Abstract
Hydrogen sulfide (H2S) is a gaseous vasodilator produced by endothelial cells. Mechanisms by which H2S induces vasodilatation are unclear. We tested the hypothesis that H2S dilates cerebral arterioles by modulating local and global intracellular Ca2+ signals in smooth muscle cells. High-speed confocal imaging revealed that Na2S, an H2S donor, increased Ca2+ spark frequency ∼1.43-fold and decreased global intracellular Ca2+ concentration ([Ca2+]i) by ∼37 nm in smooth muscle cells of intact piglet cerebral arterioles. In contrast, H2S did not alter Ca2+ wave frequency. In voltage-clamped (−40 mV) cells, H2S increased the frequency of iberiotoxin-sensitive, Ca2+ spark-induced transient Ca2+-activated K+ (KCa) currents ∼1.83-fold, but did not alter the amplitude of these events. H2S did not alter the activity of single KCa channels recorded in the absence of Ca2+ sparks in arteriole smooth muscle cells. H2S increased SR Ca2+ load ([Ca2+]SR), measured as caffeine (10 and 20 mm)-induced [Ca2+]i transients, ∼1.5-fold. H2S hyperpolarized (by ∼18 mV) and dilated pressurized (40 mmHg) cerebral arterioles. Iberiotoxin, a KCa channel blocker, reduced H2S-induced hyperpolarization by ∼51%. Iberiotoxin and ryanodine, a ryanodine receptor channel inhibitor, reduced H2S-induced vasodilatation by ∼38 and ∼37%, respectively. In summary, our data indicate that H2S elevates [Ca2+]SR, leading to Ca2+ spark activation in cerebral arteriole smooth muscle cells. The subsequent elevation in transient KCa current frequency leads to membrane hyperpolarization, a reduction in global [Ca2+]i and vasodilatation.
Key points
Hydrogen sulfide (H2S), a gas produced by endothelial cells, relaxes smooth muscle cells within the vascular wall to increase organ blood flow and lower systemic blood pressure.
Mechanisms by which H2S produces vasodilatation in the cerebral circulation are unclear.
We demonstrate that H2S increases the quantity of calcium ions (Ca2+) contained with the sarcoplasmic reticulum (SR), the intracellular Ca2+ store, of cerebral arteriole smooth muscle cells.
This elevation in SR Ca2+ stimulates the generation of local intracellular Ca2+ signals called Ca2+ sparks, which in turn activate Ca2+-sensitive potassium (KCa) channels on the cell membrane, leading to membrane hyperpolarization and vasodilatation.
Elucidating this novel mechanism of H2S-induced vasodilatation is important to better understand physiological control of blood flow within the brain.
Introduction
Hydrogen sulfide (H2S), a physiological gasotransmitter, is generated in mammalian cells through the metabolism of l-cysteine by cystathionine β-synthase and cystathionine γ-lyase (Porter et al. 1974; Allsop & Watts, 1975; Wang, 2002). H2S modulates blood flow, blood pressure, synaptic neurotransmission, immune response, hormone secretion and muscle relaxation (Fiorucci et al. 2005; Leffler et al. 2006; Austgen et al. 2011). H2S induces vasodilatation in many different vascular beds, including rat mesenteric arteries, aorta, tibial arteries and piglet cerebral arterioles (Cheng et al. 2004; Liu & Bian, 2010; Schleifenbaum et al. 2010; Leffler et al. 2011; Liang et al. 2011). Several vascular ion channels have been reported to be involved in H2S-induced vasodilatation, including ATP-sensitive K+ (KATP) channels, Ca2+-activated K+ (KCa) channels, KCNQ channels and L-type Ca2+ channels (Cheng et al. 2004; Dombkowski et al. 2004; Schleifenbaum et al. 2010; Leffler et al. 2011; Zuidema et al. 2010; Liang et al. 2011). However, smooth muscle cell ion channels that are specifically targeted by H2S are unclear as are the mechanisms by which H2S modulates these proteins.
In smooth muscle cells, ion channels generate and regulate local and global intracellular Ca2+ signals, which can control vascular contractility (Jaggar et al. 2000). Conversely, local and global intracellular Ca2+ signals can regulate the activity of plasma membrane ion channels, which feed back to modify local and global intracellular Ca2+ signalling (Jaggar et al. 2000). The regulation of local and global Ca2+ signals by H2S in vascular smooth muscle cells is unclear. Establishing such regulation may reveal mechanisms by which this gaseous vasodilator controls vascular contractility.
Three primary Ca2+ signals occur in arterial smooth muscle cells, termed Ca2+ sparks, Ca2+ waves and global [Ca2+]i (Jaggar et al. 2000). Ca2+ sparks occur due to the concerted opening of multiple sarcoplasmic reticulum (SR) ryanodine receptor (RyR) channels (Nelson et al. 1995; Jaggar et al. 2000). Ca2+ sparks activate nearby plasma membrane KCa channels, leading to transient KCa currents that hyperpolarize the membrane potential. Membrane hyperpolarization reduces voltage-dependent Ca2+ channel activity, leading to a reduction in global intracellular Ca2+ concentration and vasodilatation (Nelson et al. 1995). Ca2+ waves are propagating SR Ca2+ release events that occur due to the activation of SR inositol trisphosphate-gated Ca2+ release channels and RyR channels (Jaggar, 2007). Global [Ca2+]i is the spatially homogeneous [Ca2+]i to which plasma membrane Ca2+ influx and SR Ca2+ release can contribute (Jaggar et al. 2000). Voltage-dependent L-type Ca2+ (CaV1.2) channels are a major contributor to global [Ca2+]i (Jaggar et al. 2000). Local Ca2+ gradients, termed Ca2+ sparklets, are generated by the opening of voltage-dependent Ca2+ channels and contribute to global [Ca2+]i (Santana & Navedo, 2009).
Here, we investigated the regulation of local and global [Ca2+]i signals by H2S in cerebral arteriole smooth muscle cells, the underlying mechanisms, and the involvement in H2S-induced cerebral arteriole dilatation. Data indicate that H2S elevates [Ca2+]SR, which stimulates Ca2+ sparks, leading to an increase in transient KCa current frequency, a reduction in global [Ca2+]i and vasodilatation. In contrast, H2S did not alter the activity of Ca2+ waves or directly regulate single KCa channels. These data define a novel mechanism of action of H2S and indicate that an elevation in SR Ca2+ load ([Ca2+]SR)is a specific mechanism of vasodilatation induced by this gasotransmitter.
