Abstract
Leucine is a nutrient regulator of muscle protein synthesis by activating mTOR and possibly other proteins in this pathway. The purpose of this study was to examine the role of leucine in the regulation of human myofibrillar protein synthesis (MPS). Twenty-four males completed an acute bout of unilateral resistance exercise prior to consuming either: a dose (25 g) of whey protein (WHEY); 6.25 g whey protein with total leucine equivalent to WHEY (LEU); or 6.25 g whey protein with total essential amino acids (EAAs) equivalent to WHEY for all EAAs except leucine (EAA-LEU). Measures of MPS, signalling through mTOR, and amino acid transporter (AAT) mRNA abundance were made while fasted (FAST), and following feeding under rested (FED) and post-exercise (EX-FED) conditions. Leucinaemia was equivalent between WHEY and LEU and elevated compared to EAA-LEU (P = 0.001). MPS was increased above FAST at 1–3 h post-exercise in both FED (P < 0.001) and EX-FED (P < 0.001) conditions with no treatment effect. At 3–5 h, only WHEY remained significantly elevated above FAST in EX-FED (WHEY 184%vs. LEU 55% and EAA-LEU 35%; P = 0.036). AAT mRNA abundance was increased above FAST after feeding and exercise with no effect of leucinaemia. In summary, a low dose of whey protein supplemented with leucine or all other essential amino acids was as effective as a complete protein (WHEY) in stimulating postprandial MPS; however only WHEY was able to sustain increased rates of MPS post-exercise and may therefore be most suited to increase exercise-induced muscle protein accretion.
Key points
Essential amino acids (EAAs) stimulate increased rates of myofibrillar protein synthesis (MPS).
Leucine is a key regulator of MPS in rodents; however, its importance relative to the other EAAs is not clear.
About 20 g of protein maximally stimulates MPS after resistance exercise in young men, but we do not know if smaller doses can be made better by adding certain amino acids.
We report that a suboptimal dose of whey protein (6.25 g) supplemented with either leucine or a mixture of EAAs without leucine stimulates MPS similar to 25 g of whey protein under resting conditions; however, only 25 g of whey sustains exercise-induced rates of MPS.
Adding leucine or a mixture of EAAs without leucine to a suboptimal dose of whey is as effective as 25 g whey at stimulating fed rates of MPS; however, 25 g of whey is better suited to increase resistance exercise-induced muscle anabolism.
Introduction
Ingestion or infusion of amino acids stimulates an increase in skeletal muscle protein synthesis (Bennet et al. 1989; Bohe et al. 2001, 2003; Atherton et al. 2010a), an effect that is enhanced by prior resistance exercise (Tipton et al. 1999a; Wilkinson et al. 2007; Moore et al. 2009a,b; Tang et al. 2009; West et al. 2009). The essential amino acids (EAAs) are primarily responsible for this stimulation of muscle protein synthesis, with no apparent requirement for the non-essential amino acids (Smith et al. 1998; Tipton et al. 1999b; Borsheim et al. 2002; Volpi et al. 2003). Several animal studies have demonstrated that leucine independently stimulates muscle protein synthesis by activating components of the mammalian target of rapamycin (mTOR) signalling cascade (Anthony et al. 2000a,b, 2002; Bolster et al. 2004; Crozier et al. 2005). This activation appears critical for both the contraction (Drummond et al. 2009), and EAA-mediated (Dickinson et al. 2011) increase in muscle protein synthesis. Thus, leucine has been investigated as a pharmaconutrient with the potential to promote increases in muscle protein synthesis (Koopman et al. 2005, 2006, 2008; Katsanos et al. 2006; Rieu et al. 2006; Tipton et al. 2009; Glynn et al. 2010) and lean tissue mass (Verhoeven et al. 2009; Leenders et al. 2011). Nonetheless, while some studies indicate a role for leucine in the regulation of human muscle protein synthesis (Smith et al. 1992; Katsanos et al. 2006; Rieu et al. 2006), other studies have not found an enhanced rate of muscle protein synthesis following leucine infusion (Nair et al. 1992), after increasing the amount of leucine within a mixed EAA solution (Glynn et al. 2010), or by the addition of free leucine to a protein containing supplement (Koopman et al. 2008; Tipton et al. 2009).
There is a dose-dependent relationship between amino acid (Bohe et al. 2003; Cuthbertson et al. 2005) and protein (Moore et al. 2009a) provision and muscle protein synthesis. We previously reported that ∼20 g of isolated egg protein (containing ∼8.6 g EAAs and ∼1.7 g leucine) stimulated muscle protein synthesis after resistance exercise above that observed with both 5 g and 10 g of protein but was not further stimulated with ingestion of 40 g of protein, indicating that 20 g of egg protein is saturating for muscle protein synthesis after resistance exercise (Moore et al. 2009a). These data are consistent with previous reports of a dose-dependent relationship between EAA ingestion and myofibrillar protein synthesis (MPS) up to a maximal stimulation at ∼10 g EAAs (containing ∼2.1 g leucine; Cuthbertson et al. 2005). These dose–response data may provide insight into why other studies (Koopman et al. 2008; Tipton et al. 2009; Glynn et al. 2010) did not report a benefit of additional leucine on muscle protein synthesis when a sufficient amount of EAAs and/or leucine is provided.
Given what we know about the ingested protein dose–response of muscle protein synthesis (Bohe et al. 2003; Cuthbertson et al. 2005; Moore et al. 2009a), the aim of the present investigation was to examine the effects of supplementing a ‘suboptimal’ dose of whey protein (6.25 g whey containing ∼0.75 g of leucine) with additional leucine (LEU), or a mixture of EAAs with no leucine (EAA-LEU) on MPS at rest and following acute resistance exercise compared to a dose (25 g containing ∼3.0 g of leucine) of whey protein (WHEY) which is sufficient to induce a maximal stimulation of muscle protein synthesis after resistance exercise (Moore et al. 2009a). The suboptimal protein dose (6.25 g) was chosen to represent one-quarter of the 25 g dose in the WHEY treatment. We hypothesized that LEU would result in a stimulation of MPS equivalent to WHEY in both feeding (FED) and combined feeding and resistance exercise (EX-FED) conditions. Alternatively, we hypothesized that EAA-LEU would result in an increase in MPS in both the FED and EX-FED conditions, but the response would be significantly less than both LEU and WHEY due to the lower leucine content. In an attempt to gain insight into the mechanistic underpinnings of the response of MPS, we also examined changes in the phosphorylation status of protein targets of the Akt-mTOR pathway and in the mRNA abundance of select amino acid transporters (AATs) that have recently been shown to be regulated by EAAs (Drummond et al. 2010) and resistance exercise (Drummond et al. 2011).
Methods
Participants and ethical approval
Twenty-four recreationally active, young adult male participants (22 ± 0.6 years; 1.80 ± 0.02 m; 76.4 ± 2.0 kg; BMI 24.3 ± 0.6 kg m−2) voluntarily agreed to participate in the study. Participants were deemed healthy based on responses to a routine health screening questionnaire. Each participant was informed of the purpose of the study, the associated experimental procedures, and any potential risks prior to providing written consent. The study was approved by the Hamilton Health Sciences Research Ethics Board and conformed to the standards for the use of human subjects in research as outlined in the most recent update of the Declaration of Helsinki. The study also conformed to the standards established by the Canadian Tri-Council Policy on the ethical use of human subjects (2010).
