Abstract
Exercise training (ET) improves endurance capacity by increasing both skeletal muscle mitochondrial number and function, as well as contributing to favourable cardiac remodelling. Interestingly, some of the benefits of regular exercise can also be mimicked by the naturally occurring polyphenol, resveratrol (RESV). However, it is not known whether RESV enhances physiological adaptations to ET. To investigate this, male Wistar rats were randomly assigned to a control chow diet or a chow diet that contained RESV (4 g kg−1 of diet) and subsequently subjected to a programme of progressive treadmill running for 12 weeks. ET-induced improvements in exercise performance were enhanced by 21% (P < 0.001) by the addition of RESV to the diet. In soleus muscle, ET + RESV increased both the twitch (1.8-fold; P < 0.05) and tetanic (1.2-fold; P < 0.05) forces generated during isometric contraction, compared to ET alone. In vivo echocardiography demonstrated that ET + RESV also increased the resting left ventricular ejection fraction by 10% (P < 0.05), and reduced left ventricular wall stress compared to ET alone. These functional changes were accompanied by increased cardiac fatty acid oxidation (1.2-fold; P < 0.05) and favourable changes in cardiac gene expression and signal transduction pathways that optimized the utilization of fatty acids in ET + RESV compared to ET alone. Overall, our findings provide evidence that the capacity for fatty acid oxidation is augmented by the addition of RESV to the diet during ET, and that this may contribute to the improved physical performance of rats following ET.
Key points
Resveratrol, an antioxidant found in red wine, has beneficial effects on cardiac and skeletal muscle function, similar to the effects of endurance exercise training.
Combining resveratrol supplementation with exercise training augments the beneficial effects of exercise alone.
We show that endurance capacity is enhanced in rats whose diet includes resveratrol during a 12 week endurance-training programme.
Increased endurance was associated with increases in skeletal muscle force, cardiac function, and oxidative metabolism.
Our results establish that resveratrol is an effective ergogenic aid that enhances exercise performance over exercise alone.
Introduction
For both clinical and non-clinical reasons, various strategies have been employed to improve physical performance through augmenting exercise with nutritional and/or hormonal supplements. The intent of these supplements is to increase muscle mass, endurance and power (Spriet & Gibala, 2004) above what is observed with exercise alone. With endurance-type exercise training (ET), physical performance can be improved secondary to increased skeletal muscle biogenesis through the optimization of fuel selection for endurance activities (Holloszy & Coyle, 1984). These exercise-induced modifications in energy metabolism not only may benefit skeletal muscle function but are also associated with improved cardiac function, especially during maximal workloads (Goodwin & Taegtmeyer, 2000). Therefore, dietary supplements taken during ET that augment the physiological effects of exercise in skeletal and cardiac muscles may improve exercise performance beyond exercise alone.
Resveratrol (RESV) is a naturally occurring polyphenol that has been demonstrated to have a wide range of benefits for the cardiovascular system (Dolinsky & Dyck, 2011). Interestingly, RESV has also been shown to increase skeletal muscle mitochondrial biogenesis and fatty acid oxidation as well as exercise performance in mice (Baur et al. 2006; Lagouge et al. 2006), in a manner that is similar to that observed with ET alone (Lagouge et al. 2006). In the absence of ET, supplementation with RESV increases the exercise performance of aged mice (Murase et al. 2009) and mice fed a Western diet (Lagouge et al. 2006), suggesting that RESV can stimulate pathways similar to exercise. While RESV has been touted as an exercise mimetic, it remains to be clarified whether the beneficial effects of RESV can improve exercise performance beyond exercise alone.
In addition to increased skeletal muscle mitochondrial biogenesis and enhanced fatty acid oxidation, ET-mediated improvements in exercise performance can be explained by enhanced efficiency of oxygen extraction at the level of skeletal muscle (Hoppeler & Weibel, 1998). Moreover, improved oxygen delivery to skeletal muscled is likely to occur secondary to enhanced cardiac performance. Based on the fact that RESV has been suggested to be an exercise mimetic, these effects may also be induced by RESV supplementation. While the effects of RESV on skeletal muscle are well described (Baur et al. 2006; Lagouge et al. 2006), the effects of RESV in the heart appear to be primarily restricted to the prevention of pathological conditions, including cardiac hypertrophy, ischaemic heart disease and heart failure (reviewed in Dolinsky & Dyck, 2011). Despite our understanding about the role of ET and RESV on skeletal and cardiac muscle, the effect of RESV on skeletal muscle and the heart in animals undergoing ET has not been investigated.
In the study presented here, we show that supplementing the diet of ET rats with RESV improves their exercise performance, increases the force response of isolated muscles during isometric contraction and increases whole body oxidative metabolism. At the level of the heart, supplementation with RESV improved several parameters of left ventricular (LV) function and energy homeostasis through alterations in signal transduction pathways and gene expression profiles. Based on these findings, we conclude that RESV is an ergogenic aid that improves ET via changes in skeletal muscle function and cardiac performance, but also improves energy metabolism.
Methods
Materials
Antibodies utilized in this study were purchased from MitoSciences (Eugene, OR, USA), Cell Signaling Technology (Danvers, MA, USA), or Santa Cruz Biotechnology (Santa Cruz, CA, USA). Most other reagents and chemicals were purchased from Sigma. Resveratrol was purchased from Lalilab (Durham, NC, USA).
Animal care and diets
The University of Alberta Animal Policy and Welfare Committee adheres to the principles for biomedical research involving animals developed by the Council for International Organizations of Medical Sciences (CIOMS). Fifty 8-week-old male Wistar rats were obtained from Charles River Laboratories (Sherbrooke, QC, Canada). Rats were housed two per cage and maintained under a 12 h light–dark cycle (06.00–18.00 h). Throughout a 12 week period between the ages of 10 and 22 weeks, rats had free access to drinking water and were fed ad libitum with either an AIN93G standard chow diet (Table 1), or an AIN93G standard chow diet that contained 4 g RESV per kg diet (Dyets Inc., Bethlehem, PA, USA), a dosage that is equivalent to ∼146 mg resveratrol (kg body weight)−1 day−1, which is consistent with previous studies (Lagouge et al. 2006; Dolinsky et al. 2011). We did not observe any negative consequences from supplementing the diets of the rats with RESV at this dose. Rats were killed using sodium pentobarbital within 24 h of their final session of ET.