Methods
Tissue preparation
All procedures used were approved by the University of Tennessee Health Science Center Animal Care and Use Committee. The authors have read, and the experiments comply with the policies and regulations of The Journal of Physiology given by Drummond (2009). Newborn pigs (1–3 days old, 1–2.5 kg body weight) were anaesthetized with ketamine hydrochloride (33 mg kg−1i.m.) and acepromazine (3.3 mg kg−1i.m.). The brain was removed and maintained in ice-cold (4°C) Hepes-buffered physiological saline solution (PSS) containing (in mm): 134 NaCl, 6 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, and 10 glucose, with pH adjusted to 7.4 with NaOH. Isolated arterioles (50–200 μm) were dissected from the brain and cleaned to remove basolateral connective tissue. Individual smooth muscle cells were dissociated from cerebral arterioles using a procedure similar to that previously described (Jaggar, 2001). Arterioles were endothelium denuded by intraluminal injection of air for 1 min, as previously described (Adebiyi et al. 2008). Unless stated otherwise, all procedures were performed at room temperature (22–25°C).
Confocal Ca2+ imaging
Piglet cerebral arterioles (∼2 mm in length) were placed in Hepes-buffered PSS containing 10 μm fluo-4 AM and 0.05% Pluronic F-127 for 20 min at room temperature followed by a 30 min wash with Hepes-buffered PSS to allow indicator deesterification. Bath solution that contained (in mm): 110 NaCl, 30 KCl, 10 Hepes, 2 CaCl2, 1 MgCl2, and 10 glucose (adjusted to pH 7.4 with NaOH) was used to depolarize arteries to approximately −40 mV, as previously described (Jaggar, 2001). Fluorescence images were collected using a Noran OZ laser-scanning confocal microscope (Noran Instruments, Middleton, WI, USA) and a ×60 water immersion objective (1.2 NA) attached to a TE300 microscope (Nikon), as described previously (Jaggar, 2001). Fluo-4 AM was illuminated at 488 nm using a krypton–argon laser, and emitted light >500 nm was captured. Images (56.3 × 52.8 μm) were recorded every 16.67 ms (i.e. 60 images per second). Custom analysis software (kindly provided by Dr M. T. Nelson, University of Vermont) was used to detect Ca2+ signals in smooth muscle cells. For detection of Ca2+ sparks, an area 1.54 × 1.54 μm (7 × 7 pixels, i.e. 2.37 μm2) in each image (F) was divided by a baseline (F0) that was determined by averaging 10 images without Ca2+ spark activity. The entire image area was analysed to detect Ca2+ sparks. A Ca2+ spark was identified as a local increase in F/F0 that was >1.2. Arterial Ca2+ spark frequency (measured in Hz) was calculated by averaging values from at least two different areas of the same arteriole. Ca2+ waves were defined as an elevation in F/F0 >1.2 that propagated for at least 20 μm. Ca2+ waves were detected by placing 2.2 × 2.2 μm (10 × 10 pixels, i.e. 4.84 μm2) in each smooth muscle cell and by using a method similar to that for Ca2+ spark detection. Changes in local or global [Ca2+]i were calculated using the pseudoratio method: [Ca2+] = KR/(K/([Ca2+]rest+ 1 –R)). K is the apparent affinity of fluo-4 AM for Ca2+ (770 nm; Woodruff et al. 2002), R is the fractional fluorescence increase (F/F0), and [Ca2+]rest is [Ca2+]i at F0. [Ca2+]rest was 224 nm, as previously determined by ratiometric imaging of fura-2 in newborn cerebral arterioles (Li et al. 2006). Global [Ca2+]i fluorescence was calculated from the same images used for Ca2+ spark and wave analysis and was the mean pixel value of 100 different images acquired during a 10 s period (Cheranov & Jaggar, 2004; Xi et al. 2005; Cheranov & Jaggar, 2006).
Patch-clamp electrophysiology
Isolated smooth muscle cells were allowed to adhere to a glass coverslip in the bottom of a chamber for 10 min before experimentation. K+ currents were measured using the amphotericin B perforated-patch configuration with an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA). The bath solution was Hepes-buffered PSS, and the pipette solution contained (in mm): 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 Hepes, and 0.05 EGTA, with pH adjusted to 7.2 using KOH. Membrane currents were filtered at 1 kHz and digitized at 4 kHz. In each patch under each condition, transient KCa current frequency and amplitude or KCa channel activity was calculated from at least 5 min of continuous gap-free data.
Sarcoplasmic reticulum Ca2+ load measurements
[Ca2+]SR was estimated by measuring the amplitude of [Ca2+]i transients induced by caffeine (10 or 20 mm), a RyR channel activator (Cheranov & Jaggar, 2002, 2004; Xi et al. 2005). Endothelium-denuded arterioles were incubated in Hepes-buffered PSS containing 5 μm fura-2 AM and 0.1% Pluronic F-127 for 30 min at room temperature. After wash, arteries were allowed to de-esterify the indicator for 15 min. Fura-2 AM was alternately excited with 340 or 380 nm light using a xenon arc lamp and a personal computer-driven hyperswitch (Ionoptix, Milton, MA, USA). Background corrected ratios were collected every 1 s at 510 nm using a Dage MTI integrating CCD camera (IonOptix).
Pressurized artery diameter measurements
A cerebral arteriole segment ∼2 mm in length was cannulated at each end in a temperature-controlled perfusion chamber (Living Systems Instrumentation, Burlington, VT, USA). The chamber was continuously perfused with PSS of the following composition (mm): 112 NaCl, 4.8 KCl, 24 NaHCO3, 1.8 CaCl2, 1.2 MgSO4, 1.2 KH2PO4 and 10 glucose, equilibrated with a mixture of 21% O2, 5% CO2 and 74% N2, and maintained at 35°C. Steady-state changes in intravascular pressure were achieved by elevating and lowering an attached reservoir and monitored using a pressure transducer. Intraluminal PSS was static during experiments. Arterioles were observed with a charge-coupled device camera attached to an inverted microscope (Nikon TS 100). Arteriole diameter was measured by using the automatic edge-detection function of IonWizard software (Ionoptix, Milton, MA, USA) and digitized at 1 Hz using a personal computer. Tested compounds were applied via chamber perfusion to maintain constant concentration during experiments.
Pressurized artery membrane potential measurements
Arterioles were maintained at 40 mmHg for 2 h to ensure steady-state myogenic tone had occurred prior to obtaining membrane potential recordings. Membrane potential was measured by impaling arterioles with glass microelectrodes filled with 3 m KCl (50–90 mΩ) from the adventitial side. Membrane potential was recorded using a WPI FD223 amplifier, pCLAMP 9.2 software (Molecular Devices) and a personal computer. Successful intracellular impalements required a rapid negative potential change upon insertion; a stable voltage for at least 25 s; a fast positive voltage deflection upon removal, and a <10% change in tip resistance after impalement.
