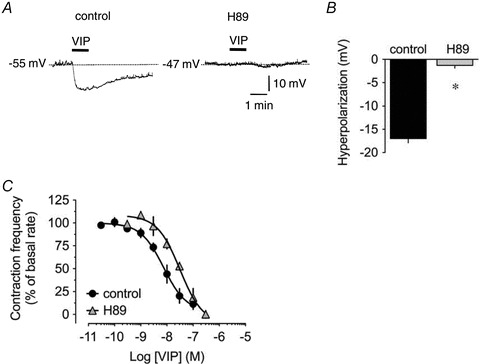
A, original intracellular microelectrode recordings displaying the inhibitory effect of 10 μm H89 on the hyperpolarizing response to 500 nm VIP applied for 1 min (horizontal bars). B, summary graph of the effect of H89 on VIP-induced hyperpolarization (n = 4). Columns are mean ± SEM expressed as percentage of control VIP hyperpolarization obtained during the same impalement. *P < 0.05 versus control. C, effect of 10 μm H89 (n = 4) on the concentration-dependent pumping inhibition induced by VIP.
Discussion
The current study reveals the presence of VIP in guinea pig mesenteric lymphatic vessels and demonstrates a role for the peptide in the modulation of lymphatic contractile activity. Exogenously applied VIP markedly decreased lymphatic pumping in vitro, via a mechanism suggested to involve stimulation of VPAC2 expressed at the surface of the lymphatic muscle cells, consequently activating the adenylyl cyclase–cAMP–PKA pathway. Our findings also demonstrate that KATP channels are downstream effectors of the VIP-induced response. Increased opening probability of these channels causes a hyperpolarization that is responsible, at least in part, for the VIP-induced inhibition of lymphatic pumping.
Our findings are consistent with an earlier report showing that VIP was able to relax lymphatic bovine mesenteric lymphatic vessel strips maintained under isometric tension (Ohhashi et al. 1983). Although in the experimental conditions of this study, bovine lymphatics were not displaying rhythmical contractions, VIP was effective in a concentration range similar to that reported in the present study (6 to 300 nm).
VIP is described as a potent vasodilator of systemic blood vessels. The pathway through which it causes smooth muscle relaxation is via binding to the VIP receptors VPAC1 and VPAC2 (Laburthe & Couvineau, 2002). Our pharmacological assessment strongly suggests VPAC2 as the main receptor involved in the effects VIP induced in lymphatic vessels. Indeed, these effects were significantly inhibited by PACAP6-38, a VPAC2/PAC1 antagonist and VIP6-28, a VPAC1/VPAC2 antagonist (however, VIP-induced hyperpolarization was not affected by the latter), but were not affected by [D-p-Cl-Phe6,Leu17]-VIP, a VPAC1 antagonist (Dickinson & Fleetwood-Walker, 1999). Involvement of VPAC1 could be further ruled out as the selective VPAC1 agonist [Ala11,22,28]-VIP did not mimic VIP action on lymphatic pumping or membrane potential. Expression of VPAC2 in lymphatic vessels was also confirmed by our biochemical and immunofluorescence data. Quantitative real-time PCR assessment showed a consistent VPAC2 mRNA transcript level in the wall of mesenteric lymphatic vessels that was similar to what we measured in guinea pig mesenteric artery. Immunoreactivity to VPAC2 was observed mainly in lymphatic muscle, but also in the endothelium. Despite a lack of pharmacological action on contractile activity, we showed, through PCR and immunofluorescence studies, that guinea pig mesenteric lymphatic vessels also expressed VPAC1. In contrast to VPAC2, this receptor seemed to be more exclusively associated with the lymphatic endothelium. Similar findings have been reported in porcine basilar arteries, where VPAC1 was expressed in the endothelium and VPAC2 in the outer layer of the smooth muscle (Grant et al. 2006). Expression of both VIP receptors was also demonstrated by immunohistochemistry in tissue sections of the aorta and pulmonary artery as well as in cultured smooth muscle cells from these vessels (St Hilaire et al. 2010). In contrast, only VPAC1 expression was reported in rat cerebral arteries and arterioles, and these receptors were observed in the plasmalemma of circular smooth muscles, but not in the endothelium (Fahrenkrug et al. 2000).