Table 1.
Diet compositions
| AIN-93G control diet | AIN-93G supplemented with resveratrol | |||
|---|---|---|---|---|
| g per kg diet | kcal per g diet | g per kg diet | kcal per kg diet | |
| Casein | 200 | 3.58 | 200 | 3.58 |
| l-Cysteine | 3 | 4 | 3 | 4 |
| Sucrose | 96 | 4 | 96 | 4 |
| Cornstarch | 397.5 | 3.6 | 397.5 | 3.6 |
| Dextrinized cornstarch (90–94% tetrasaccharides) | 132 | 3.8 | 132 | 3.8 |
| Soybean oil | 70 | 9 | 70 | 9 |
| t-Butylhydroquinone | 0.014 | 0 | 0.014 | 0 |
| Cellulose | 50 | 0 | 50 | 0 |
| Choline bitartrate | 2.5 | 0 | 2.5 | 0 |
| Mineral mix no. 210025 | 35 | 0.88 | 35 | 0.88 |
| Vitamin mix no. 310025 | 10 | 3.9 | 10 | 3.9 |
| Resveratrol | 0 | 0 | 4 | 0 |
* Information obtained from Dyets Inc. Bethlemen, PA, USA
Exercise training and evaluation of endurance capacity
A calibrated motor driven rodent treadmill equipped with electrical stimulation for aversive foot shock (Columbus Instruments, Columbus, OH, USA) was used for endurance training and to determine the exercise performance in rats. At 9 weeks of age, Wistar rats were acclimated to treadmill running. ET began at 10 weeks of age whereby rats performed 60 min of daily progressive treadmill running that began at 10 m min−1, 0% incline and was systematically increased up to 20 m min−1 at 0% incline, similar to well established protocols (Fenning et al. 2003). Endurance training continued 5 days week−1 for a period of 12 weeks. A combination of electrical stimulation and air puff was used to encourage rodents to run. Exercise performance was assessed in 22-week-old rats, following 12 weeks of diets and training, as described above. Exercise performance was determined from a graded exercise test to exhaustion performed at 0% incline, beginning with 10 m min−1 for 1 min, 11 m min−1 for 1 min, 12 m min−1 for 1 min, 13 m min−1 for 2 min, 15 m min−1 for 5 min, 17 m min−1 for 5 min and 20 m min−1 until exhaustion. Exercise performance was determined by the time to exhaustion. A rat was deemed to be fatigued when it was no longer able to continue to run on the treadmill as judged by the rat spending >50% of time or >30 consecutive seconds on the electrical stimulus and resistant to prodding by the air puff. Sedentary control rats were also acclimated to the treadmill and handled 5 days per week, but did not receive daily endurance training.
Isometric muscle measurements
Muscle force measurements of the soleus muscles were performed as described previously (Gallo et al. 2004). Rats were anaesthetized with ketamine, administered intraperitoneally. Incisions were made along the dorsum of the right and left hindlimbs and the muscles were exposed. A purpose-made nerve cuff (AS 632 Cooner Wire Co., Chatsworth, CA, USA) was placed around each sciatic nerve for electrical stimulation. The distal tendons of each muscle were isolated and individually secured with 2.0 silk to a Kulite strain gauge (model KH-102). Skin incisions were then sutured before beginning functional measurements. Core body temperature was maintained at 37°C with a heating pad and monitored with a rectal thermometer. Animals were placed in a prone position and secured with clamps at the knee and ankle joints. Before each series of recordings, the optimal resting length required to generate maximum isometric force was determined for each muscle. Maximum twitch (in mN) and tetanic forces (in mN) were sequentially recorded in the right and then left soleus muscle. Twitch force was determined as the average of peak forces generated by five individual twitches elicited at 1 Hz. Tetanic force was determined by high frequency stimulation (100 Hz for 200–400 ms) that resulted in a contraction reaching peak force followed by a plateau at this level. Fatigue index was measured using the standard Burke protocol (Burke et al. 1973) where intermittent isometric contractions were evoked using 40 Hz stimulation for 325 ms, repeated every second for 2 min. The fatigue index is the ratio of initial to final force measured during stimulation. The SAG ratio measures the ability to maintain whole muscle force during an unfused tetanic contraction. Isometric contractions were evoked using a stimulation rate determined by the time to peak (TTP) of the twitch force (1/TTP × 1.25) for 800 ms. To calculate SAG the peak force in the first 100 ms was divided by the peak force for the last stimulus. Muscles with a large proportion of type I slow-twitch fibres typically have a SAG ratio ≥1.0 while muscles composed of large proportions of type IIa and IIb fibres have a ratio <1.0. All measurements were amplified, filtered, acquired and analysed using a Digidata 1200 system, Axoscope (v 8.1) and Clampfit (v 9.0) software (Axon Instruments, Union City, CA, USA).
In vivo assessment of cardiac and vascular parameters
Following 11 weeks of diets and/or exercise, transthoracic echocardiography was performed to determine LV morphometry and function in anaesthetized 21-week-old rats using a Vevo 770 small rodent ultrasound (Visual Sonics, Toronto, ON, Canada), as previously described (Dolinsky et al. 2010). LV wall stress was calculated according to a validated equation (Murakami et al. 2002). Non-invasive BP measurements were made on conscious, but midly restrained rats, as detailed previously (Dolinsky et al. 2010) using a tail cuff system, according to manufacturer's instructions (IITC Life Science, Woodland Hills, CA, USA).
Ex vivo assessment of cardiac function and oxidative metabolism
Whole hearts were excised from rats following 12 weeks of diets and ET and were perfused in the working mode with 5 mmol l−1[U-14C]glucose, 1.2 mmol l−1[9,10-3H]palmitate prebound to 3% delipidated BSA, and 50 μU l−1 insulin. Initially, hearts were perfused aerobically for 30 min at a constant preload pressure of 15 mmHg and afterload pressure of 80 mmHg (normal workload). Subsequently, hearts were perfused with an increased afterload of 140 mmHg and perfusate containing isoproterenol (300 nmol l−1) for an additional 30 min (high workload). Glucose and palmitate oxidation rates were measured throughout the perfusion. At the end of the perfusion, hearts were frozen in liquid nitrogen and stored at –80°C.