Statistical analysis
Values are reported as means ± SEM; n refers to the number of events analysed, unless otherwise specified. Student's t test was used for comparison of paired data, except for statistical analysis of Ca2+ spark frequency and amplitude and Ca2+ wave frequency, where a non-parametric Wilcoxon's matched pairs test was used. ANOVA with the Student–Newman–Keuls post hoc test was used for multiple group comparison. P < 0.05 was considered significant.
Chemicals
Fluo-4 AM, fura-2 AM and Pluronic F-127 were purchased from Molecular Probes (Eugene, OR, USA). Iberiotoxin was purchased from California Peptide Research Inc. (Napa, CA, USA). Unless otherwise stated, all other chemicals were obtained from Sigma Chemical (St Louis, MO, USA).
Results
H2S activates Ca2+ sparks and reduces global [Ca2+]i in smooth muscle cells of intact cerebral arterioles
The regulation of local and global Ca2+ signals by H2S was measured in smooth muscle cells of piglet cerebral arteriole segments. For this study, we used Na2S as an H2S donor. Na2S (10 μm) increased mean Ca2+ spark frequency from ∼2.8 Hz to 4.0 Hz, or 1.43-fold (Fig. 1A and B). In contrast, H2S did not change mean Ca2+ spark amplitude or Ca2+ wave frequency (Fig. 1C and D). H2S decreased mean global F/F0 by ∼16%, which translates to a reduction in global intracellular Ca2+ concentration [Ca2+]i from 224 ± 29 nm (Li et al. 2006) to 187 ± 12 nm (Fig. 1E). These data indicate that H2S elevates Ca2+ spark frequency and reduces global [Ca2+]i, but does not alter Ca2+ waves in cerebral arteriole smooth muscle cells.
Figure 1. Hydrogen sulfide (H2S) regulates local and global Ca2+ signals in cerebral arteriole smooth muscle cells.
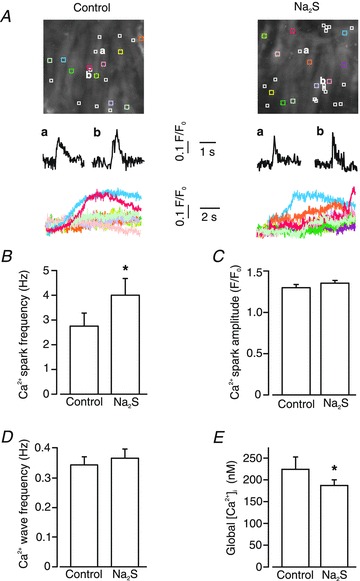
A, confocal images in upper panels illustrate average fluo-4 fluorescence in smooth muscle cells in the same arteriole in control and Na2S (10 μm). White boxes illustrate locations where sparks occurred during 10 s of imaging. Coloured boxes illustrate locations from where corresponding normalized fluorescence (F/F0) over time traces from each cell shown in the lower panels were determined. Middle panels show two representative Ca2+ sparks that occurred at locations labelled as a and b in each condition. Lower panels indicate intracellular Ca2+ changes that occurred in corresponding coloured boxes over time with Ca2+ waves evident in a proportion of cells. H2S increased Ca2+ spark frequency (B), but did not change Ca2+ spark amplitude (C) (n = 8 for each condition). D, H2S did not alter Ca2+ wave frequency (n = 8 for each condition). E, H2S decreased global [Ca2+]i from 224 ± 29 nm to 187 ± 12 nm (n = 8). *P < 0.05 vs. control.
H2S activates transient KCa currents in isolated arteriole smooth muscle cells
Ca2+ sparks activate transient KCa currents in cerebral artery and arteriole smooth muscle cells (Jaggar et al. 2000, 2002). H2S regulation of Ca2+ spark-induced transient KCa currents was measured in isolated cerebral arteriole smooth muscle cells using the perforated patch-clamp configuration. At a membrane potential of −40 mV, Na2S (10 μm) increased mean transient KCa current frequency from ∼0.23 to 0.42 Hz, or 1.83-fold (Fig. 2A and B). In contrast, Na2S did not alter mean transient KCa current amplitude (Fig. 2A and C). To further investigate Na2S regulation of transient KCa current amplitude, events were divided into small (<25 pA), medium (25–50 pA) and large (>50 pA) amplitude groups, as we have done previously (Li et al. 2008). Na2S did not significantly alter the amplitude distribution of these groups (Fig. 2A and D). When applied in the presence of Na2S, iberiotoxin, a selective KCa channel blocker, essentially abolished transient KCa currents, reducing mean frequency and amplitude to ∼0.5 and 10.2% of that in Na2S (Fig. 2B and C). These data indicate that H2S-induced Ca2+ spark activation leads to an elevation in transient KCa current frequency, but does not alter transient KCa current amplitude, in cerebral arteriole smooth muscle cells.
Figure 2. H2S increases transient KCa current frequency in cerebral arteriole smooth muscle cells.
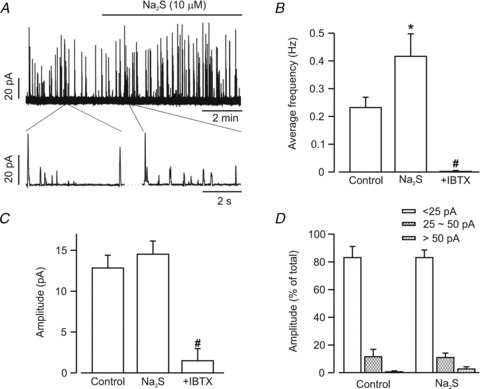
A, representative recording illustrating Na2S (10 μm) induced an elevation in transient KCa current frequency, but not amplitude. B, mean transient KCa current frequency in the same cells in control (n = 11 cells) and Na2S (10 μm, n = 11 cells), and in Na2S+iberiotoxin (IBTX, 100 nm, n = 5 cells). C, transient KCa current mean amplitude was not altered by Na2S (n = 11 cells), but was reduced by iberiotoxin (IBTX, n = 5 cells). D, Na2S did not change the proportion of transient KCa currents in each transient KCa current amplitude category (n = 11 cells for control and Na2S). All currents were recorded at a steady holding potential of −40 mV. *P < 0.05 vs. control. #P < 0.05 vs. Na2S.