Expression of VPAC2 in the lymphatic endothelium may suggest this cell layer as an intermediary in VIP actions. Indeed, in several vascular beds, vasodilatation caused by VIP has been suggested to be fully or partially mediated by endothelium-derived NO (Hattori et al. 1992; Jovanovic et al. 1998; Grant et al. 2006; Anaid et al. 2007; Shahbazian et al. 2007; Zhang et al. 2010). In guinea pig mesenteric lymphatics, NO does not seem to be involved in VIP-induced responses, as neither pump inhibition nor hyperpolarization was affected by the NOS inhibitor l-NNA. Yet, a small inhibition of VIP-induced decrease in contraction frequency was observed in the presence of indomethacin and in de-endothelized vessels. These findings suggest that in addition to directly acting on the lymphatic muscle to inhibit pumping, VIP also stimulates the endothelium to release prostaglandins, which, by further altering the muscle contractile activity, indirectly contributing to the overall VIP-induced pumping inhibition. Cyclooxygenase metabolites have been shown to be constitutively produced by the lymphatic endothelium, where their release following stimulation by agonists and luminal flow led to prominent modulation of lymphatic pumping (Yokoyama & Ohhashi, 1993; von der Weid et al. 1996; Mizuno et al. 1998; Gao et al. 1999; Koller et al. 1999; Chan et al. 2004). It must be noted that, as demonstrated by Scallan & Huxley (2010), the mesenteric lymphatic vessel wall has a relatively high permeability. This would explain that VIP, or other agonists as shown in previous studies (von der Weid et al. 1996; Chan et al. 2004), applied via the superfusing solution, has no issue diffusing through the mesenteric layer and the lymphatic vessel wall to reach the endothelium and induce an endothelium-dependent effect.
VIP receptors belong to the large family of heptahelical G-protein-coupled receptors mainly coupled to Gs and the adenylyl cyclase–cAMP–PKA signalling pathway (reviewed in Laburthe & Couvineau, 2002). Our findings also strongly support a major role for PKA in mediating VIP-induced responses, as both hyperpolarization and pumping inhibition were inhibited by H89.
Stimulation of smooth muscle by VIP has been consistently correlated with hyperpolarization due to the opening of ion channels, such as ATP-sensitive K+ (KATP) channels, calcium-activated K+ (KCa) channels or voltage-dependent K+ (Kv) channels (Kishi et al. 1996, 2000; Kawasaki et al. 1997; Quayle et al. 1997; Jury & Daniel, 1999). Activation of these channels leads to membrane hyperpolarization, decreased Ca2+ entry via voltage-gated Ca2+ channels and ultimately relaxation (Standen et al. 1989; Nelson & Quayle, 1995; Quayle et al. 1995, 1997). In the present study, VIP-induced decrease in lymphatic pumping was accompanied by a large hyperpolarization of the lymphatic muscle membrane potential and our data suggest a main role for KATP channels in these actions, because both responses were significantly altered by glibenclamide. We also demonstrated that the pore-forming subunit, Kir6.1, and the regulatory subunit, SUR2B, which specifically constitute KATP channels in smooth muscle (reviewed in Flagg et al. 2010), were expressed at the mRNA level in guinea pig mesenteric lymphatic vessels in amounts comparable to mesenteric artery, whereas mRNA of the Kir6.2 subunit was not detected. These findings substantiate our earlier pharmacological demonstration of the presence of KATP channels in these lymphatic muscles (von der Weid, 1998). Activation of KATP channels by VIP has been reported in rat mesenteric artery, where the response could be blocked by glibenclamide (Yang et al. 2008). In that study, VIP also activated a current in HEK293 cells expressing the KATP channel subunits Kir6.1 and SUR2B, with an ED50 of 10 nm, identical to that obtained in the present study. Also, similar to our findings showing a decrease in the VIP-induced hyperpolarization with H89, VIP activation of the channel was blocked by the PKA inhibitor Rp-cAMP (Yang et al. 2008). PKA is known to phosphorylate KATP channels on both the pore-forming and regulatory subunits resulting in the up-regulation of channel activity (Quinn et al. 2004; Shi et al. 2007, 2008). It must be noted that the ability of H89 to reduce VIP-induced pumping