Glucose and insulin tolerance tests
Following a 5 h fast, rats were injected intraperitoneally with a 50% glucose solution (2 g (kg body weight)−1) for the glucose tolerance test (GTT). Glucose plasma levels were determined using an ACCU-CHEK Advantage glucometer (Roche Diagnostics, Laval, QC, Canada) using blood collected from the tail at baseline and following glucose injection (at 15, 30, 60, 90 and 120 min). For the insulin tolerance test (ITT), human recombinant insulin (Novolin) was used to prepare an insulin-saline solution (1 mU (kg body weight)−1) that was injected intraperitoneally after a 2 h fast. As described above, blood glucose from the tail was measured at baseline and following insulin injection (at 15, 30, 60, 90 and 120 min).
Indirect calorimetry, food intake and physical activity
After 10 weeks of diets and/or exercise, measurements of food intake and whole body energy metabolism were performed using a Comprehensive Lab Animal Monitoring System (Oxymax/CLAMS; Columbus Instruments, Columbus, OH, USA). Following an initial 24 h acclimatization period, rats were monitored every 13 min for 24 h for a complete 12 h dark (active)–12 h light (inactive) cycle and oxygen consumption (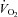 ), CO2 production (
), CO2 production (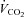 ) and heat production were measured. The respiratory exchange ratio (RER =
) and heat production were measured. The respiratory exchange ratio (RER = 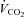 /
/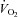 ) was used to estimate the relative contribution of fat and carbohydrate to whole-body energy metabolism in rats in vivo. Lipid and glucose oxidation were calculated using validated equations (Peronnet & Massicotte, 1991). Physical activity was monitored by dual axis detection (X, Z) using infra-red photocell technology. Total physical activity was calculated by adding Z-counts (rearing or jumping) to total counts associated with ambulatory movement and typical behaviour (grooming, scratching, etc.).
) was used to estimate the relative contribution of fat and carbohydrate to whole-body energy metabolism in rats in vivo. Lipid and glucose oxidation were calculated using validated equations (Peronnet & Massicotte, 1991). Physical activity was monitored by dual axis detection (X, Z) using infra-red photocell technology. Total physical activity was calculated by adding Z-counts (rearing or jumping) to total counts associated with ambulatory movement and typical behaviour (grooming, scratching, etc.).
Tissue homogenization, assays and immunoblotting
Tissues were collected and frozen in liquid nitrogen. Subsequently, frozen tissue homogenates were prepared in ice-cold sucrose homogenation buffer (20 mm Tris-HCl (pH 7.4), 50 mm NaCl, 50 mm NaF, 5 mm sodium pyrophosphate, 0.25 m sucrose) containing protease and phosphatase inhibitor cocktails (Sigma), 1 mm dithiothreitol and 20 mm sodium orthovanadate as previously described (Koonen et al. 2010). In brief, tissues were homogenized on ice in a 20 s burst and the homogenate centrifuged at 1000 g for 20 min at 4°C to remove nuclei and cellular debris. Protein concentrations were determined using a Bradford protein assay. Citrate synthase activity was assessed at 30°C in homogenates (1:20 dilution) using 0.1 mm oxaloacetate as substrate. Protein (15–20 μg) was subjected to 10% SDS-PAGE, transferred to nitrocellulose, immunoblotted with antibodies and visualized using the Perkin-Elmer enhanced chemiluminescence Western blotting detection system.
Determination of plasma free fatty acids, and triacylglycerol
Plasma from rats following a 16 h fast were collected in the presence of EDTA and immediately stored on ice to inhibit lipase activity without the use of chemical inhibition. Lipids were extracted from 200 μl of plasma or tissue lysates by the method of Folch (Folch et al. 1957). Triacylglycerol (TG), and free fatty acids were separated by fast protein liquid chromatography according to the method described by Christie (1985).
Affymetrix microarray analysis of heart tissues
GeneChip Rat Gene 1.0 ST microarrays were used for gene expression analysis (Affymetrix, Santa Clara, CA, USA). Total RNA was pooled from the hearts of five animals from either the ET + RESV or ET + Control group using a GenElute kit (Sigma, Oakville, ON, Canada) and prepared for microarray hybridization as per manufacturer's instructions. RNA quality was confirmed (RIN>8.0) on a 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). Results were collected and analysed using Partek Express software (Partek, St. Louis, MO, USA) and Robust Multi-Array Averaging. Functional annotation analysis was performed using the DAVID Bioinformatics Resource 6.7 (http://david.abcc.ncifcrf.gov/) (Huang da et al. 2009).
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). Comparisons between treatment groups were performed using the Student's unpaired, two-tailed t test, or two-way analysis of variance (ANOVA) with a Bonferroni post hoc test of pairwise comparisons between groups, where appropriate. A probability value of <0.05 was considered significant.
Results
Resveratrol increases the endurance capacity of Wistar rats
Male Wistar rats were divided into four groups, which included sedentary rats fed either a standard diet or a diet supplemented with RESV, as well as ET and ET + RESV groups (Fig. 1). Compared to sedentary rats, ET alone significantly reduced body weight in Wistar rats fed either the standard diet or the diet supplemented with RESV (Fig. 2A). In agreement with previous studies that used aged mice (Murase et al. 2009) or mice fed a Western diet (Lagouge et al. 2006), we found that supplementing the diets of sedentary rats with RESV resulted in a significant improvement (∼25%) in exercise performance (Fig. 2B and C). As expected, 12 weeks of ET on its own dramatically increased the endurance of rats compared to sedentary rats (Fig. 2B and C). Interestingly, compared to ET alone, ET + RESV increased exercise performance a further ∼20% (Fig. 2B and C), demonstrating that the addition of RESV improves exercise performance beyond ET alone.
Figure 1. Experimental design.