Experimental design
Approximately 1–2 weeks prior to participating in the experimental infusion trial, study participants underwent unilateral strength testing of the knee-extensor muscles. Participants performed a 10 repetition maximum (10-RM) test of both standard seated knee-extension (Atlantis Precision Series C-105) and seated leg press (Maxam Strength, Hamilton, Ontario, Canada) exercise with their dominant leg. In addition, each participant underwent a whole-body dual-energy X-ray absorptiometry scan (QDR-4500A; Hologic; software version 12.31) to measure body composition. The study participants physical characteristics are shown in Table 1. Participants were assigned to one of three post-exercise nutritional treatment groups (described below) that were counter-balanced for bodyweight.
Table 1.
Participants' characteristics
| WHEY | LEU | EAA-LEU | |
|---|---|---|---|
| Age (years) | 22.1 (0.8) | 21.5 (1.1) | 22.5 (1.3) |
| Height (m) | 1.8 (0.02) | 1.8 (0.02) | 1.8 (0.02) |
| Weight (kg) | 77.3 (3.9) | 76.5 (3.9) | 75.4 (2.7) |
| BMI (kg m−2) | 25.0 (1.2) | 24.2 (1.2) | 23.8 (0.7) |
| Fat-free mass (kg) | 63.2 (2.9) | 64.6 (3.8) | 63.4 (2.4) |
| Body fat (%) | 17.9 (2.2) | 16.1 (2.4) | 16.5 (1.2) |
Values are mean ± SEM (n = 8 per treatment group).
Study participants were provided with a pre-packaged standardized diet that was consumed the day prior to the experimental infusion trial. Diets were designed to provide sufficient energy to maintain energy balance as determined by the Harris–Benedict equation and were adjusted using a moderate activity factor (1.4–1.6) to account for participants reported physical activity patterns. The macronutrient distribution was 55% carbohydrates, 30% lipids, and 15% protein. The study participants were told to refrain from physical exercise for 72 h prior to the experimental infusion trial and to consume their evening meal no later than 22.00 h.
Infusion protocol
Participants reported to the lab at ∼06.00 h on the morning of the experimental infusion trial in an overnight postabsorptive state. A catheter was inserted into an antecubital vein and a baseline blood sample was taken before initiating a 0.9% saline drip to keep the catheter patent to allow for repeated arterialized blood sampling over the course of the experimental trial. Arterialized blood samples (Copeland et al. 1992) were obtained repeatedly over the course of the infusion trial by wrapping a heating blanket around the forearm. Blood samples were collected into 4 ml heparinized evacuated tubes and chilled on ice. A second catheter was placed in the antecubital vein of the opposite arm before initiating a primed continuous infusion (0.05 μmol kg−1 min−1; 2.0 μmol kg−1 prime) of [ring-13C6]phenylalanine (Cambridge Isotope Laboratories, Woburn, MA, USA). The infusate was passed through a 0.2 μm filter before entering the participant's bloodstream. Our research group has recently validated a method (Burd et al. 2011) in which the resting (fasted) fractional synthetic rate (FSR) of MPS is calculated based on the 13C enrichment of a pre-infusion baseline blood sample obtained from tracer-naive participants, and a single biopsy taken following a period of tracer incorporation (Miller et al. 2005; Mittendorfer et al. 2005; Tang et al. 2009, 2011; West et al. 2009; Burd et al. 2010b). This method assumes that the 13C enrichment of a mixed plasma protein fraction reflects the 13C enrichment of muscle protein (Heys et al. 1990). Thus, the baseline rate of MPS was calculated using a pre-infusion baseline blood sample and a single resting skeletal muscle biopsy sample obtained ∼2.5 h after the onset of the primed constant infusion. Participants then performed an acute bout of unilateral resistance exercise consisting of four sets of 10–12 repetitions of both seated knee-extension (Atlantis Precision Series C-105) and leg-press (Maxam Fitness, Hamilton, Ontario, Canada) exercise at ∼95% of their previously determined 10-RM with an inter-set rest-interval of 2 min. Immediately following completion of the resistance exercise, participants were administered 1 of 3 post-exercise nutrient treatments orally in a single-blinded fashion and bilateral biopsy samples were obtained at 1, 3, and 5 h post-exercise recovery from a FED and EX-FED leg. Muscle biopsies were obtained from the vastus lateralis muscle using a 5 mm Bergström needle modified for manual suction under 2% xylocaine local anaesthesia. Biopsy samples were immediately freed from visible blood, fat and connective tissue, and immediately frozen in liquid nitrogen for further analysis as previously described (West et al. 2009; Burd et al. 2010a). Each biopsy sample was obtained from a separate incision ∼4–5 cm apart. Each participant underwent a total of seven skeletal muscle biopsies: four from the rested leg, and three from the exercised leg. Specific details of the infusion protocol are outlined in Fig. 1.
Figure 1. Schematic diagram of the experimental protocol.
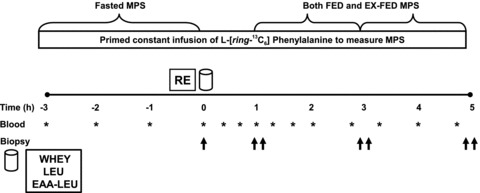
Study participants consumed either EAA-LEU, LEU, or WHEY (see Methods) in single-blinded fashion (n = 8 per treatment group) immediately following resistance exercise. Exercise consisted of 4 sets each of unilateral seated knee extension and leg press. Asterisk indicates blood sample; single upward arrow indicates unilateral biopsy; double upward arrow indicates bilateral biopsy.
Drink composition
Study participants were administered protein/amino acid based nutrient solutions in a blinded manner. The amino acid/protein composition of each of the three nutrient treatments is outlined in Table 2. Briefly, the three nutrient treatments were as follows: WHEY, which consisted of: 25 g whey protein isolate (total leucine = 3.0 g); LEU: 6.25 g whey protein isolate supplemented with free-form leucine (total leucine = 3.0 g); EAA-LEU: 6.25 g whey protein isolate supplemented with free-form EAAs but without added leucine (total EAAs = to WHEY for each individual EAAs except leucine which was 0.75 g). The whey protein isolate (biPro, Davisco Foods, Le Sueur, MN, USA) was independently tested (Telmark, Matawan, NJ, USA) in triplicate for content analysis. The free-form essential amino acids used were as follows: l-leucine, l-isoleucine, l-valine, l-histidine, l-phenylalanine, l(+)-lysine, l-threonine and l-methionine (Sigma-Aldrich, St Louis MO, USA). All nutrient solutions were prepared with 300 ml of water (see Table 2). To minimize disturbances in isotopic equilibrium following amino acid ingestion, nutrient solutions were enriched to 4% with tracer according to a phenylalanine content of 3.5% in whey protein. Our research group has recently shown this method to be valid for maintaining isotopic steady state in both the plasma free and muscle intracellular free precursor pools after protein ingestion and resistance exercise (Burd et al. 2011).