H2S does not directly activate KCa channels in cerebral arteriole smooth muscle cells
In piglet cerebral arteriole smooth muscle cells, a proportion (∼40%) of Ca2+ sparks do not activate a transient KCa current (Jaggar et al. 2002). Carbon monoxide, a gasotransmitter that elevates KCa channel Ca2+ sensitivity induces coupling of KCa channels to these smaller amplitude Ca2+ sparks (Jaggar et al. 2002; Xi et al. 2004; Li et al. 2006). To investigate the possibility that H2S stimulates transient KCa currents through an effect on KCa channels, the regulation of single KCa channels by H2S was measured in isolated cerebral arteriole smooth muscle cells. Thapsigargin (100 nm), a SR Ca2+-ATPase (SERCA) inhibitor, was used to deplete [Ca2+]SR and abolish transient KCa currents. From a control activity (NPo) of 0.35 ± 0.1, H2S did not alter KCa channel activity (95 ± 17% of control, P > 0.05; Fig. 3). These data indicate that H2S does not activate KCa channels, further supporting other data here that H2S activates KCa channels solely by elevating Ca2+ spark frequency.
Figure 3. H2S does not alter single KCa channel activity in intact arteriole smooth muscle cells.
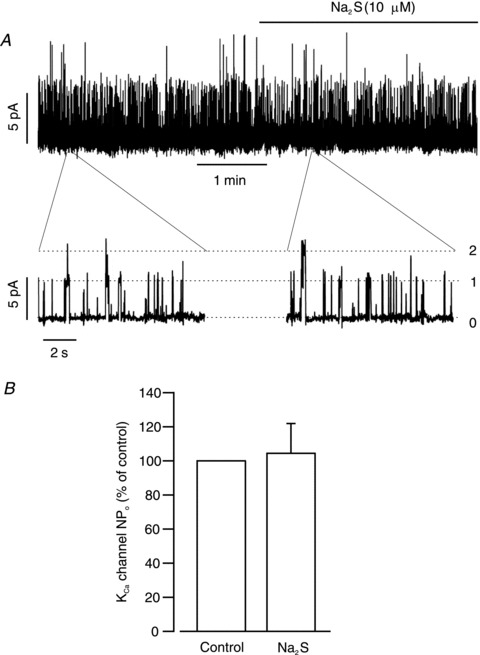
A, representative trace illustrating that H2S did not change single KCa channel activity in an arteriole smooth muscle cell. B, mean data (n = 6 cells). All currents were recorded at a steady holding potential of 0 mV. Cells were pre-treated with thapsigargin (100 nm) to inhibit Ca2+ spark-induced transient KCa currents.
Na2S elevates sarcoplasmic reticulum Ca2+ load
[Ca2+]SR regulates Ca2+ spark frequency (Wellman et al. 2001; Cheranov & Jaggar, 2002). Therefore, the regulation of [Ca2+]SR by H2S was studied by measuring the amplitude of caffeine-induced [Ca2+]i transients in endothelium-denuded piglet cerebral arterioles. Caffeine (10 mm)-stimulated [Ca2+]i transients under control conditions (Fig. 4A and B). Na2S increased caffeine (10 mm)-induced [Ca2+]i transients to 161 ± 13% (1st application) and 153 ± 11% (2nd application) of control (n = 5 arterioles, P < 0.05; Fig. 4). Conceivably, Na2S may elevate caffeine-induced [Ca2+]i transients by increasing RyR caffeine sensitivity. To investigate this possibility, experiments were repeated using a higher caffeine concentration (20 mm). Na2S similarly increased caffeine (20 mm)-induced [Ca2+]i transients to 149 ± 14% (1st application) and 167 ± 12% (2nd application) of control (n = 5 arterioles, P < 0.05). Data were not significantly different when comparing the first and second caffeine applications at 10 and 20 mm (P > 0.05 for each). These data indicate that H2S elevates [Ca2+]SR.
Figure 4. H2S elevates [Ca2+]SR.
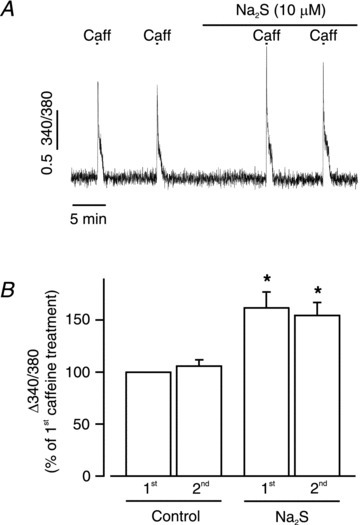
A, original trace illustrating that Na2S (10 μm) increased caffeine (Caff, 10 mm)-induced [Ca2+]i transients in an isolated piglet cerebral arteriole. 340/380, 340 to 380 nm ratio. B, mean change in fura-2 AM ratio in two sequential control conditions and Na2S (n = 5 arterioles). *P < 0.05 vs. 1st caffeine-induced [Ca2+]i transient.
H2S induces iberiotoxin-sensitive hyperpolarization in pressurized cerebral arterioles
To investigate the functional significance of H2S-induced transient KCa current activation, the membrane potential of pressurized arterioles was measured using glass microelectrodes. At an intravascular pressure of 40 mmHg, mean arteriole membrane potential was ∼−30.3 mV (Fig. 5A and B). Na2S (10 μm) hyperpolarized pressurized arterioles to ∼−47.9 mV, or by 17.6 mV (Fig. 5A and B). Application of iberiotoxin, a selective KCa channel blocker, in the presence of Na2S returned mean membrane potential to ∼−39.0 mV, inhibiting the Na2S-induced hyperpolarization by ∼51% (Fig. 5A and B).
Figure 5. H2S induces iberiotoxin-sensitive hyperpolarization in pressurized arterioles.
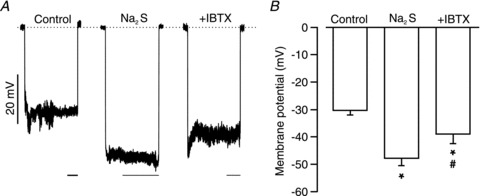
A, original recordings of membrane potential upon microelectrode impalement in pressurized (40 mmHg) arterioles in control, Na2S (10 μm) and Na2S (10 μm) + iberiotoxin (100 nm). Dotted line indicates 0 mV. Scale bars are 20 s. B, mean data (control, n = 6; Na2S, n = 5; Na2S + iberiotoxin, n = 5). *P < 0.05 when compared with control and # when compared with Na2S.