Following acclimation to the treadmill, 10-week-old male Wistar rats were randomly divided into four groups, which included sedentary rats or exercise trained (ET; level treadmill running 20 m min−1 for 60 min, 5 days week−1 for 12 weeks) rats that received control AIN-93G diet (Control) or the AIN-93G diet supplemented with resveratrol (RESV; 4 g RESV/kg food). Glucose tolerance test (GTT). Insulin tolerance test (ITT). Rats were subjected to experimental procedures at the indicated time points.
Figure 2. Resveratrol (RESV) improves the endurance capacity of exercise-trained (ET) rats.
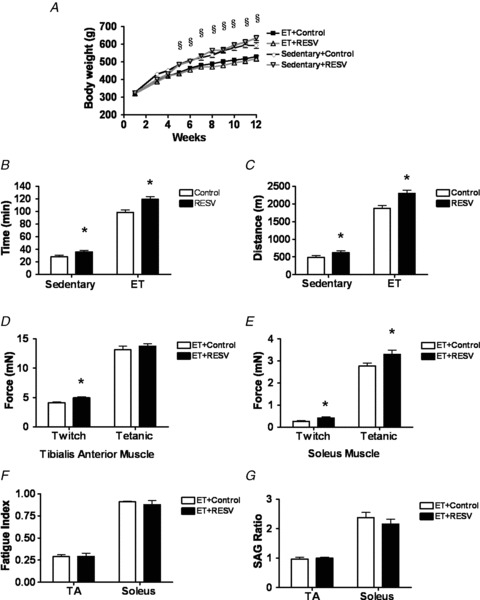
A, body weight. B and C, time to exhaustion (B) and distance run (C) during a treadmill exercise test after the 12 weeks of ET. D and E, twitch and tetanic forces in the tibialis anterior (TA) muscle (D) and soleus muscle (E). F and G, the fatigue index (F) and SAG ratio (G) during isometric muscle contractions. Data are presented as the mean ± SEM of n = 10 rats. Significant difference: *P < 0.05 Control vs. RESV; §P < 0.05 Sedentary vs. ET using a two-way ANOVA and Bonferroni post hoc test.
To explore the skeletal muscle adaptations that may have contributed to the increased exercise performance achieved with RESV, we measured isometric muscle twitch and tetanic forces from the tibialis anterior (TA) and soleus muscles in response to single and high frequency stimulation of the sciatic nerve. In TA muscle, the combination of ET + RESV significantly increased the twitch force ∼18% compared to ET alone (Fig. 2D), while improvements in tetanic muscle force were not different between ET and ET + RESV. However, in soleus muscle both the twitch and tetanic forces were significantly increased by ∼58% and ∼22%, respectively, in ET + RESV rats compared to ET alone (Fig. 2E). These results demonstrated that RESV supplementation during ET increases the isometric force production by the skeletal muscle. Next, we assessed muscle fatigue during two minutes of stimulation. Despite the increased strength of the soleus and TA muscles in the ET + RESV group, the fatigue index and SAG ratio were not different between ET alone and ET + RESV (Fig. 2F and G) suggesting that RESV did not increase the endurance capacity of isolated skeletal muscles. Therefore, the increased endurance observed in ET + RESV rats could not be directly attributed to resistance to muscle fatigue. This observation is consistent with the finding that in humans, running performance is more strongly correlated with cardiovascular performance than muscle-fibre type distribution (Foster et al. 1978).
Resveratrol improves cardiac function when administered during exercise training
As ET + RESV increased the endurance of rats without improving the fatigue properties of skeletal muscle, we speculated that adaptations in the cardiovascular system contributed to this effect. Consistent with this hypothesis, the increased exercise performance observed in ET + RESV rats was associated with significantly improved LV ejection fraction (Fig. 3A) and fractional shortening (Table 2). Moreover, the decreased isovolumic relaxation time (IVRT; Fig. 3B) and the increased ratio of the peak mitral flow velocity (E-wave) to the peak velocity of the late filling wave of atrial contraction (A-wave), termed E/A ratio (Fig. 3C), indicate a significant improvement in LV diastolic function in the ET + RESV rats compared to ET alone.
Figure 3. Resveratrol (RESV) improves the cardiac function of exercise-trained (ET) rats.
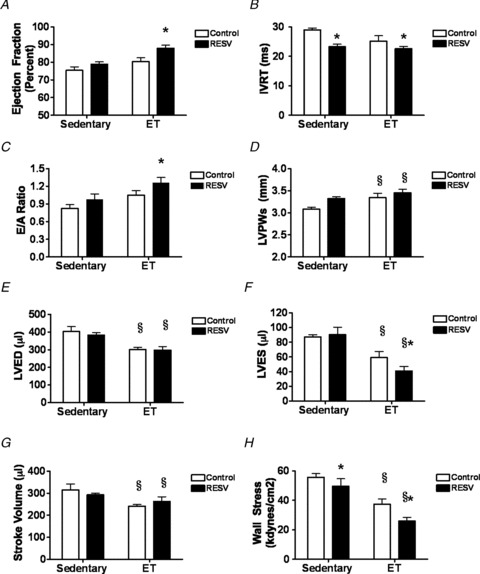
Following 12 weeks of exercise training, in vivo cardiac function was measured by echocardiography. A, cardiac ejection fraction. B, intraventricular relaxation time (IVRT). C, the E-wave/A-wave ratio. D, left ventricular (LV) posterior wall (LVPW) thickness. E, LV end diastolic (LVED) volume. F, LV end systolic (LVES) volume. G, stroke volume. H, LV wall stress. Data are presented as the mean ± SEM of n = 6 rats. Significant difference: *P < 0.05 Control vs. RESV; §P < 0.05 Sedentary vs. ET using a two-way ANOVA and Bonferroni post hoc test.
Table 2.