Table 2.
Total and essential amino acid content of the nutritional treatments
| Nutritional treatment | |||
|---|---|---|---|
| WHEY | LEU | EAA-LEU | |
| Alanine (g) | 1.15 | 0.29 | 0.29 |
| Arginine (g) | 0.53 | 0.13 | 0.13 |
| Aspartic acid (g) | 2.80 | 0.70 | 0.70 |
| Cystine (g) | 0.78 | 0.19 | 0.19 |
| Glutamic acid (g) | 4.10 | 1.03 | 1.03 |
| Glycine (g) | 0.43 | 0.11 | 0.11 |
| Proline (g) | 1.05 | 0.26 | 0.26 |
| Serine (g) | 0.63 | 0.16 | 0.16 |
| Tyrosine (g) | 0.88 | 0.22 | 0.22 |
| Tryptophan (g) | 0.68 | 0.17 | 0.17 |
| Histidine (g)* | 0.55 | 0.14 | 0.55 |
| Isoleucine (g)* | 1.35 | 0.34 | 1.35 |
| Leucine (g)* | 3.00 | 3.00 | 0.75 |
| Lysine (g)* | 2.70 | 0.68 | 2.70 |
| Methionine (g)* | 0.58 | 0.14 | 0.58 |
| Phenylalanine (g)* | 0.88 | 0.22 | 0.88 |
| Threonine (g)* | 1.10 | 0.28 | 1.10 |
| Valine (g)* | 1.38 | 0.34 | 1.38 |
| Total (g) | 24.57 | 8.40 | 12.55 |
| ΣEAAs (g) | 11.54 | 5.14 | 9.29 |
| ΣNEAAs (g) | 13.03 | 3.26 | 3.26 |
Content included as an essential amino acid (EAA). NEAA, non-essential amino acid.
Analytical methods
Blood glucose was measured using a blood glucose meter (OneTouch Ultra 2, Lifescan Inc., Milpitas, CA, USA). Blood amino acid concentrations were analysed by high performance liquid chromatography (HPLC) as described previously (Wilkinson et al. 2007). Plasma l-[ring-13C6] phenylalanine enrichment was determined as previously described (Glover et al. 2008). Plasma insulin concentration was measured using a commercially available immunoassay kit (ALPCO Diagnostics, Salem, NH, USA).
Muscle samples (∼40–50 mg) were homogenized on ice in buffer (10 μl mg−1 25 mm Tris 0.5% v/v Triton X-100 and protease/phosphatase inhibitor cocktail tablets (Complete Protease Inhibitor Mini-Tabs, Roche, Indianapolis, IN, USA; PhosSTOP, Roche Applied Science, Mannhein, Germany)). Samples were then centrifuged at 15,000 g for 10 min 4°C. The supernatant was removed and protein concentrations were determined via the Bradford Assay. The pellet containing the myofibrillar proteins was stored at −80°C until future processing. Working samples of equal concentration were prepared in Laemmli buffer (Laemmli, 1970). Equal amounts (20 μg) of protein were loaded onto 10% or 15% SDS-polyacrylamide gels for separation by electrophoresis. Proteins were then transferred to a polyvinylidene fluoride membrane, blocked (5% skim milk) and incubated overnight at 4°C in primary antibody: phospho-AktSer473 (1:1000, Cell Signaling Technology, no. 9271) phospho-mTORSer2448 (1:1000, Cell Signaling Technology, no. 2971) phospho-p70S6kThr389 (1:500, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; no. 11759), phospho-4E-BP1Thr37/46 (1:1000, Cell Signaling Technology, no. 9459), phospho-Erk1/2Tyr202/204 (1:1000, Cell Signaling Technology, no. 9101), and phospho-p38Thr180/Tyr182 (1:1000, Cell Signaling Technology, no. 9215). Membranes were then washed and incubated in secondary antibody (1 h at room temperature) before detection with chemiluminescence (SuperSignalWest Dura Extended Duration Substrate, ThermoScientific, no. 34075) on a FluorChem SP Imaging system (Alpha Innotech, Santa Clara, CA, USA). Phosphorylation status was expressed relative to α-tubulin abundance (1:2000, Sigma-Alderich no. T6074) and is presented for each protein as a fold-change from rested fasted conditions (FAST). Images were quantified by spot densitometry using ImageJ software (National Institute of Health, USA).
RNA was isolated from muscle using the phenol/chloroform method as previously described (Philp et al. 2010). RNA was quantified using an Epoch Multi-Volume Spectrophotometer (BioTek, Winooski, VT, USA) at 260 and 280 nm. Firststrand cDNA was synthesized on a Thermo Hybaid cycler (Thermo Scientific) from 1 μg of RNA using the reverse transcription system (Promega, Southampton, UK) according to the manufacturer's instructions.
Quantitative real-time PCR was performed to measure relative mRNA expression using an Eppendorf Light Cycler PCR machine, SYBR Green PCR plus reagents (Sigma-Aldrich), and previously published primers for LAT1, CD98, PAT1, GCN2 and ATF4 (Drummond et al. 2010, 2011). Ten-microlitre PCR reactions were assayed in triplicate on a 96-well heat-sealed PCR plate (Thermo Fisher Scientific). Each reaction contained 5 μl of SYBR Green Taq, 1 μl of forward and reverse primers, and 3 μl of cDNA (1:10 dilution). Target gene expression was calculated relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and expressed normalized to basal (FAST) values. Absolute cycle threshold (CT) for GAPDH was unchanged by any of the treatments (data not shown).
Muscle biopsy samples were processed as previously described (Moore et al. 2009b). Briefly, to determine the intracellular enrichment, ∼20–25 mg of muscle was homogenized in 0.6 m perchloric acid. Free amino acids in the resulting supernatant fluid were then passed over an ion-exchange resin (Dowex 50WX8–200 resin Sigma-Aldrich) and converted to their heptafluorobutyric derivatives for analysis via gas chromatography–mass spectrometry (models 6890 GC and 5973 MS; Hewlett-Packard) by monitoring ions 316 and 322 after electron ionization. To determine muscle free intracellular amino acid concentrations, samples were processed as previously described (Wilkinson et al. 2007). Briefly, muscle samples were derivatized and analysed by HPLC (HPLC: Waters model 2695; column: Waters Nova-Pak C18, 4 μm; detector: Waters 474 scanning fluorescence detector). This method achieved separation of 19 of the 20 physiological amino acids, with the exception of tryptophan (not included in the analysis). To determine myofibrillar protein-bound enrichments, a separate piece (∼40–50 mg) of muscle was homogenized in a standard buffer containing protease and phosphatase inhibitors as described above under ‘Immunoblotting’. The supernatant fluid was collected for Western blot analysis as described above, and the pellet was further processed to extract myofibrillar proteins as previously described (Moore et al. 2009b). The resulting myofibrillar ‘enriched’ protein pellet was hydrolysed in 6 m HCl at 110°C overnight. Subsequently, the free amino acids were purified using ion-exchange chromatography and converted to their N-acetyl-n-propyl ester derivatives for analysis by gas chromatography combustion isotope ratio mass spectrometry (GC-C-IRMS: Hewlett Packard 6890; IRMS model Delta Plus XP, Thermo Finnagan, Waltham, MA USA).