H2S dilates cerebral arterioles by activating RyR and KCa channels
Edge-detection myography was performed to study contractility regulation by H2S and involvement of Ca2+ spark and KCa channel activation in pressurized arterioles. At 40 mmHg, arterioles constricted from a mean passive diameter of 231 ± 10 μm to a myogenic diameter of 132 ± 8 μm, or by 43% (n = 11). Na2S (10 μm) increased mean arteriole diameter by ∼33 μm (Fig. 6A and C). Iberiotoxin (100 nm) or ryanodine, a RyR channel blocker (10 μm), did not alter the diameter of pressurized arterioles when applied alone (Fig. 6B and D). In contrast, iberiotoxin partially reversed Na2S-induced vasodilatation from ∼32 to 20 μm, or by 38% (Fig. 6A and B). Ryanodine also partially reversed Na2S-induced vasodilatation from ∼35 to 22 μm, or by 37% (Fig. 6C and D). These data indicate that H2S-induced cerebral arteriole vasodilatation occurs in part via Ca2+ spark-induced KCa channel activation in piglet cerebral arteriole smooth muscle cells.
Figure 6. H2S dilates pressurized (40 mmHg) piglet cerebral arterioles via RyR and KCa channel activation.
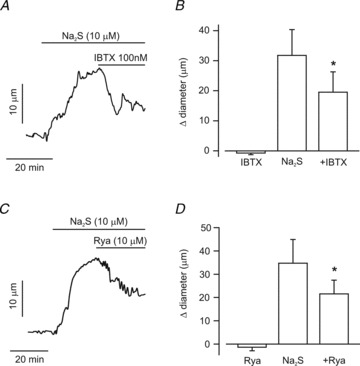
A, representative trace illustrating that iberiotoxin (IBTX, 100 nm), partially reverses vasodilatation induced by Na2S (10 μm). B, mean data (IBTX, n = 5; Na2S and Na2S + IBTX, n = 9 for each). C, representative trace illustrating that ryanodine (Rya, 10 μm) partially reverses vasodilatation induced by Na2S (10 μm). D, mean data (Rya, Na2S, and Na2S + Rya, n = 6 for each). *P < 0.05 vs. Na2S.
Discussion
Here, we investigated the regulation of local and global intracellular Ca2+ signals by H2S in smooth muscle cells of piglet cerebral arterioles. Major findings of this study are that (1) H2S activates Ca2+ sparks but not Ca2+ waves, and reduces global intracellular Ca2+ concentration ([Ca2+]i), (2) H2S activation of Ca2+ sparks leads to an increase in transient KCa current frequency, (3) H2S increases [Ca2+]SR, (4) H2S does not directly activate KCa channels, (5) H2S hyperpolarizes pressurized cerebral arterioles via KCa channel activation, and (6) H2S dilates pressurized cerebral arterioles and this dilatation is partially reversed by RyR and KCa channel blockers. Collectively, these data indicate that H2S elevates [Ca2+]SR, which stimulates Ca2+ sparks that increase transient KCa currents, leading to membrane hyperpolarization, a reduction in global [Ca2+]i and vasodilatation.
H2S is generated endogenously in a wide variety of mammalian tissues, including brain, liver, heart, aorta and kidney (Ubuka, 2002). Physiological concentrations of H2S in rat and human blood are between 5 and 50 μm (Zhao et al. 2001; Ubuka, 2002; Li et al. 2005; Elsey et al. 2010). In piglet cerebrospinal fluid, we measured a mean H2S concentration during stimulation with hypercapnia of approximately 4 μm (Leffler et al. 2011). Here, we used Na2S, a commonly used H2S donor, to elevate extracellular H2S. Recent phase 1 human clinical trials have also used Na2S as a potential therapeutic treatment for myocardial infarction (Li et al. 2009). Na2S at 10 μm generates ∼5 μm H2S gas in physiological saline solution at room temperature within 1 min (Liang et al. 2011). Therefore, the Na2S concentration studied here produces H2S concentrations within the physiological range. In donating H2S, Na2S contributes only micromolar Na+ as an additional atom. Bath solutions used for our experiments contain 140 mm Na+. A 10 μm Na+ elevation will have insignificant effects on Ca2+ sparks, transient KCa currents and arteriole contractility. We have previously shown that Na2S and NaHS, another H2S donor, produce quantitatively similar KATP current activation in cerebral arteriole smooth muscle cells, indicating that these donors act through H2S donation (Liang et al. 2011).
In arterial smooth muscle cells, a single Ca2+ spark activates multiple KCa channels, leading to a transient KCa current (Nelson et al. 1995). Asynchronous transient KCa currents hyperpolarize arterial membrane potential, thereby reducing voltage-dependent Ca2+ channel activity and [Ca2+]i, leading to vasodilatation (Jaggar et al. 2000). Several vasodilators, including those that activate adenylyl cyclase or soluble guanylyl cyclase, activate Ca2+ sparks (Porter et al. 1998; Cheranov & Jaggar, 2006; Mandala et al. 2007; Li et al. 2008). Such Ca2+ spark stimulation elevates transient KCa current frequency, leading to membrane hyperpolarization, a reduction in voltage-dependent Ca2+ channel activity, a decrease in [Ca2+]i, and vasodilatation (Jaggar & Nelson, 2000; Cheng & Lederer, 2008). Here, we provide the first direct evidence that H2S activates Ca2+ sparks in arteriole smooth muscle cells. H2S increased Ca2+ spark frequency, but did not alter Ca2+ spark amplitude. Consistent with this result, H2S increased mean transient KCa current frequency, but did not alter mean amplitude. We have previously demonstrated that transient KCa current amplitude is not normally distributed in piglet cerebral arteriole smooth muscle cells (Li et al. 2008). Therefore, H2S-induced changes in transient KCa current amplitude may not be revealed by comparing mean data. Previous studies from our laboratory indicated that although carbon monoxide (CO) elevates the effective coupling of KCa channels to Ca2+ sparks, astrocyte-derived CO did not alter transient KCa current mean amplitude (Jaggar et al. 2002; Xi et al. 2004; Li et al. 2008). Categorizing transient KCa currents into three amplitude groups indicated that CO increased the frequency of smaller amplitude transient KCa currents more than larger events, thereby depressing the mean amplitude (Li et al. 2008). The emergence of a new population of transient KCa currents occurs due to CO-induced coupling of previously uncoupled smaller amplitude Ca2+ sparks to KCa channels (Jaggar et al. 2002). Similar to our previous study, data here indicate that the largest proportion of transient KCa currents are smaller events (<25 pA) in cerebral arteriole smooth muscle cells (Li et al. 2008). In contrast to CO, which elevates KCa channel sensitivity to Ca2+ sparks (Jaggar et al. 2002; Li et al. 2008), H2S did not alter transient KCa current amplitude distribution. These data indicate that H2S does not elevate effective coupling of KCa channels to Ca2+ sparks. Consistent with a lack of effect of H2S on transient KCa current mean amplitude or amplitude distribution, H2S also did not alter single KCa channel activity in intact arteriole smooth muscle cells. These data provide further support for our finding that H2S activates transient KCa currents specifically by elevating Ca2+ spark frequency. In contrast to our findings, NaHS, another H2S donor, at 0.1–1 mm, had no effect on transient KCa currents, but inhibited transient KCa currents at a very high concentration (4 mm) in guinea-pig gastric myocytes (Zhao et al. 2009).