Cardiovascular characteristics
| Sed + Control | Sed + RESV | ET + Control | ET + RESV | |
|---|---|---|---|---|
| Fractional shortening (%) | 47.7 ± 1.2 | 48.9 ± 2.0 | 51.0 ± 1.9 | 56.3 ± 2.5*§ |
| IVSs (mm) | 3.06 ± 0.03 | 3.24 ± 0.08 | 3.35 ± 0.09§ | 3.42 ± 0.08§ |
| LVIDs (mm) | 4.40 ± 0.06 | 4.27 ± 0.16 | 3.69 ± 0.09§ | 3.29 ± 0.12*§ |
| Heart Rate (bpm) | 396 ± 19.0 | 407 ± 14.0 | 378 ± 23.0 | 377 ± 10.0 |
| Cardiac output (ml min−1) | 110.1 ± 11.3 | 110.2 ± 3.4 | 95.7 ± 5.0 | 107.5 ± 10.4 |
| Systolic pressure (mmHg) | 148.5 ± 6.6 | 147.5 ± 7.2 | 139.2 ± 5.9 | 132.9 ± 6.1 |
| Diastolic pressure (mmHg) | 118.5 ± 10.1 | 116.9 ± 11.0 | 107.3 ± 7.0 | 92.4 ± 9.1 |
| Plasma TG (mg dl−1) | 153.2 ± 30.6 | 95.7 ± 19.1 | 93.1 ± 13.8 | 71.5 ± 9.6 § |
| Plasma free fatty acid (mm) | 0.47 ± 0.07 | 0.38 ± 0.02 | 0.47 ± 0.07 | 0.39 ± 0.04 |
Values are the means ± SEM of n = 5–6 rats. Significant difference:
P < 0.05 Control vs. Resveratrol (RESV);
P < 0.05 Sedentary (Sed) vs. Exercise Training (ET) using a two-way ANOVA and Bonferronic post hoc test.
As ET + RESV improved cardiac function to a greater extent than ET alone, we also investigated whether there was beneficial cardiac remodelling in ET + RESV rats. Although we have previously shown that RESV blunts pathological cardiac hypertrophy (Chan et al. 2008; Dolinsky et al. 2009), RESV treatment did not prevent physiological cardiac remodelling in ET rats. Instead, ET + RESV induced a modest, though non-significant trend towards increased LV posterior wall (LVPW) thickness compared to ET alone (Fig. 3D). Additionally, ET reduced the LV internal dimension (Table 2) and also lowered LV end diastolic (LVED) volume (Fig. 3E) and the LV end systolic (LVES) volume (Fig. 3F) in the presence or absence of RESV. Despite ET, both the heart rate and cardiac output were not significantly altered between groups (Table 2), which may be attributed to the echocardiographic assessment being performed in anaesthetized rats. As such, exercise promoted positive cardiac remodelling, which probably contributed to reduced stroke volume (Fig. 3G) and decreased wall stress (Fig. 3H) in the absence of altered LV afterload (as suggested by the absence of changes in systolic and diastolic blood pressures; Table 2). Interestingly, the additive effect of several small changes in LV structure in the ET + RESV rats appeared to have a combined effect that reduced LV wall stress by ∼30% beyond ET alone (Fig. 3H). Together, these structural and functional alterations in hearts from ET + RESV rats are consistent with what would be expected from rats that had undergone more intense ET and thus may contribute to the enhanced exercise performance observed in these rats (Kemi et al. 2008).
Resveratrol improves whole body insulin sensitivity and oxidative metabolism in exercise trained rats
Improved insulin sensitivity is a hallmark feature of endurance training (Goodpaster et al. 2001; Dumortier et al. 2003). Based on this, we further hypothesized that ET-mediated improvements in insulin sensitivity and glucose disposal would be enhanced by the addition of RESV to the diet. Impressively, we observed that supplementing the diets of rodents with RESV during ET further improved both glucose (Fig. 4A) and insulin (Fig. 4B) performance of rats beyond those achieved with ET alone. Given that the metabolic effects of ET + RESV were additive to those observed with ET alone, we further characterized the effects of RESV on whole body in vivo oxidative metabolism. To do this, we used indirect calorimetry as a surrogate marker of energy substrate utilization by muscle. In the dark cycle when rats are most active, oxygen consumption was greater in ET + RESV rats compared to ET rats (Fig. 4C). Furthermore, the respiratory exchange ratio (RER) in ET + RESV rats was significantly lower at all points in the light-dark cycle compared to ET alone (Fig. 4D), suggesting improved metabolic flexibility with ET + RESV. These indirect calorimetry findings are consistent with the inference that fat oxidation was significantly higher in ET + RESV rats compared to ET alone (Fig. 3E), despite similar rates of glucose oxidation (Fig. 4F). These alterations in whole body energy metabolism observed with RESV supplementation appeared to act directly on oxidative metabolism since the consumption of food (Fig. 4G) and the basal activity (Fig. 4H) were not different between the groups. Since enhanced endurance is influenced by increased mitochondrial fatty acid oxidation (Holloszy & Coyle, 1984) and metabolism by skeletal muscle, it is possible that supplementation with RESV during ET may provide supplemental adaptations to genes involved in mitochondrial oxidative capacity and/or fat oxidation.
Figure 4. Resveratrol (RESV) improves whole-body glucose homeostasis and fatty acid oxidation in exercise trained (ET) rats.
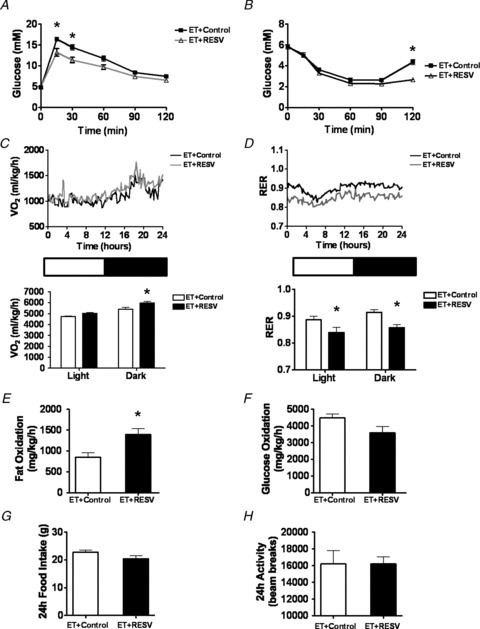
A and B, glucose (A) and insulin (B) tolerance tests. C and D, oxygen consumption (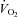 ) (C) and respiratory exchange ratio (RER) (D) were measured by indirect calorimetry. E and F, the amount of whole-body fat (E) and glucose oxidation (F) was calculated from the calorimetry data. G, food consumption was measured over a 24 h period. H, physical activity was monitored by dual axis detection using infra-red photocell technology over a 24 h period. Data are presented as the mean ± SEM of n = 6 rats. Significant difference: *P < 0.05 ET + Control vs. ET + RESV using a Student's t test.