Calculations
The fractional synthetic rate (FSR) of MPS was calculated using the standard precursor-product equation:
where Eb is the enrichment of bound (myofibrillar) protein, EIC is the average enrichment of the intracellular free amino acid precursor pool of two muscle biopsies, and t is the tracer incorporation time in hours. The utilization of ‘tracer naive’ subjects allowed us to use a pre-infusion blood sample (i.e. a mixed plasma protein fraction) as the baseline enrichment (E1b) for calculation of resting (i.e. fasted) FSR (Miller et al. 2005; Mittendorfer et al. 2005; Tang et al. 2009). This approach is based on the fact that the ‘natural’13C enrichment (δ13CPDB) in blood is the same as that of muscle protein; an assumption recently confirmed by our research group (West et al. 2009) and others (Heys et al. 1990).
Statistics
Anthropometric measures and strength tests were compared using a one-factor (treatment) ANOVA. Blood amino acids (leucine, branch-chain amino acids (BCAAs), EAAs, total amino acids), plasma insulin and blood glucose were analysed using a two-factor (treatment × time) repeated measures ANOVA. Blood leucine AUC was analysed using a one-factor (treatment) ANOVA. Plasma enrichments were analysed using a two-factor (treatment × time) repeated measures ANOVA and linear regression. Intracellular precursor pool enrichments were analysed using a two-factor (treatment × time) repeated measures ANOVA for each condition (i.e. FED and EX-FED), a two-factor ANOVA (treatment × condition) at each time point (1, 3 and 5 h), and linear regression. Intracellular amino acids, protein phosphorylation, mRNA expression, and myofibrillar FSR were analysed using a two-factor (treatment × time) repeated measures ANOVA for each condition and a two-factor ANOVA (treatment × condition) at each time point. Protein phosphorylation and mRNA abundance are expressed as fold-change from FAST. Tukey's post hoc analysis was performed whenever a significant F ratio was found to isolate specific differences. Statistical analyses were performed using SigmaStat 3.1 software (Systat Software Inc., Point Richmond, CA, USA). Values are expressed as means ± standard error of the mean (SEM), and means were considered to be statistically different for P values < 0.05.
Results
Participant characteristics
Participant characteristics are shown in Table 1. There were no differences between treatment groups for any anthropometric variable measured.
Exercise variables
There were no differences between treatment groups for participant's unilateral 10-RM test when measured for seated leg-press (P = 0.68) or knee extension exercise (P = 0.78). Further, the exercise volume, defined as the product of exercise load (kg) and repetitions (i.e. load × repetitions) was not different per set between treatment groups for either seated leg press (P = 0.78) or knee extension exercise (P = 0.78; data not shown).
Blood glucose, plasma insulin and blood amino acid concentrations
Baseline blood glucose averaged 5.3 ± 0.1 mmol l−1 in each treatment group, and did not differ between treatment groups (P = 0.81). Plasma insulin concentration peaked at 40 min post-treatment administration in all treatment groups before declining. However, insulin concentration following WHEY remained elevated above LEU at 1 h, and both LEU and EAA-LEU at 2 h post-treatment (see Fig. 1 in the online Supplemental Material).
Blood leucine concentrations showed a large but transient increase following LEU as compared to WHEY, with WHEY demonstrating a more moderate but sustained increase (Fig. 2A). In brief, LEU was significantly increased above WHEY at 40 and 60 min, while WHEY was elevated above LEU at 80, 100 and 120 min post-treatment administration. Despite these differences, area under the leucine curve was not different between LEU and WHEY; however, both treatments were significantly greater than EAA-LEU (P = 0.001 Fig. 2A inset).
Figure 2. Mean (±SEM) blood concentrations (μmol l−1) of leucine (A), branched chain amino acids (BCAAs) (B), essential amino acids (EAAs) (C) and total amino acids (D) following EAA-LEU, LEU and WHEY treatments.
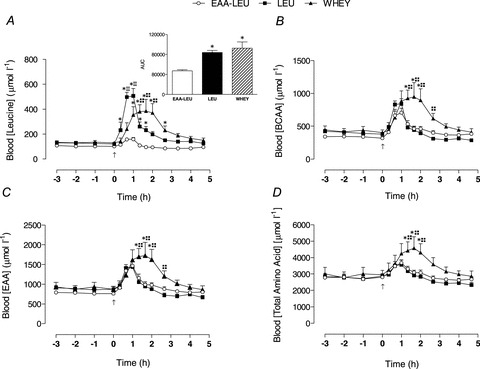
Inset shows the area under the curve (AUC). Upward arrow indicates time of treatment administration. *Significantly greater than EAA-LEU (P < 0.05); +significantly greater than LEU (P < 0.05); ‡significantly greater than WHEY (P < 0.05).
Blood BCAAs increased after treatment administration, peaking at ∼1 h for LEU and EAA-LEU. WHEY was significantly increased above LEU and EAA-LEU from 80 to 120 min, and LEU at 160 min after ingestion (Fig. 2B). Blood EAAs (including leucine) showed a similar interaction (treatment × time) effect (P < 0.001) with WHEY being elevated above LEU and EAA-LEU from 80 to 120 min and LEU at 160 min post-treatment administration (Fig. 2C). Blood total amino acid showed a significant interaction (P < 0.001) effect such that WHEY was significantly increased above LEU and EAA-LEU from 80 to 120 min post-treatment administration (Fig. 2D).
Plasma and intracellular free phenylalanine enrichments
Plasma free phenylalanine enrichments were not different between treatments (P = 0.66) and were stable across time (P = 0.34). The slope of plasma free phenylalanine enrichment by time was also not different from zero in any treatment group (EAA-LEU P = 0.95; LEU P = 0.11; WHEY P = 0.40) (see Supplemental Fig. 2). Similarly, intracellular free phenylalanine enrichments were not different between treatments and were stable across time in both FED (time, P = 0.92; treatment, P = 0.90) and EX-FED (time, P = 0.30; treatment P = 0.88) conditions when measured at 1, 3 and 5 h post-exercise. Further there were no differences between conditions at 1 h (P = 0.90), 3 h (P = 0.42), or 5 (P = 0.98). The slope of intracellular free phenylalanine enrichment by time in both FED and EX-FED conditions was also not different from zero in any treatment group, indicating measurements were made at an isotopic plateau (EAA-LEU FED P = 0.77, EX-FED P = 0.41; LEU FED P = 0.84, EX-FED P = 0.68; WHEY FED P = 0.56, EX-FED P = 0.84; see Supplemental Fig. 3).
Myofibrillar protein synthesis
FED rates of MPS were increased above FAST when measured 1–3 h post-exercise recovery (P < 0.001; EAA-LEU = 0.063 ± 0.008; LEU = 0.068 ± 0.006; WHEY = 0.061 ± 0.009). By 3–5 h post-exercise recovery, FED rates of MPS had returned to values not different from FAST, with no differences between treatment groups at any time point (P = 0.74) (Fig. 3A). Similarly, EX-FED rates of MPS were increased above FAST over 1–3 h post-exercise recovery (P = 0.001; EAA-LEU = 0.069 ± 0.012; LEU = 0.068 ± 0.014; WHEY = 0.064 ± 0.007). However, the rates of MPS remained increased above FAST at 3–5 h exercise recovery only after ingestion of WHEY versus LEU and EAA-LEU (EAA-LEU = 0.050 ± 0.005; LEU = 0.048 ± 0.012; WHEY = 0.088 ± 0.010; Fig. 3B).