H2S has been reported to both activate and inhibit KCa channels. Previous studies indicated that H2S activated KCa channels in different tissues (Sitdikova et al. 2010; Zuidema et al. 2010; Jackson-Weaver et al. 2011). Iberiotoxin blocked H2S-induced vasodilatation in rat mesenteric arteries, decreased H2S-induced KCa channel activation in rat pituitary tumour cell outside-out patches, and reversed an H2S-induced elevation in whole-cell KCa current in cultured human microvascular endothelial cells (Sitdikova et al. 2010; Zuidema et al. 2010; Jackson-Weaver et al. 2011). In contrast, H2S inhibited carotid body glomus cell KCa channels and recombinant KCa channels expressed in human embryonic kidney cells (Telezhkin et al. 2009; Li et al. 2010). Explanations for these different observations may include channel subunit composition, including whether and which β subunit isoforms are present and the α:β ratio, cell type and species studied, H2S concentrations used, and whether experiments were performed in intact cells or inside-out membrane patches, which may modify pH and oxidation state, and thus the proportion of H2S to HS−. It is also important to note that we have shown 300 μm and 1 mm Na2S induce an alkaline shift in pH from 7.4 to 7.54 and 7.75, respectively, in physiological saline solution (Liang et al. 2011). In contrast, lower concentrations of H2S donors, similar to those used here, do not alter pH. Therefore, pH alterations may underlie results in studies where high concentrations of H2S donor or H2S gas have been used. Of note, alkaline pH shifts Ca2+ sparks to waves in arterial smooth muscle cells and has been demonstrated to induce vasoconstriction (Austin & Wray, 1993; Heppner et al. 2002). Intracellular acidification also inhibits KCa channels by reducing Ca2+ sensitivity and open time in rabbit tracheal smooth muscle cells (Kume et al. 1990).
An elevation in [Ca2+]SR increases Ca2+ spark frequency in cardiac myocytes and smooth muscle cells (Satoh et al. 1997; Cheranov & Jaggar, 2002). To examine mechanisms by which H2S increased Ca2+ spark frequency, we measured H2S regulation of [Ca2+]SR. Na2S increased the amplitude of [Ca2+]i transients produced by two different millimolar caffeine concentrations similarly. These data indicate that Na2S elevates [Ca2+]SR. In contrast, Na2S did not alter global [Ca2+]i in these experiments. This is expected, because for these experiments the bath solution contained 6 mm K+, in contrast to the experiments shown in Fig. 1 where the bath solution contained 30 mm K+ to depolarize smooth muscle cells to physiological voltage. In 6 mm K+, unpressurized arterial potential is ∼−60 mV, and global [Ca2+]i, Ca2+ spark frequency, and KCa channel activity is low. Under this condition, an Na2S-induced elevation in Ca2+ sparks and thus, KCa channel activity would not be expected to further reduce global [Ca2+]i. These data indicate that an Na2S-induced elevation in [Ca2+]SR stimulates an increase in Ca2+ spark frequency, and thus transient KCa current frequency. Mechanisms by which H2S elevates [Ca2+]SR are likely to involve SERCA activation. Phospholamban is an endogenous inhibitor of SERCA (Hutter et al. 2002; Toyoshima et al. 2003). Protein kinase C, cAMP/cGMP-dependent protein kinases and Ca2+–calmodulin-dependent protein kinase II phosphorylate phospholamban, leading to an increase in SERCA activity (Wegener et al. 1986; Simmerman et al. 1986; Karczewski et al. 1997). H2S can also S-sulfhydrate cysteine residues, including in KATP channels, which elevates channel activity (Mustafa et al. 2011). Conceivably, H2S may activate SERCA through direct sulfhyrdration or via regulation of an upstream SERCA regulator, including phospholamban. It is possible that H2S also activates RyR channels and thus, Ca2+ sparks, by a SR Ca2+ load-independent mechanism. Future studies will investigate further the mechanisms by which H2S activates RyR channels to elevate Ca2+ spark frequency.
Consistent with H2S-induced Ca2+ spark and transient KCa current activation, our data indicate that H2S hyperpolarizes pressurized arterioles and reduces global [Ca2+]i in arteriole smooth muscle cells. These data are consistent with the established concept that transient KCa current activation leads to membrane hyperpolarization, which reduces voltage-dependent Ca2+ channel activity, and therefore, global [Ca2+]i (Jaggar & Nelson, 2000; Cheng & Lederer, 2008). Recent evidence indicated that NaHS, an H2S donor, produced iberiotoxin-sensitive membrane hyperpolarization in mesenteric arteries (Jackson-Weaver et al. 2011). In contrast, NaHS hyperpolarized coronary arteries via a 4-aminopyridine-sensitive mechanism (Cheang et al. 2010). Here, H2S activated Ca2+ sparks and reduced global [Ca2+]i, but did not alter Ca2+ waves. RyR channels can contribute to Ca2+ waves in arterial smooth muscle cells (Jaggar & Nelson, 2000). Conceivably, effects of H2S on Ca2+ waves may be concentration dependent, with higher non-physiological H2S concentrations, which can induce an alkaline shift in pH, stimulating RyR channels sufficiently to increase Ca2+ wave frequency (Heppner et al. 2002).