) (C) and respiratory exchange ratio (RER) (D) were measured by indirect calorimetry. E and F, the amount of whole-body fat (E) and glucose oxidation (F) was calculated from the calorimetry data. G, food consumption was measured over a 24 h period. H, physical activity was monitored by dual axis detection using infra-red photocell technology over a 24 h period. Data are presented as the mean ± SEM of n = 6 rats. Significant difference: *P < 0.05 ET + Control vs. ET + RESV using a Student's t test.
Resveratrol alters cardiac function in ex vivo perfused hearts from exercise trained rats
In order to further characterize the effects of RESV on cardiac function during ET, we utilized an isolated perfused working heart model to assess LV function and myocardial substrate utilization in the absence of potentially confounding systemic factors. Baseline parameters of ex vivo LV function were measured in hearts perfused for 30 min at a preload of 15 mmHg and an afterload of 80 mmHg (normal workload). To mimic the increase in cardiac workload induced by exercise, rat hearts were perfused at an 1.75-fold increased afterload of 140 mmHg and subjected to adrenergic stimulation with isoproterenol (300 nmol l−1) to more closely mimic high workload (i.e. ET) conditions (Dyck et al. 2004). Although our ex vivo heart perfusions do not exactly mimic what is occurring in vivo in response to exercise (i.e. no increase in preload), the high workload perfusion protocol increased both heart rate (HR) and the peak systolic pressure (PSP) and thus cardiac function (HR × PSP) was increased in both groups of rats using this protocol (Fig. 5A). However, several parameters of ex vivo cardiac function such as LV developed pressure (LVDP; Fig. 5B), coronary flow (Fig. 5C) and cardiac work (Fig. 5D) were significantly increased in the ET + RESV rats at normal cardiac workload and high workload conditions, compared with ET alone. Because cardiac work was increased in the ET + RESV rats, this finding confirmed our in vivo finding that the LV ejection fraction and systolic function was improved compared to ET alone (Fig. 3A). Furthermore, the enhanced LV systolic performance may explain the increased exercise tolerance observed in the ET + RESV rats compared to ET on its own.
Figure 5. Resveratrol (RESV) increases cardiac performance and elevates fatty acid oxidation in the isolated perfused working hearts of exercise trained (ET) rats.
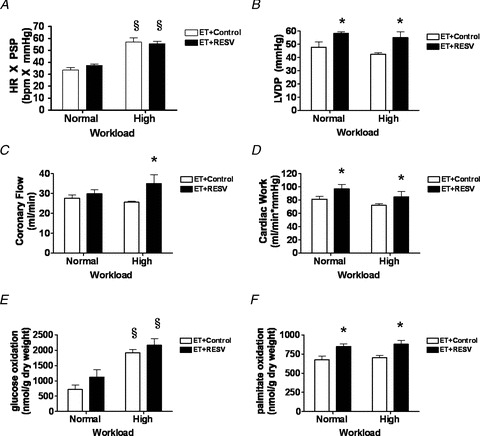
Cardiac function was measured in ex vivo perfused working rat hearts at both normal and high workload conditions. A, heart rate (HR) × peak systolic pressure (PSP). B, left-ventricular developed pressure (LVDP). C, coronary flow. D, cardiac work. E and F, glucose (E) and palmitate (F) oxidation rates were measured in perfused hearts at both normal and high workloads. Data are presented as the mean ± SEM of n = 6 hearts in each group. Significant difference *P < 0.05 ET + Control vs. ET + RESV; §P < 0.05 Normal vs. High Workload using a two-way ANOVA and Bonferroni post hoc test.
Resveratrol alters cardiac energy metabolism in ex vivo perfused hearts from exercise trained rats
In order to directly assess the effects of RESV supplementation on cardiac energy metabolism we compared [3H]palmitate and [14C]glucose oxidation rates between ET + RESV and ET groups. While cardiac glucose oxidation was not significantly different under normal or high workload conditions (Fig. 5E), fatty acid oxidation rates were significantly increased in the hearts of ET + RESV rats under both normal and high workload conditions compared to ET alone (Fig. 5F). These results demonstrate that RESV supplementation enhanced cardiac muscle fatty acid oxidative capacity and this appeared to contribute to the increased cardiac performance under high workload conditions. Based on these findings, our data suggest that RESV increases the ability of the heart to adapt to elevated workloads that would be induced by exercise.
Resveratrol regulates cardiac fatty acid metabolic processes via altered gene expression and signal transduction pathways in exercise trained rats
In order to explore the mechanisms responsible for the ability of RESV to improve cardiac function, we performed microarray analysis of the heart tissues of ET and ET + RESV rats. In the heart, 77 genes were up-regulated at least 1.5-fold and 254 genes were down-regulated at least 1.5-fold in response to RESV. Clusters of genes involved in the PPAR signalling pathway (P < 0.0001) and fatty acid metabolic processes were significantly upregulated (P < 0.0002; Table 3). The PPAR-regulated genes included stearoyl-coenzyme A desaturase (both Scd1 and Scd4), perilipin (Plin), adiponectin (Adipoq) and uncoupling proteins (both Ucp1 and Ucp3). In addition, a key gene involved in TG synthesis, diacylglycerol acyltransferase-2 (Dgat2) was increased. Genes involved in the oxidation of fatty acids and catabolic pathways were also upregulated including, cAMP-dependent protein kinase IIβ (Prkar2b), pyruvate dehydrogenase kinase 4 (Pdk4), NADH dehydrogenase subunit 6 (ND6), and transketolase (Tkt). Though the majority of the genes that were down-regulated in heart by RESV do not have a known function, the expression of several pro-inflammatory genes was reduced, including down-stream effectors of the toll-like receptor signalling pathway. These included Cd80, interferon regulatory factor (Irf7) and interferon inducible protein (Ip10) (Table 4). Corresponding with the alterations in substrate metabolism and the reduction of inflammatory mediators, cardiac adiponectin (Adipoq) expression was increased by RESV. Together, these findings suggest that RESV supplementation during ET not only alters cardiac energy metabolism but also reduces cardiac inflammation.