Figure 3. Mean (±SEM) fractional synthetic rate (FSR) (% h−1) calculated during FAST, and over both early (1–3 h), and late (3–5 h) time periods of post-exercise recovery in both FED (A) and EX-FED (B) conditions after EAA-LEU, LEU and WHEY treatments.
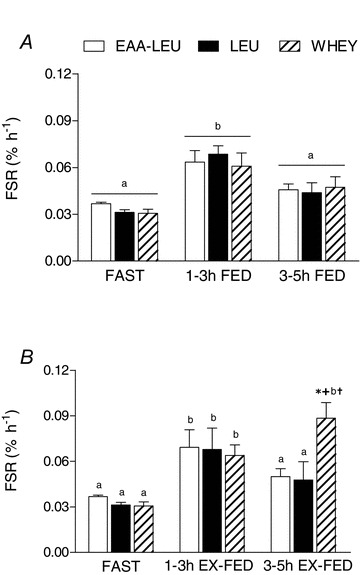
Times with different letters are significantly different from each other within that treatment and condition. *Significantly greater than EAA-LEU within that time and condition (P < 0.05); +significantly greater than LEU within that time and condition (P < 0.05); †significantly greater than FED condition at that time point (P < 0.05).
Intracellular amino acids
Intracellular leucine concentration was increased above FAST at 1 h post-exercise recovery in LEU and WHEY; however the increase in LEU was significantly greater than both WHEY and EAA-LEU (Fig. 4A). A similar response was observed in the EX-FED condition (P < 0.001); however, intracellular leucine concentrations in EAA-LEU decreased below basal levels at 3 and 5 h post-exercise recovery and were significantly lower than both LEU and WHEY at these time points (Fig. 4B). Intracellular BCAA in the FED condition with EAA-LEU showed concentrations that were increased above LEU at 3 h, and both LEU and WHEY at 5 h post-exercise recovery (Fig. 4C). Similar results were observed in the EX-FED treatment whereby EAA-LEU showed concentrations that were increased above LEU and WHEY at both 3- and 5 h post-exercise recovery (P < 0.001) (Fig. 4D). Intracellular EAAs in the FED condition showed a main effect (P < 0.001) for treatment, with concentrations in EAA-LEU being greater than both LEU and WHEY (Fig. 4E). In the EX-FED condition, a main effect of time (P < 0.001) demonstrated an increase above FAST at 1 h followed by a decrease below FAST at 5 h post-exercise recovery, such that at 5 h, the intracellular EAAs concentration in FED was greater than EX-FED (P = 0.009) (Fig. 4F).
Figure 4. Mean (±SEM) intracellular concentrations (μmol l−1) of leucine (A and B), branched chain amino acids (BCAAs) (C and D) and essential amino acids (EAAs) (E and F) measured during FAST and at 1, 3 and 5 h post-exercise recovery in both FED and EX-FED conditions following EAA-LEU, LEU and WHEY treatments.
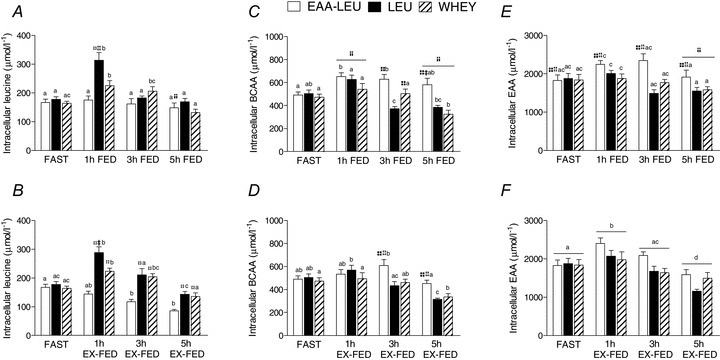
Times with different letters are significantly different from each other within that treatment and condition. *Significantly greater than EAA-LEU within that time and condition (P < 0.05); +significantly greater than LEU within that time and condition (P < 0.05); ‡significantly greater than WHEY within that time and condition (P < 0.05); †significantly greater than EX-FED condition at that time-point (P < 0.05).
Amino acid transporter mRNA expression
The mRNA expression of ATF4 was increased above FAST in both FED (P < 0.001) and EX-FED (P < 0.001) conditions at 1, 3 and 5 h post-exercise recovery (main effect for Time) with no differences between treatment groups (Supplemental Fig. 4A–B). GCN2 mRNA expression demonstrated a significant (P = 0.004) interaction effect in the FED condition whereby gene expression was increased to a greater extent in LEU vs. EAA-LEU at 3 h and both EAA-LEU and WHEY at 5 h post-exercise recovery (Supplemental Fig. 4C). In the EX-FED condition, there was a main effect for time (P = 0.003) (Supplemental Fig. 4D). There were no differences between treatments in the mRNA expression of CD98 (SLC3A2) in either FED or EX-FED conditions; however the increase at 5 h post-exercise recovery was significantly greater in EX-FED vs. FED (P = 0.003) (Fig. 5A and B). Similarly, there were no treatment effects for the mRNA expression of LAT1 (SLC7A5) in either FED or EX-FED conditions, however the increase at 5 h post-exercise was greater in EX-FED vs. FED (P = 0.025) (Fig. 5C and D). Lastly, there was a main effect (P 0.031) for treatment when examining changes in the mRNA expression of PAT1 (SLC36A1) in the EX-FED condition whereby WHEY was significantly greater (P = 0.031) than EAA-LEU (Fig. 5E and F).
Figure 5. Mean (±SEM) mRNA expression of CD98 (SLC3A2) (A and B), LAT1 (SLC7A5) (C and D), and PAT1 (SLC36A1) (E and F) (expressed as fold-difference from FAST) at 1, 3 and 5 h post-exercise recovery in both FED and EX-FED conditions following EAA-LEU, LEU and WHEY treatments.
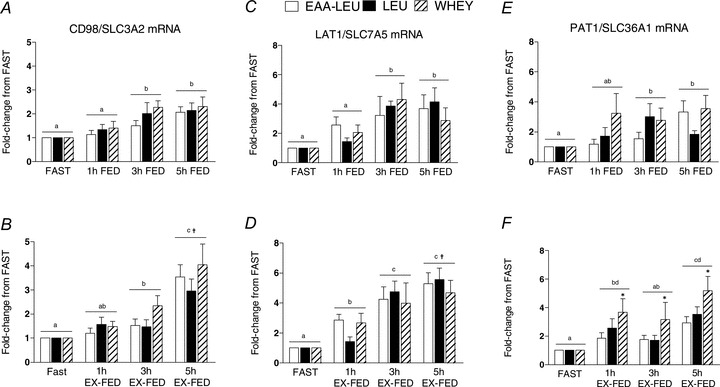
Times with different letters are significantly different from each other within that treatment and condition. *Significantly greater than EAA-LEU within that time and condition (P < 0.05); †significantly greater than EX-FED condition at that time point (P < 0.05).