In pressurized piglet cerebral arterioles, iberiotoxin or ryanodine alone did not alter arterial diameter. This finding is consistent with previous measurements performed in vitro and in vivo indicating that baseline Ca2+ spark and KCa channel activity are too low in piglet cerebral arteriole smooth muscle cells to oppose pressure-induced vasoconstriction (Jaggar et al. 2002; Ahmed et al. 2004; Kanu & Leffler, 2007). We show that H2S produces hyperpolarization and vasodilatation in pressurized arterioles. Iberiotoxin partially reversed H2S-induced hyperpolarization and iberiotoxin and ryanodine both attenuated H2S-induced vasodilatation. These data indicate that H2S stimulates Ca2+ sparks that activate KCa channels, leading to hyperpolarization and vasodilatation. We have previously demonstrated that H2S-induced vasodilatation in pressurized piglet cerebral arterioles is also partially inhibited by glibenclamide, a KATP channel-specific inhibitor (Liang et al. 2011). Therefore, our data indicate that H2S dilates by activating both KCa and KATP channels in smooth muscle cells. Our previous in vivo study showed that glibenclamide can completely block H2S (10 μm)-induced piglet pial arteriole dilatation in cranial window experiments (Leffler et al. 2011). The greater effect of glibenclamide in vivo could be explained by different experiment conditions and potential impact of other cells. The cranial windows are surgically implanted and closed. Under cranial window, cerebral arterioles are in contact with meninges, neurons and particularly astrocytes. Topically applied H2S may affect not only pial arterioles but also astrocytes or neurons. Those cells also could affect pial arterial dilatation. Oxygen partial pressure is ∼40 mmHg in cranial window experiments, but ∼150 mmHg in the pressurized arterial experiments. Finally, the pressurized arterioles in the present study had mean diameters of ∼150 μm, while the arterioles measured in vivo were much smaller at ∼50 μm. Any or all of these differences may allow detection of a role for KCa channels in dilatation to H2S that was not detected in the previous study in vivo. Collectively, these data indicate that H2S can dilate piglet cerebral arterioles via the activation of both KCa and KATP channels, the former mediated through Ca2+ spark activation. Recent evidence indicates that KATP channels are the primary vasodilatory target of H2S in non-pressurized mouse and rat aorta and mesenteric arteries (Cheng et al. 2004; Mustafa et al. 2011). Therefore, vascular origin, size, age and species may underlie different vasodilatory mechanisms for H2S and specific ion channels targeted.
In summary, we demonstrate that H2S elevates [Ca2+]SR, which stimulates Ca2+ sparks, leading to an increase in transient KCa current frequency in cerebral arteriole smooth muscle cells. The H2S-induced elevation in KCa channel activity produces membrane hyperpolarization that reduces global [Ca2+]i, leading to vasodilatation.
Acknowledgments
This research was supported by NIH/NHLBI grants HL67061, HL94378 and HL110347 to J.H.J., and HL34059 and HL42851 to C.W.L. We thank Dr Marie Dennis Leo for comments on the manuscript.
Glossary
Abbreviations
- RyR
ryanodine receptor
- SERCA
SR Ca2+-ATPase
- SR
sarcoplasmic reticulum
Author contributions
G.H.L. performed and analysed experiments, contributed to study design and wrote the manuscript; Q.X. performed experiments; J.H.J. and C.W.L. designed the study and wrote the manuscript. All authors approved the final version.
References
- Adebiyi A, McNally EM, Jaggar JH. Sulfonylurea receptor-dependent and -independent pathways mediate vasodilation induced by ATP-sensitive K+ channel openers. Mol Pharmacol. 2008;74:736–743. doi: 10.1124/mol.108.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Waters CM, Leffler CW, Jaggar JH. Ionic mechanisms mediating the myogenic response in newborn porcine cerebral arteries. Am J Physiol Heart Circ Physiol. 2004;287:H2061–H2069. doi: 10.1152/ajpheart.00660.2004. [DOI] [PubMed] [Google Scholar]
- Allsop J, Watts RW. Methionine adenosyltransferase, cystathionine β-synthase and cystathionine γ-lyase activity of rat liver subcellular particles, human blood cells and mixed white cells from rat bone marrow. Clin Sci Mol Med Suppl. 1975;48:509–513. doi: 10.1042/cs0480509. [DOI] [PubMed] [Google Scholar]
- Austgen JR, Hermann GE, Dantzler HA, Rogers RC, Kline DD. Hydrogen sulfide augments synaptic neurotransmission in the nucleus of the solitary tract. J Neurophysiol. 2011;106:1822–1832. doi: 10.1152/jn.00463.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C, Wray S. Extracellular pH signals affect rat vascular tone by rapid transduction into intracellular pH changes. J Physiol. 1993;466:1–8. [PMC free article] [PubMed] [Google Scholar]
- Cheang WS, Wong WT, Shen B, Lau CW, Tian XY, Tsang SY, Yao X, Chen ZY, Huang Y. 4-Aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascul Pharmacol. 2010;53:94–98. doi: 10.1016/j.vph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- Cheranov SY, Jaggar JH. Sarcoplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient KCa currents. J Physiol. 2002;544:71–84. doi: 10.1113/jphysiol.2002.025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheranov SY, Jaggar JH. Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries. J Physiol. 2004;556:755–771. doi: 10.1113/jphysiol.2003.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheranov SY, Jaggar JH. TNF-α dilates cerebral arteries via NAD(P)H oxidase-dependent Ca2+ spark activation. Am J Physiol Cell Physiol. 2006;290:C964–C971. doi: 10.1152/ajpcell.00499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombkowski RA, Russell MJ, Olson KR. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol Regul Integr Comp Physiol. 2004;286:R678–R685. doi: 10.1152/ajpregu.00419.2003. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsey DJ, Fowkes RC, Baxter GF. Regulation of cardiovascular cell function by hydrogen sulfide (H2S) Cell Biochem Funct. 2010;28:95–106. doi: 10.1002/cbf.1618. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di SM, Morelli A, Cirino G, Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Santana LF, Nelson MT. Alkaline pH shifts Ca2+ sparks to Ca2+ waves in smooth muscle cells of pressurized cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283:H2169–H2176. doi: 10.1152/ajpheart.00603.2002. [DOI] [PubMed] [Google Scholar]