inhibition was modest compared to the blockade by glibenclamide. We had already observed in earlier studies that 10 μm H89 was less effective than 1 or 10 μm glibenclamide at blocking the response to inhibitory agonists (see, for example, Rehal et al. 2009). Although in the present study, EC50 values obtained in the presence of H89 and glibenclamide were not significantly different (P = 0.0726, Student's unpaired t test), this observation is intriguing and may reflect either an opening of KATP channels independent of PKA activation, or an efficacy of H89 to inhibit PKA lower than that of glibenclamide to block the KATP channels. Indeed, a cAMP-dependent effect independent of PKA activation has been described through a pathway involving exchange proteins directly activated by cyclic AMP or Epacs. Importantly in the context of the present study, Epacs have been shown to mediate cAMP-dependent, but PKA-independent modulation of KATP channels in rat aortic smooth muscle (Purves et al. 2009). We examined whether Epacs could be involved in VIP-induced inhibition of lymphatic pumping by using the Epac inhibitor, brefeldin A (Zhong & Zucker, 2005), but could not demonstrate any effect of the inhibitor on the of VIP-induced pumping inhibition (n = 5, authors’ unpublished data). This finding argues against an involvement of Epacs in the inhibitory response to VIP, and although activation of KATP channels via other signalling pathways cannot be totally excluded, we favour the idea that the VIP effect is mainly mediated by PKA and that the difference observed between the inhibitory effect of glibenclamide and H89 is due to a lower efficacy of the latter. Glibenclamide IC50 has been reported to be about 10–40 nm when SUR2B is associated with the Kir6.1 subunit (Russ et al. 1999). IC50 of H89 for PKA, however, is 135 nm in an in vitro cell assay (Davies et al. 2000). Importantly in that paper, the authors noted that, like many protein kinase inhibitors, H89 competes with ATP for its binding site and the concentration of H89 required to inhibit PKA in any assay depends on the concentration of ATP; the higher this concentration, the higher the concentration of H89 necessary to inhibit PKA. As the vessels were contracting, we can assume ATP concentration would be elevated and the efficacy of H89 decreased.
The potent pumping inhibition VIP induces in isolated lymphatic vessels and the expression of VPACs in these vessels suggest important physiological function(s) for the peptide in vivo, and position it as a critical modulator of lymph drainage. To fulfil this role, VIP needs to be present in proximity to the lymphatics. A potential source of VIP could be the peptidergic fibres innervating the mesenteric lymphatics. Indeed, and consistent with descriptions of sparse peptidergic innervation of lymphatic vessels (Guarna et al. 1991; Wang et al. 1995), a faint VIP immunoreactivity has been reported in fibres surrounding bovine mesenteric lymphatics (Ohhashi et al. 1983). However, detection of VIP immunoreactive fibres has been controversial. VIP immunoreactivity was not found in the intestinal mesentery of the small and large intestine of the sheep, while it was observed in the mucosa, submucosa, smooth muscles and plexuses (Wathuta, 1986). In our own experience, although we were able to reveal VIP-positive fibres in whole-mount preparations of guinea pig ileum, we could not detect any VIP immunostaining in and around mesenteric lymphatic vessel in whole-mount mesenteric preparations of the same animals (n = 4, data not shown). Yet, our ELISA data strongly suggest the presence of VIP in mesenteric lymphatics. However, this finding does not provide information on the origin of VIP and the cells which produce it, i.e. nerve varicosities, muscle cells, endothelial cells or other cells such as immune cells potentially present in close proximity to the lymphatic vessels. In addition to the important potential reservoir constituted by nerve varicosities and resident immune cells, VIP could be released from immune cells such as dendritic cells, macrophages and lymphocytes trafficking within the lymph. Indeed, our data showing higher VIP levels in samples of unflushed lymphatic vessels, still containing lymph, strongly support immune cells as potential VIP producers.