Muscle signalling
Protein kinase B (p-AktSer473) was increased at 1 h in both FED (P < 0.001) and EX-FED (P = 0.001) conditions with no effect of treatment (Fig. 6A and B). Similarly, p-mTORSer2448 was significantly elevated at 3 h in FED (P = 0.037) (Fig. 6C), and 1, 3 and 5 h in EX-FED (P < 0.001) (Fig. 6D). Phosphorylation of p70S6kThr 389 showed a significant interaction in both FED (P = 0.008) and EX-FED (P = 0.013) conditions. In FED, LEU and WHEY were significantly elevated above EAA-LEU at 3 h and 5 h (Fig. 6E), while in EX-FED, both LEU and WHEY were increased above EAA-LEU at 3 h, while LEU was increased above EAA-LEU at 5 h post-exercise recovery (Fig. 6F). Phosphorylation of p-4E-BP1Thr 37/46 in FED was increased above FAST at 1 and 3 h post-exercise recovery (P < 0.001) but returned to basal by 5 h (Supplemental Fig. 5A). However, in EX-FED phosphorylation was increased at 1, 3 and 5 h, (P < 0.001) such that at 5 h the increase in EX-FED was greater than FED (P = 0.001) (Supplemental Fig. 5B). Lastly, p-extracellular regulated kinase 1/2Thr202/Tyr204 (Supplemental Fig. 5C and D) and p-p38Thr180/Tyr182 (Supplemental Fig. 5E and F) MAPK were unchanged in the FED condition but showed time dependent increases in the EX-FED condition (ERK, P = 0.001; p38, P < 0.001) with no effect of treatment. As such, ERK 1/2 was significantly increased in EX-FED vs. FED at 1, 3, and 5 h, while p38 was higher in EX-FED vs. FED at 1 h post-exercise recovery. See Supplemental Figure 6 for representative blot images for each protein target.
Figure 6. Mean (±SEM) phosphorylation status of AktSer473 (A and B), mTORSer2448 (C and D) and p70S6kThr389 (E and F) (expressed as fold-difference from FAST) at 1, 3 and 5 h post-exercise recovery in both FED (top panel) and EX-FED (bottom panel) conditions following EAA-LEU, LEU and WHEY treatments.
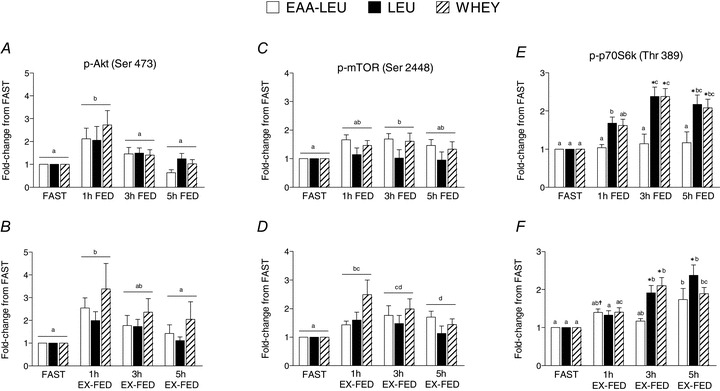
Times with different letters are significantly different from eachother within that treatment and condition. *Significantly greater than EAA-LEU within that time and condition (P < 0.05); +significantly greater than LEU within that time and condition (P < 0.05); ‡significantly greater than WHEY within that time and condition (P < 0.05).
Discussion
In this study we report that a dose of whey protein, previously shown to be less than maximally effective for stimulating muscle protein synthesis after resistance exercise (Moore et al. 2009a), supplemented with leucine (LEU) resulted in an early (1–3 h post-exercise recovery) increase in both FED and EX-FED rates of MPS equal to that seen following ingestion of 25 g of whey protein (WHEY). Contrary to our hypothesis, supplementation of a low dose of whey protein with a mixture of EAAs devoid of leucine (EAA-LEU treatment) also resulted in a robust early stimulation of MPS that was not different from that achieved after LEU and WHEY. However, despite similar early responses of MPS, EX-FED rates over 3–5 h were only sustained following WHEY, whereas EX-FED rates of MPS in both LEU and EAA-LEU had decreased to values not significantly different from FAST. Interestingly, these differences between 3 and 5 h occurred despite blood amino acid concentrations that had returned to basal levels in all treatments. In the absence of exercise we did not see a difference in the rates of MPS between treatments indicating that signalling events as well as amino acid supply were all more than adequate to stimulate a full and robust response that rose and fell within the 4 h incorporation time period, as we (Moore et al. 2009b) and others (Atherton et al. 2010a) have shown previously.
Both in vitro (Buse & Reid, 1975) and in vivo (Anthony et al. 1999, 2000a,b; Crozier et al. 2005; Escobar et al. 2005, 2006) evidence from animals supports a role for leucine as a nutrient regulator of muscle protein synthesis, capable of phosphorylating proteins involved in mRNA translation initiation, primarily through the mTOR signalling pathway including 4E-BP1, p70S6k and rpS6 (Anthony et al. 2000b; Suryawan et al. 2008). It is currently unclear whether MPS is regulated by changes in extracellular (Bohe et al. 2003), or intracellular (Biolo et al. 1995) EAAs and/or leucine availability. In rodents, the leucine content of a meal and the subsequent postprandial leucinaemia direct the peak activation of muscle protein synthesis such that feeding proteins containing a higher proportion of leucine results in a greater plasma leucine concentration and subsequently, a greater increase in muscle protein synthesis (Norton et al. 2009). Our current findings do not support the notion that the postprandial stimulation of MPS is directly proportional only to the rise in blood leucine (Rennie et al. 2006; Norton et al. 2009) under rested or post-exercise conditions in young men. Specifically, we observed pronounced differences in blood leucine concentration (Fig. 2A) that were apparently of little consequence to either the FED or EX-FED MPS response when measured over 1–3 h post-exercise (Fig. 3A and B). Thus, in humans, peak activation of MPS does not appear to be driven by leucinaemia. Potentially, amino acid transport across the sarcolemma (Hundal & Taylor, 2009) and intracellular amino acid availability (Biolo et al. 1995) may be important in the regulation of MPS.