Table 3.
Gene annotation clusters exhibiting enrichment by resveratrol in the heart
| Gene Ontology Classification | Enrichment Score | Count | Percentage | P value |
|---|---|---|---|---|
| PPAR signalling pathway | 3.09 | 6 | 8.8 | 0.00001 |
| Fatty acid metabolic processes | 2.65 | 7 | 10.3 | 0.00002 |
Table 4.
Selected rat heart genes that are altered (>1.5-fold) by the addition of resveratrol to exercise training
| Ref Seq | Symbol | Gene name | Fold change |
|---|---|---|---|
| PPAR signalling pathway | |||
| NM139192 | Scd1 | Stearoyl-coenzyme A desaturase-1 | 8.8 |
| NM012682 | Ucp1 | Uncoupling protein-1 | 7.3 |
| NM144744 | Adipoq | Adiponectin | 3.8 |
| NM013094 | Plin | Perilipin | 1.9 |
| ENSRNOT00000017834 | Scd4 | Stearoyl-coenzyme A desaturase-4 | 1.7 |
| NM013167 | Ucp3 | Uncoupling protein-3 | 1.7 |
| Fatty Acid Metabolic Processes | |||
| ENSRNOT00000051268 | ND6 | NADH dehydrogenase subunit-6 | 2.5 |
| NM001030020 | Prkar2b | cAMP-dependent protein kinase 2b | 2.3 |
| NM001012345 | Dgat2 | Diacylglycerol acyltransferase-2 | 1.7 |
| NM022592 | Tkt | Transketolase | 1.6 |
| NM053551 | Pdk4 | Pyruvate dehydrogenase kinase-4 | 1.5 |
| NM001135583 | Fa2h | Fatty acid 2-hydoxylase | −1.6 |
| Toll-like receptor signalling pathway | |||
| NM001033691 | Irf7 | Interferon regulatory factor-7 | −1.5 |
| NM012926 | Cd80 | Cd80 | −1.8 |
| NM139089 | IP10 | Interferon-inducible protein 10 | −1.6 |
| Other genes | |||
| NM012703 | Thrsp | Thyroid hormone responsive protein | 5.1 |
| NM019292 | Car3 | Carbonic anhydrase-3 | 2.3 |
| NM134326 | Alb | Albumin | 2.2 |
| ENSRNOT00000065224 | Thbs4 | Thrombospondin-4 | −1.9 |
| NM020096 | Ifit1 | Interferon induced protein-1 | −3.5 |
Both exercise (Coven et al. 2003; Musi et al. 2005) and RESV (Chan et al. 2008; Dolinsky et al. 2009) have been shown to activate the energy-sensing kinase AMP-activated protein kinase (AMPK) in the heart. Since levels of AMPK phosphorylation at threonine 172 correlate with the activity of AMPK (Dolinsky & Dyck, 2006), we measured AMPK phosphorylation at this site by immunoblot analysis in order to determine whether the addition of RESV to ET further activated AMPK. As expected, immunoblot analysis revealed that ET + RESV modestly elevated AMPK phosphorylation (∼1.6-fold), compared to ET alone (Fig. 6A). As a surrogate marker of AMPK activity, we also measured the phosphorylation of acetyl-CoA carboxylase (ACC) because it is directly phosphorylated by AMPK. Phosphorylation of ACC at serine 79 was increased ∼1.4-fold in the hearts of ET + RESV rats compared to ET alone (Fig. 6B). Furthermore, ET + RESV increased the expression of PGC1-α (Fig. 6C), a transcriptional regulator of mitochondrial biogenesis and function. Increased PGC1-α appeared to contribute to the increased expression of electron transport chain complexes I, III and IV (Fig. 6D), and citrate synthase activity (Fig. 6E) in the hearts of ET + RESV rats compared to ET alone. While plasma TG levels were reduced by RESV (Table 2), these alterations did not significantly affect plasma free fatty acid (Table 2) or cardiac TG levels (Fig. 6F). Taken together with our ex vivo (Fig. 5) and in vivo (Fig. 4) data, these findings suggest that RESV enhances exercise performance through increased cardiac fatty acid metabolism.
Figure 6. Resveratrol (RESV) modulates cardiac energy metabolism in exercise trained (ET) rats via altered signal transduction pathways and increased mitochondrial function.
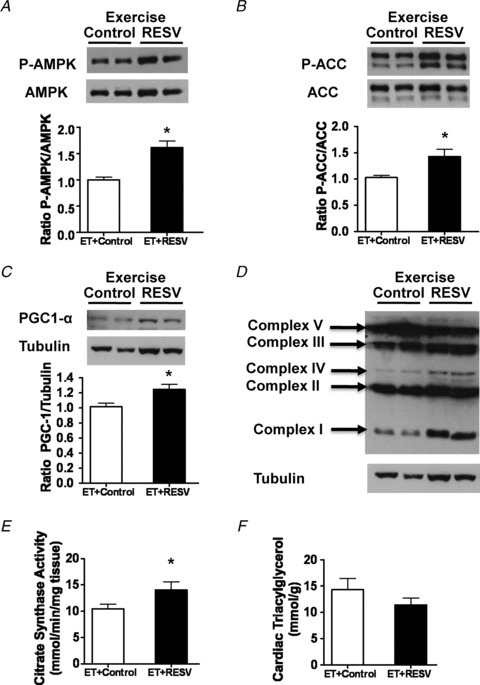
Immunoblot analysis was performed on homogenates of hearts from ET + Control and ET + RESV Wistar rats. A, levels of phosphorylated threonine-172 AMPK (P-AMPK) were quantified by densitometry and normalized against total AMPK. B, levels of phosphorylated serine-79 ACC (P-ACC) were quantified by densitometry and normalized against total ACC. C, levels of PGC-1α normalized against tubulin. D, levels of electron transport chain complexes compared to tubulin. E, citrate synthase assay. F, cardiac triacylglycerol levels. Values are the mean ± SEM of n = 6 rats in each group. *Significant difference (P < 0.05) between ET + Control and ET + RESV rats using a Student's t-test.