While VIP is the main agonist of VPAC2, other peptides, such as pituitary adenylate cyclase activating polypeptide (PACAP) 27 and PACAP38, also potently activate this receptor (reviewed in Dickinson & Fleetwood-Walker, 1999). By stimulating VPAC2, these peptides could also play a physiological role in contributing to lymphatic pumping inhibition. This possibility has yet to be examined and the presence of these peptides in the vicinity of the lymphatics established. Whether the situation is different during inflammation, where the number of immune cells circulating in the lymph and their potential to produce and release VIP and/or PACAPs is expected to augment, would also need to be examined, as no data are currently available. Lymphatic pumping inhibition induced by these peptides may thus compromise lymph drainage, oedema resolution and immune cell trafficking, and contribute to the deleterious effects of inflammation by potentially delaying the initiation of an adequate immune response. On the other hand, impaired lymph drainage could confine inflammatory mediators, antigens and activated immune cells to the site of inflammation, avoiding the development of an uncontrolled immune response (Wu et al. 2005). Whether the extent of the immune response can be modulated through lymphatic pumping, or the volume of lymph drained, certainly deserves further investigations, as understanding these processes should help to better interpret the causes and consequences of oedema and compromised immune responses.
Acknowledgments
We would like to acknowledge the support of the Snyder Institute Live Cell Imaging Facility, which is funded by an equipment and infrastructure grant from the Canadian Foundation for Innovation (CFI) and the Alberta Science and Research Authority. We also would like to thank K. Loutzenhiser and C. MacNaughton for their help with the immunofluorescence procedures and C. Rous for his valuable technical assistance. This study was supported by grants from the Crohn's and Colitis Foundation of Canada and the Canadian Institutes of Health Research.
Glossary
Abbreviations
- KATP channel
ATP-sensitive K+ channel
- PACAP
pituitary adenylate cyclase activating polypeptide
- PKA
protein kinase A
- PSS
physiological saline solution
- STD
spontaneous transient depolarizations
- VIP
vasoactive intestinal peptide
Author contributions
All experiments were undertaken at the University of Calgary. P.-Y.vdW. led in the conception and design of the project, in the analysis of the data, and wrote the manuscript. He also performed the electrophysiology experiments with help from M.R. S.R. and P.D. contributed equally to the vessel contraction measurement experiments with help from S.L. S.R. obtained the ELISA and molecular biology data together with R.M., and M.S.I. carried out the immunofluorescence experiments with help from S.R. All authors contributed to the critical review of drafts of the manuscript and gave final approval of the version to be published.
References
- Anaid S, Petkov V, Baykuscheva-Gentscheva T, Hoeger H, Painsipp E, Holzer P, Mosgoeller W. Involvement of endothelial NO in the dilator effect of VIP on rat isolated pulmonary artery. Regul Pept. 2007;139:102–108. doi: 10.1016/j.regpep.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Benoit JN, Zawieja DC. Effects of f-Met-Leu-Phe-induced inflammation on intestinal lymph flow and lymphatic pump behavior. Am J Physiol Gastrointest Liver Physiol. 1992;262:G199–G202. doi: 10.1152/ajpgi.1992.262.2.G199. [DOI] [PubMed] [Google Scholar]