Previous reports have demonstrated that ∼10 g of EAAs is sufficient to maximally stimulate MPS under both resting and post-exercise conditions in young healthy subjects (Cuthbertson et al. 2005; Moore et al. 2009a). We observed that LEU resulted in an early (1–3 h post-exercise) stimulation of MPS equal to that of WHEY, despite containing only ∼45% of the total EAA content (11.5 g vs. 5.1 g). This suggests that leucine can potently stimulate MPS; however, we observed a similar rise in MPS in the EAA-LEU treatment as that seen with LEU and WHEY despite containing only ∼25% of the leucine of LEU and WHEY (WHEY = 3.0 g; LEU = 3.0 g; vs. EAA-LEU = 0.75 g leucine). Thus, we speculate that in young healthy individuals, the leucine content provided by ∼6.25 g of whey protein (∼0.75 g) appears to be sufficient to activate and induce a maximal stimulation of MPS provided adequate amounts of the other EAAs are provided (i.e. amounts equivalent to ∼25 g whey protein or ∼8.5 g EAAs). Alternatively, there may be other EAAs, in addition to leucine, that can stimulate MPS. For example, valine, phenylalanine and threonine have been shown to increase human muscle protein synthesis when administered as a flooding dose (Smith et al. 1998). Further, the effect of each individual EAA on mTORC1 signalling in C2C12 myotubes showed that EAAs in addition to leucine can enhance both p70S6k and rpS6 phosphorylation (Atherton et al. 2010b), suggesting that other EAAs in addition to leucine can activate proteinsd synthetic signalling pathways.
We previously reported that a sustained elevation of MPS occurs when resistance exercise is followed by the immediate provision of 25 g of whey protein (Moore et al. 2009b; West et al. 2011) despite aminoacidaemia equivalent to basal levels. In agreement with these findings, WHEY was able to sustain the EX-FED response over 3–5 h post-exercise recovery in the present study while MPS in both LEU and EAA-LEU had declined to resting values. These results suggest that the ability of amino acids to sustain the contraction mediated increase in MPS is not solely dependent on leucine availability as leucine AUC was matched between LEU and WHEY. However, WHEY was associated with a protracted aminoacidaemia as compared to LEU and EAA-LEU (Fig. 2A–D), which may have acted as a signal to extend the EX-FED response of MPS. Alternatively, while non-essential amino acids (NEAAs) are not necessary to ‘turn on’ MPS and/or direct the magnitude of the response (Smith et al. 1998; Tipton et al. 1999b; Borsheim et al. 2002; Volpi et al. 2003), there were large differences in the amount of total NEAAs provided in each treatment (WHEY = 13.0 g; LEU = 3.3 g; EAAs = 3.3 g). Hence, it is conceivable that NEAAs may be required to sustain elevated rates of MPS under conditions of a higher ‘anabolic drive’ stimulated by resistance exercise compared to feeding alone. Under such conditions, more NEAAs may be required to serve as substrates necessary for the synthesis of new muscle proteins or other functions; further studies are necessary to examine this hypothesis.
The precise mechanism(s) underpinning the observed changes in MPS following resistance exercise and amino acid intake appear to involve activation of the Akt/mTOR signalling cascade (Anthony et al. 2000b; Cuthbertson et al. 2005; Atherton et al. 2010a; Dickinson et al. 2011). We observed an increase in the phosphorylation status of AktSer473, an upstream regulator of mTOR, at 1 h post-exercise recovery. Consistent with this finding, we also observed a significant increase in the phosphorylation status of mTORSer2448, which was evident earlier and was sustained for longer in the EX-FED vs. FED condition (Fig. 6C and D), and both p70S6kThr389 and 4E-BP1Thr 37/46, downstream targets of mTOR involved in translation initiation. Notably, however, while the phosphorylation status of p70S6kThr389 was markedly increased above fasted conditions following both LEU and WHEY, no changes were observed following EAA-LEU, except at 5 h EX-FED when MPS was no longer significantly elevated (Fig. 6E and F). These findings suggest that leucine is a potent regulator of p70S6kThr389 signalling (Atherton et al. 2010b; Glynn et al. 2010), and corroborate previous findings demonstrating that single point-in-time changes in signalling molecule phosphorylation do not always reflect changes in dynamic measures of protein synthesis (Greenhaff et al. 2008; Glynn et al. 2010).
In an attempt to further elucidate how protein/amino acids and resistance exercise interact to affect MPS we measured the mRNA abundance of select skeletal muscle amino acid transporters (AATs) and members of the general amino acid control pathway including general control non-repressed (GCN2) and activating transcription factor (ATF4). The transcription factor ATF4 has been reported to upregulate AAT (Harding et al. 2003), and can itself be upregulated in response to GCN2 activation (Ameri & Harris, 2008) and anabolic stimuli such as amino acid and insulin sufficiency (Adams, 2007; Malmberg & Adams, 2008). In agreement, select AATs have recently been shown to be upregulated in human muscle in response to EAA intake (Drummond et al. 2010) and resistance exercise (Drummond et al. 2011). We observed a large increase in gene expression for the AAT LAT1 (SLC7A5), PAT1 (SLC36A1), and CD98 (SLC3A2) consistent with previous reports (Drummond et al. 2010; Drummond et al. 2011), as well as time dependent increases in ATF4. Thus, the increases in amino acid and insulin availability may have acted as a signal to increase ATF4 expression, allowing for the subsequent upregulation of AAT expression. Our findings further demonstrate that changes in the mRNA expression of these transporters are not dependent upon the level of leucine intake after resistance exercise, and also that combined feeding and exercise appear to prolong the increase in gene expression compared to feeding alone (Fig. 5A–B and C–D). We did not measure changes in protein content of these transporters following feeding and resistance exercise, and it remains possible that the nutritional treatments may have demonstrated a differential response at the protein level. Further research is needed to elucidate the functional and physiological significance of changes in these transporters following EAAs and resistance exercise.
In conclusion, our model allowed us to address the specific role of total meal leucine content versus that of EAAs found in a dose of protein that maximally stimulates MPS after resistance exercise. We report that both LEU and EAA-LEU were as effective as WHEY at stimulating both FED and EX-FED rates of MPS over 1–3 h post-exercise recovery. These findings demonstrate that while leucine is potent in its ability to stimulate MPS, only a relatively small amount (0.75 g) is required to achieve a maximal stimulation of MPS when other EAAs are provided in larger quantities (∼8.5 g). However, only WHEY, containing both EAAs and NEAAs, was able to sustain the elevated rates of MPS 3–5 h after resistance exercise and therefore may be a better choice to support resistance exercise induced anabolism. The increase in the phosphorylation of p70S6kThr389 following treatment administration was associated with leucine intake (i.e. increased in WHEY and LEU) but not MPS. We conclude that supplementing a suboptimal dose of whey protein (6.25 g) with leucine, or a mixture of EAAs without leucine, is an effective strategy to stimulate rates of postprandial MPS comparable to the response elicited following ingestion of 25 g of whey protein, and suggest that only a small amount (∼0.75 g) of leucine is required to stimulate MPS in young healthy individuals when ample amounts of other EAAs are provided. These findings may have important implications for individuals unable to tolerate a full protein meal.
Acknowledgments
We thank Tracy Rerecich, Amy Hector, Leigh Breen and Todd Prior for their technical assistance and Randy Burd for his assistance in acquiring the whey protein used in this study. We thank the study participants for their time and effort. T.A.C.V. was supported by a Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarship. This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) and Canadian Institutes of Health Research (CIHR) to S.M.P. This trial is registered at clinicaltrials.gov as: NCT01492010.