- Hutter MC, Krebs J, Meiler J, Griesinger C, Carafoli E, Helms V. A structural model of the complex formed by phospholamban and the calcium pump of sarcoplasmic reticulum obtained by molecular mechanics. Chembiochem. 2002;3:1200–1208. doi: 10.1002/1439-7633(20021202)3:12<1200::AID-CBIC1200>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Jackson-Weaver O, Paredes DA, Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca2+-activated potassium channels. Circ Res. 2011;108:1439–1447. doi: 10.1161/CIRCRESAHA.110.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- Jaggar JH. Smooth muscle sparklet Cav channels defined: 1.2 is the number. Am J Physiol Heart Circ Physiol. 2007;293:H1317–H1319. doi: 10.1152/ajpheart.00613.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, E S, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res. 2002;91:610–617. doi: 10.1161/01.res.0000036900.76780.95. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1528–C1539. doi: 10.1152/ajpcell.2000.279.5.C1528. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Kanu A, Leffler CW. Carbon monoxide and Ca2+-activated K+ channels in cerebral arteriolar responses to glutamate and hypoxia in newborn pigs. Am J Physiol Heart Circ Physiol. 2007;293:H3193–H3200. doi: 10.1152/ajpheart.00274.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski P, Kuschel M, Baltas LG, Bartel S, Krause EG. Site-specific phosphorylation of a phospholamban peptide by cyclic nucleotide- and Ca2+/calmodulin-dependent protein kinases of cardiac sarcoplasmic reticulum. Basic Res Cardiol. 1997;92(Suppl 1):37–43. doi: 10.1007/BF00794066. [DOI] [PubMed] [Google Scholar]
- Kume H, Takagi K, Satake T, Tokuno H, Tomita T. Effects of intracellular pH on calcium-activated potassium channels in rabbit tracheal smooth muscle. J Physiol. 1990;424:445–457. doi: 10.1113/jphysiol.1990.sp018076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Basuroy S, Jaggar JH, Umstot ES, Fedinec AL. Hydrogen sulfide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol. 2011;300:H440–H447. doi: 10.1152/ajpheart.00722.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol. 2006;100:1065–1076. doi: 10.1152/japplphysiol.00793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Adebiyi A, Leffler CW, Jaggar JH. KCa channel insensitivity to Ca2+ sparks underlies fractional uncoupling in newborn cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H1118–H1125. doi: 10.1152/ajpheart.01308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, Leffler CW. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle cell KCa channels. Circ Res. 2008;102:234–241. doi: 10.1161/CIRCRESAHA.107.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- Li L, Hsu A, Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation – a tale of three gases! Pharmacol Ther. 2009;123:386–400. doi: 10.1016/j.pharmthera.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Li Q, Sun B, Wang X, Jin Z, Zhou Y, Dong L, Jiang LH, Rong W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid Redox Signal. 2010;12:1179–1189. doi: 10.1089/ars.2009.2926. [DOI] [PubMed] [Google Scholar]
- Liang GH, Adebiyi A, Leo MD, McNally EM, Leffler CW, Jaggar JH. Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am J Physiol Heart Circ Physiol. 2011;300:H2088–H2095. doi: 10.1152/ajpheart.01290.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YH, Bian JS. Bicarbonate-dependent effect of hydrogen sulfide on vascular contractility in rat aortic rings. Am J Physiol Cell Physiol. 2010;299:C866–C872. doi: 10.1152/ajpcell.00105.2010. [DOI] [PubMed] [Google Scholar]
- Mandala M, Heppner TJ, Bonev AD, Nelson MT. Effect of endogenous and exogenous nitric oxide on calcium sparks as targets for vasodilation in rat cerebral artery. Nitric Oxide. 2007;16:104–109. doi: 10.1016/j.niox.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Porter PN, Grishaver MS, Jones OW. Characterization of human cystathionine β-synthase. Evidence for the identity of human L-serine dehydratase and cystathionine β-synthase. Biochim Biophys Acta. 1974;364:128–139. doi: 10.1016/0005-2744(74)90140-5. [DOI] [PubMed] [Google Scholar]
- Porter VA, Bonev AD, Knot HJ, Heppner TJ, Stevenson AS, Kleppisch T, Lederer WJ, Nelson MT. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. Am J Physiol. 1998;274:C1346–C1355. doi: 10.1152/ajpcell.1998.274.5.C1346. [DOI] [PubMed] [Google Scholar]
- Santana LF, Navedo MF. Molecular and biophysical mechanisms of Ca2+ sparklets in smooth muscle. J Mol Cell Cardiol. 2009;47:436–444. doi: 10.1016/j.yjmcc.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Blatter LA, Bers DM. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am J Physiol. 1997;272:H657–H668. doi: 10.1152/ajpheart.1997.272.2.H657. [DOI] [PubMed] [Google Scholar]
- Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, Crean CS, Luft FC, Huang Y, Schubert R, Gollasch M. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986;261:13333–13341. [PubMed] [Google Scholar]
- Sitdikova GF, Weiger TM, Hermann A. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflugers Arch. 2010;459:389–397. doi: 10.1007/s00424-009-0737-0. [DOI] [PubMed] [Google Scholar]
- Telezhkin V, Brazier SP, Cayzac S, Muller CT, Riccardi D, Kemp PJ. Hydrogen sulfide inhibits human BKCa channels. Adv Exp Med Biol. 2009;648:65–72. doi: 10.1007/978-90-481-2259-2_7. [DOI] [PubMed] [Google Scholar]
- Toyoshima C, Asahi M, Sugita Y, Khanna R, Tsuda T, Maclennan DH. Modeling of the inhibitory interaction of phospholamban with the Ca2+ ATPase. Proc Natl Acad Sci U S A. 2003;100:467–472. doi: 10.1073/pnas.0237326100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T. Assay methods and biological roles of labile sulfur in animal tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:227–249. doi: 10.1016/s1570-0232(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Wegener AD, Simmerman HK, Liepnieks J, Jones LR. Proteolytic cleavage of phospholamban purified from canine cardiac sarcoplasmic reticulum vesicles. Generation of a low resolution model of phospholamban structure. J Biol Chem. 1986;261:5154–5159. [PubMed] [Google Scholar]
- Wellman GC, Santana LF, Bonev AD, Nelson MT. Role of phospholamban in the modulation of arterial Ca2+ sparks and Ca2+-activated K+ channels by cAMP. Am J Physiol Cell Physiol. 2001;281:C1029–C1037. doi: 10.1152/ajpcell.2001.281.3.C1029. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, Fain GL. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol. 2002;542:843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res. 2005;97:354–362. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of α-subunits. Am J Physiol Heart Circ Physiol. 2004;286:H610–H618. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- Zhao P, Huang X, Wang ZY, Qiu ZX, Han YF, Lu HL, Kim YC, Xu WX. Dual effect of exogenous hydrogen sulfide on the spontaneous contraction of gastric smooth muscle in guinea-pig. Eur J Pharmacol. 2009;616:223–228. doi: 10.1016/j.ejphar.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidema MY, Yang Y, Wang M, Kalogeris T, Liu Y, Meininger CJ, Hill MA, Davis MJ, Korthuis RJ. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of BK channels. Am J Physiol Heart Circ Physiol. 2010;299:H1554–H1567. doi: 10.1152/ajpheart.01229.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