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol. 1989;257:H2059–H2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- Chan AK, Vergnolle N, Hollenberg MD, von der Weid P-Y. Proteinase-activated receptor 2 modulates guinea-pig mesenteric lymphatic vessel pacemaker potential and contractile activity. J Physiol. 2004;560:563–576. doi: 10.1113/jphysiol.2004.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AK, von der Weid P-Y. 5-HT decreases contractile and electrical activities in lymphatic vessels of the guinea-pig mesentery: role of 5-HT 7-receptors. Br J Pharmacol. 2003;139:243–254. doi: 10.1038/sj.bjp.0705264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- Dickinson T, Fleetwood-Walker SM. VIP and PACAP: very important in pain? Trends Pharmacol Sci. 1999;20:324–329. doi: 10.1016/s0165-6147(99)01340-1. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J, Tams J, Georg B. Immunohistochemical localization of the VIP1 receptor (VPAC1R) in rat cerebral blood vessels: relation to PACAP and VIP containing nerves. J Cereb Blood Flow Metab. 2000;20:1205–1214. doi: 10.1097/00004647-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev. 2010;90:799–829. doi: 10.1152/physrev.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JL, von der Weid P-Y. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol. 2002;136:1210–1218. doi: 10.1038/sj.bjp.0704820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Zhao J, Rayner SE, van Helden DF. Evidence that the ATP-induced increase in vasomotion of guinea-pig mesenteric lymphatics involves an endothelium-dependent release of thromboxane A2. Br J Pharmacol. 1999;127:1597–1602. doi: 10.1038/sj.bjp.0702710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Delgado M. Anti-inflammatory neuropeptide receptors: new therapeutic targets for immune disorders? Trends Pharmacol Sci. 2007;28:482–491. doi: 10.1016/j.tips.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Grant S, Lutz EM, McPhaden AR, Wadsworth RM. Location and function of VPAC1, VPAC2 and NPR-C receptors in VIP-induced vasodilation of porcine basilar arteries. J Cereb Blood Flow Metab. 2006;26:58–67. doi: 10.1038/sj.jcbfm.9600163. [DOI] [PubMed] [Google Scholar]
- Guarna M, Pucci AM, Alessandrini C, Volpi N, Fruschelli M, D’Antona D, Fruschelli C. Peptidergic innervation of mesenteric lymphatics in guinea pigs: an immunocytochemical and pharmacological study. Lymphology. 1991;24:161–167. [PubMed] [Google Scholar]
- Hattori Y, Nagashima M, Endo Y, Kanno M. Glibenclamide does not block arterial relaxation caused by vasoactive intestinal polypeptide. Eur J Pharmacol. 1992;213:147–150. doi: 10.1016/0014-2999(92)90246-z. [DOI] [PubMed] [Google Scholar]
- Hosaka K, Rayner SE, von der Weid PY, Zhao J, Imtiaz MS, van Helden DF. Calcitonin gene-related peptide activates different signaling pathways in mesenteric lymphatics of guinea pigs. Am J Physiol Heart Circ Physiol. 2006;290:H813–H822. doi: 10.1152/ajpheart.00543.2005. [DOI] [PubMed] [Google Scholar]
- Imtiaz MS, Zhao J, Hosaka K, von der Weid PY, Crowe M, van Helden DF. Pacemaking through Ca2+ stores interacting as coupled oscillators via membrane depolarization. Biophys J. 2007;92:3843–3861. doi: 10.1529/biophysj.106.095687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MG. Interaction of inflammatory mediators with the lymphatic vessel. Pathol Immunopathol Res. 1987;6:177–189. doi: 10.1159/000157044. [DOI] [PubMed] [Google Scholar]
- Jovanovic A, Jovanovic S, Tulic I, Grbovic L. Predominant role for nitric oxide in the relaxation induced by vasoactive intestinal polypeptide in human uterine artery. Mol Hum Reprod. 1998;4:71–76. doi: 10.1093/molehr/4.1.71. [DOI] [PubMed] [Google Scholar]
- Jury J, Daniel EE. Activation of outward K+ currents: effect of VIP in oesophagus. Br J Pharmacol. 1999;127:553–561. doi: 10.1038/sj.bjp.0702579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki J, Kobayashi S, Miyagi Y, Nishimura J, Fujishima M, Kanaide H. The mechanisms of the relaxation induced by vasoactive intestinal peptide in the porcine coronary artery. Br J Pharmacol. 1997;121:977–985. doi: 10.1038/sj.bjp.0701206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Takeuchi T, Katayama H, Yamazaki Y, Nishio H, Hata F, Takewaki T. Involvement of cyclic AMP–PKA pathway in VIP-induced, charybdotoxin-sensitive relaxation of longitudinal muscle of the distal colon of Wistar-ST rats. Br J Pharmacol. 