Glossary
Abbreviations
- 4E-BP1
eukaryotic initiation factor 4E binding protein 1
- AAT
amino acid transporter
- Akt
protein kinase B
- ATF4
activating transcription factor 4
- AUC
area-under-the-curve
- BCAA
branch-chain amino acid
- CD98
glycoprotein CD98
- ERK 1/2
extracellular signal-regulated kinase 1/2
- EAAs
essential amino acid
- EAA-LEU
nutritional treatment consisting of 6.25 g whey protein supplemented with a mixture of essential amino acids but no leucine
- EX-FED
response to combined feeding and resistance exercise
- FAST
rested fasted condition
- FED
response to feeding
- FSR
fractional synthetic rate
- GCN2
general control nonrepressed
- LAT1
L-type amino acid transporter type 1
- LEU
nutritional treatment consisting of 6.25 g whey protein supplemented with leucine
- MPS
myofibrillar protein synthesis
- mTOR
mammalian target of rapamycin
- p38 MAPK
p38 mitogen activated protein kinase
- p70S6k
70 kDa ribosomal protein S6 kinase 1
- PAT1
proton-coupled amino acid transporter type 1
- WHEY
nutritional treatment consisting of 25 g whey protein
Author contributions
T.A.C.V. and S.M.P. contributed to the conception and the design of the experiment. All authors contributed to collection, analysis and interpretation of data. All authors contributed to drafting or revising intellectual content of the manuscript. All authors read, edited and approved the final version of the manuscript.
Supplementary material
Supplemental Figure 1
Supplemental Figure 2
Supplemental Figure 3
Supplemental Figure 4
Supplemental Figure 5
Supplemental Figure 6
References
- Adams CM. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem. 2007;282:16744–16753. doi: 10.1074/jbc.M610510200. [DOI] [PubMed] [Google Scholar]
- Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40:14–21. doi: 10.1016/j.biocel.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000a;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Anthony TG, Layman DK. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J Nutr. 1999;129:1102–1106. doi: 10.1093/jn/129.6.1102. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, Kimball SR, Jefferson LS. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes. 2002;51:928–936. doi: 10.2337/diabetes.51.4.928. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000b;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010a;92:1080–1088. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010b;38:1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1-13C]leucine. Clin Sci (Lond) 1989;76:447–454. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268:E514–520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolster DR, Vary TC, Kimball SR, Jefferson LS. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr. 2004;134:1704–1710. doi: 10.1093/jn/134.7.1704. [DOI] [PubMed] [Google Scholar]
- Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol. 2010a;588:3119–3130. doi: 10.1113/jphysiol.2010.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, West DW, Rerecich T, Prior T, Baker SK, Phillips SM. Validation of a single biopsy approach and bolus protein feeding to determine myofibrillar protein synthesis in stable isotope tracer studies in humans. Nutr Metab (Lond) 2011;8:15. doi: 10.1186/1743-7075-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010b;5:e12033. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse MG, Reid SS. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland KC, Kenney FA, Nair KS. Heated dorsal hand vein sampling for metabolic studies: a reappraisal. Am J Physiol Endocrinol Metab. 1992;263:E1010–1014. doi: 10.1152/ajpendo.1992.263.5.E1010. [DOI] [PubMed] [Google Scholar]
- Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–862. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol. 2011;111:135–142. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab. 2005;288:E914–921. doi: 10.1152/ajpendo.00510.2004. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E612–621. doi: 10.1152/ajpendo.00402.2005. [DOI] [PubMed] [Google Scholar]
- Glover EI, Oates BR, Tang JE, Moore DR, Tarnopolsky MA, Phillips SM. Resistance exercise decreases eIF2Bɛ phosphorylation and potentiates the feeding-induced stimulation of p70S6K1 and rpS6 in young men. Am J Physiol Regul Integr Comp Physiol. 2008;295:R604–610. doi: 10.1152/ajpregu.00097.2008. [DOI] [PubMed] [Google Scholar]
- Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140:1970–1976. doi: 10.3945/jn.110.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Molecular Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Heys SD, McNurlan MA, Park KG, Milne E, Garlick PJ. Baseline measurements for stable isotope studies: an alternative to biopsy. Biomed Environ Mass Spectrom. 1990;19:176–178. doi: 10.1002/bms.1200190314. [DOI] [PubMed] [Google Scholar]
- Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296:E603–613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, Wagenmakers AJ, van Loon LJ. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006;84:623–632. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJ, Kuipers H, van Loon LJ. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008;99:571–580. doi: 10.1017/S0007114507812013. [DOI] [PubMed] [Google Scholar]
- Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288:E645–653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, Saris WH, van Loon LJ. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr. 2011;141:1070–1076. doi: 10.3945/jn.111.138495. [DOI] [PubMed] [Google Scholar]
- Malmberg SE, Adams CM. Insulin signaling and the general amino acid control response. Two distinct pathways to amino acid synthesis and uptake. J Biol Chem. 2008;283:19229–19234. doi: 10.1074/jbc.M801331200. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol. 2005;563:203–211. doi: 10.1113/jphysiol.2004.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009a;89:161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009b;587:897–904. doi: 10.1113/jphysiol.2008.164087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol Endocrinol Metab. 1992;263:E928–934. doi: 10.1152/ajpendo.1992.263.5.E928. [DOI] [PubMed] [Google Scholar]
- Norton LE, Layman DK, Bunpo P, Anthony TG, Brana DV, Garlick PJ. The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. J Nutr. 2009;139:1103–1109. doi: 10.3945/jn.108.103853. [DOI] [PubMed] [Google Scholar]
- Philp A, Perez-Schindler J, Green C, Hamilton DL, Baar K. Pyruvate suppresses PGC1α expression and substrate utilization despite increased respiratory chain content in C2C12 myotubes. Am J Physiol Cell Physiol. 2010;299:C240–250. doi: 10.1152/ajpcell.00438.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ, Bohe J, Smith K, Wackerhage H, Greenhaff P. Branched-chain amino acids as fuels and anabolic signals in human muscle. J Nutr. 2006;136:264S–268S. doi: 10.1093/jn/136.1.264S. [DOI] [PubMed] [Google Scholar]
- Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol Endocrinol Metab. 1992;262:E372–376. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol Endocrinol Metab. 1998;275:E73–78. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295:E868–875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JE, Lysecki PJ, Manolakos JJ, MacDonald MJ, Tarnopolsky MA, Phillips SM. Bolus arginine supplementation affects neither muscle blood flow nor muscle protein synthesis in young men at rest or after resistance exercise. J Nutr. 2011;141:195–200. doi: 10.3945/jn.110.130138. [DOI] [PubMed] [Google Scholar]
- Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107:987–992. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Elliott TA, Ferrando AA, Aarsland AA, Wolfe RR. Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Appl Physiol Nutr Metab. 2009;34:151–161. doi: 10.1139/H09-006. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol Endocrinol Metab. 1999a;276:E628–634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Gurkin BE, Matin S, Wolfe RR. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem. 1999b;10:89–95. doi: 10.1016/s0955-2863(98)00087-4. [DOI] [PubMed] [Google Scholar]
- Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89:1468–1475. doi: 10.3945/ajcn.2008.26668. [DOI] [PubMed] [Google Scholar]
- Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr. 2011;94:795–803. doi: 10.3945/ajcn.111.013722. [DOI] [PubMed] [Google Scholar]
- West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol. 2009;587:5239–5247. doi: 10.1113/jphysiol.2009.177220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