2000;129:140–146. doi: 10.1038/sj.bjp.0702998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Takeuchi T, Suthamnatpong N, Ishii T, Nishio H, Hata F, Takewaki T. VIP- and PACAP-mediated nonadrenergic, noncholinergic inhibition in longitudinal muscle of rat distal colon: involvement of activation of charybdotoxin- and apamin-sensitive K+ channels. Br J Pharmacol. 1996;119:623–630. doi: 10.1111/j.1476-5381.1996.tb15719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller A, Mizuno R, Kaley G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1683–R1689. doi: 10.1152/ajpregu.1999.277.6.R1683. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC Receptors for VIP and PACAP. Regul Pept. 2002;108:165–173. doi: 10.1016/s0167-0115(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol Regul Integr Comp Physiol. 1998;274:R790–R796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- Moitra J, Sammani S, Garcia JG. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl Res. 2007;150:253–265. doi: 10.1016/j.trsl.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Ohhashi T, Olschowka JA, Jacobowitz DM. Vasoactive intestinal peptide inhibitory innervation in bovine mesenteric lymphatics. A histochemical and pharmacological study. Circ Res. 1983;53:535–538. doi: 10.1161/01.res.53.4.535. [DOI] [PubMed] [Google Scholar]
- Pozo D, Delgado M. The many faces of VIP in neuroimmunology: a cytokine rather a neuropeptide? FASEB J. 2004;18:1325–1334. doi: 10.1096/fj.03-1440hyp. [DOI] [PubMed] [Google Scholar]
- Purves GI, Kamishima T, Davies LM, Quayle JM, Dart C. Exchange protein activated by cAMP (Epac) mediates cAMP-dependent but protein kinase A-insensitive modulation of vascular ATP-sensitive potassium channels. J Physiol. 2009;587:3639–3650. doi: 10.1113/jphysiol.2009.173534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle JM, Bonev AD, Brayden JE, Nelson MT. Pharmacology of ATP-sensitive K+ currents in smooth muscle cells from rabbit mesenteric artery. Am J Physiol Cell Physiol. 1995;269:C1112–C1118. doi: 10.1152/ajpcell.1995.269.5.C1112. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94:1359–1366. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- Rehal S, Blanckaert P, Roizes S, von der Weid PY. Characterization of biosynthesis and modes of action of prostaglandin E2 and prostacyclin in guinea pig mesenteric lymphatic vessels. Br J Pharmacol. 2009;158:1961–1970. doi: 10.1111/j.1476-5381.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockson SG. Lymphedema. Am J Med. 2001;110:288–295. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- Russ U, Hambrock A, Artunc F, Loffler-Walz C, Horio Y, Kurachi Y, Quast U. Coexpression with the inward rectifier K+ channel Kir6.1 increases the affinity of the vascular sulfonylurea receptor SUR2B for glibenclamide. Mol Pharmacol. 1999;56:955–961. [PubMed] [Google Scholar]
- St Hilaire RC, Murthy SN, Kadowitz PJ, Jeter JR., Jr Role of VPAC1 and VPAC2 in VIP mediated inhibition of rat pulmonary artery and aortic smooth muscle cell proliferation. Peptides. 2010;31:1517–1522. doi: 10.1016/j.peptides.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Scallan JP, Huxley VH. In vivo determination of collecting lymphatic vessel permeability to albumin: a role for lymphatics in exchange. J Physiol. 2010;588:243–254. doi: 10.1113/jphysiol.2009.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian A, Petkov V, Baykuscheva-Gentscheva T, Hoeger H, Painsipp E, Holzer P, Mosgoeller W. Involvement of endothelial NO in the dilator effect of VIP on rat isolated pulmonary artery. Regul Pept. 2007;139:102–108. doi: 10.1016/j.regpep.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Shi Y, Cui N, Shi W, Jiang C. A short motif in Kir6.1 consisting of four phosphorylation repeats underlies the vascular KATP channel inhibition by protein kinase C. J Biol Chem. 2008;283:2488–2494. doi: 10.1074/jbc.M708769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1205–R1214. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Umarova BA, Lelekova TV, Kopylova GN, Goncharova EL, Bakaeva ZV, Samonina GE. The role of protective effects of proline-containing peptides (PGP, PG, and GP) in contractile dysfunction of mesenteric lymphatic vessels in rats with experimental acute peritonitis. Bull Exp Biol Med. 2006;142:279–282. doi: 10.1007/s10517-006-0346-2. [DOI] [PubMed] [Google Scholar]
- van Helden D. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol. 1993;471:465–479. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid P-Y. ATP-sensitive K+ channels in smooth muscle cells of guinea-pig mesenteric lymphatics: role in nitric oxide and beta-adrenoceptor agonist-induced hyperpolarizations. Br J Pharmacol. 1998;125:17–22. doi: 10.1038/sj.bjp.0702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid P-Y. Lymphatic vessel pumping and inflammation – the role of spontaneous constrictions and underlying electrical pacemaker potentials. Aliment Pharmacol Ther. 2001;15:1115–1129. doi: 10.1046/j.1365-2036.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- von der Weid P-Y, Crowe MJ, van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol. 1996;493:563–575. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid P-Y, Lee S, Dyrda P, Rehal S, Roizes S, Imtiaz MS. Inhibition of mesenteric lymphatic vessel pumping by the neuro-immuno-modulators VIP and PACAP. Inflam Res. 2011;60:S46. (abstract) [Google Scholar]
- von der Weid P-Y, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol. 2008;295:H1989–H2000. doi: 10.1152/ajpheart.00007.2008. [DOI] [PubMed] [Google Scholar]
- von der Weid P-Y, Rehal S, Dyrda P. Characterization of VIP-induced contractile inhibition in lymphatic vessels. FASEB J. 2009;23:764–765. (abstract) [Google Scholar]
- von der Weid P-Y, van Helden DF. Functional electrical properties of the endothelium in lymphatic vessels of the guinea-pig mesentery. J Physiol. 1997;504:439–451. doi: 10.1111/j.1469-7793.1997.439be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid P-Y, Zhao J, van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol. 2001;280:H2707–H2716. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- Wang XY, Wong WC, Ling EA. Ultrastructural localisation of substance P, vasoactive intestinal peptide and somatostatin immunoreactivities in the submucous plexus of guinea pig ileum. J Anat. 1995;186:187–196. [PMC free article] [PubMed] [Google Scholar]
- Wathuta EM. The distribution of vasoactive intestinal polypeptide-like, substance P-like and bombesin-like immunoreactivity in the digestive system of the sheep. Q J Exp Physiol. 1986;71:615–631. doi: 10.1113/expphysiol.1986.sp003022. [DOI] [PubMed] [Google Scholar]
- Wu TF, Carati CJ, Macnaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G566–G574. doi: 10.1152/ajpgi.00058.2006. [DOI] [PubMed] [Google Scholar]
- Wu TF, MacNaughton WK, von der Weid PY. Lymphatic vessel contractile activity and intestinal inflammation. Mem Inst Oswaldo Cruz. 2005;100:107–110. doi: 10.1590/s0074-02762005000900018. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shi Y, Guo S, Zhang S, Cui N, Shi W, Zhu D, Jiang C. PKA-dependent activation of the vascular smooth muscle isoform of KATP channels by vasoactive intestinal polypeptide and its effect on relaxation of the mesenteric resistance artery. Biochim Biophys Acta. 2008;1778:88–96. doi: 10.1016/j.bbamem.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Ohhashi T. Effects of acetylcholine on spontaneous contractions in isolated bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol. 1993;264:H1460–H1464. doi: 10.1152/ajpheart.1993.264.5.H1460. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Guo S, Zhang J, Chu X, Jiang C, Zhu D. Vasoactive intestinal polypeptide relaxes isolated rat pulmonary artery rings through two distinct mechanisms. J Physiol Sci. 2010;60:389–397. doi: 10.1007/s12576-010-0107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Zucker RS. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J Neurosci. 2005;25:208–214. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