Discussion
In this study, we sought to clarify whether RESV was beneficial for exercise performance in otherwise healthy rodents and to determine whether RESV has additional effects during ET. Several reports have demonstrated that supplementing rodent diets with RESV improved the exercise performance of high-fat fed mice (Lagouge et al. 2006) or aged mice (Murase et al. 2009). However, a recent report failed to detect improved endurance in genetically obese mice fed RESV and subsequently subjected to a single bout of exercise (Mayers et al. 2009). In contrast to that latter study, we demonstrate here that rats consuming diets supplemented with RESV throughout 12 weeks of ET were able to run longer and further than rats that were only subjected to ET. In agreement with previous findings with RESV alone (Murase et al. 2009; Momken et al. 2011), we also found that ET + RESV was associated with improved strength of the soleus and TA muscles, which may contribute to improved exercise performance in the RESV treated rats.
Although we cannot conclusively identify the precise mechanism responsible for improved exercise performance in the ET + RESV treated rats, previous work has shown that the endurance capacity of skeletal muscles are also influenced by energy homeostasis (Adhihetty et al. 2003). Moreover, RESV has been shown to increase skeletal muscle mitochondrial biogenesis and fatty acid oxidation in mice (Baur et al. 2006; Lagouge et al. 2006), thereby contributing to increased endurance (Lagouge et al. 2006). Furthermore, it was recently shown that RESV supplementation also improved mitochondrial efficiency in overweight middle-aged men (Timmers et al. 2011), demonstrating that the beneficial effects of RESV were not specific to rodents. In agreement with this, our data show that ET + RESV rats also exhibited increased whole body oxygen consumption (Fig. 4C), suggesting that skeletal muscle probably has increased oxidative capacity. Moreover, whole body fat oxidation was increased in ET + RESV rats (Fig. 4E), which could also contribute to improved aerobic exercise capacity. Based on these findings, we conclude that RESV and ET synergistically act to improve skeletal muscle oxidative capacity and metabolism and that this contributes to the enhanced endurance capacity of the skeletal muscles.
While there are direct effects of RESV and ET on skeletal muscle, exercise performance is also influenced by the efficiency of oxygen transport to skeletal muscle (Hoppeler & Weibel, 1998), which can be mediated by enhanced cardiac performance. In response to exercise, the heart increases its metabolic rate several fold while maintaining constant concentrations of high-energy phosphates (Goodwin & Taegtmeyer, 2000). Previous work has shown that RESV treatment causes metabolic alterations in the hearts of sedentary mice that resulted in increased the expression of PPARα regulated genes such as Ucp3 and Pdk4 (Barger et al. 2008). This observation also supports the finding that RESV preserved the expression of PPARα regulated genes in the failing rat heart (Rimbaud et al. 2011). While our study did not investigate how increased mitochondrial uncoupling could contribute to RESV-induced adaptations to ET, based on findings from ischemia-reperfused hearts (Hoerter et al. 2004), we speculate that increased uncoupling could limit the production of oxygen radicals during bouts of exercise. Moreover, our data show that the addition of RESV to ET produced several changes in signal transduction pathways and the gene expression profile of the heart that may contribute to positive adaptations that alter the oxidative capacity of the heart. For example, increased expression of Prkar2 prepares the heart for the lipolysis of stored triglycerides in response to adrenergic stimulus during exercise (Goldberg & Khoo, 1985). In addition, induction of Pdk4 and the activation of AMPK drive the oxidation of fatty acids for the generation of energy by the heart (Chambers et al. 2011). Consistent with this, hearts from ET + RESV rats also had higher expression levels of mitochondrial complexes, suggesting enhanced oxidative capacity. While the metabolic and haemodynamic parameters were not directly measured during exercise, our findings support the concept that the addition of RESV to ET increases cardiac performance by modifying signal transduction pathways as well as altering the mRNA expression of specific metabolic genes, thus increasing the oxidative capacity of the heart.
Interestingly, cardiac fat oxidation was increased in ET + RESV rats compared to ET rats alone (Figs. 4F), which was shown to improve cardiac performance. Previous studies have demonstrated that AMPK is activated by exercise (Coven et al. 2003; Nielsen et al. 2003; Musi et al. 2005) as well as RESV (Baur et al. 2006; Dolinsky et al. 2009, 2011; Timmers et al. 2011). Thus, we measured phosphorylation levels of AMPK and its downstream target, ACC in these hearts in order to delineate some of the mechanisms responsible for these observations. Consistent with AMPK activation promoting fat oxidation in the heart, we also observed increased AMPK and ACC phosphorylation in hearts from ET + RESV rats compared to ET rats (Fig. 6A and B). Since this is the first report showing the additive effect of RESV and ET on the phosphorylation of cardiac AMPK, the precise mechanism by which this occurs is still unknown. While RESV may act directly to stimulate AMPK during exercise, it is also possible that AMPK phosphorylation is increased by RESV-mediated augmentation of adiponectin levels indirectly resulting from enhanced Adipoq expression (Table 4). Indeed, increased Adipoq expression and elevated plasma levels of adiponectin stimulate the oxidation of fatty acids through the activation of AMPK (Yamauchi et al. 2002). Given that Adipoq is synthesized and secreted by cardiomyocytes (Pineiro et al. 2005), adiponectin could augment fat oxidation and AMPK activity via an autocrine signalling loop. Whether or not this is the mechanism by which RESV acts to increase AMPK phosphorylation in the hearts of ET rats is as yet unknown. Elevated cardiac fatty acid oxidation likely involves enhanced mitochondrial functions, as indicated by increased expression of mitochondrial complexes and citrate synthase activities (Fig. 6). ET + RESV also induced expression of PGC1-α, a well-known transcriptional regulator of mitochondrial number and function (Finck & Kelly, 2006). Based on the observation that activated AMPK stimulates PGC1-α expression/transcriptional activity (Jager et al. 2007; Iwabu et al. 2010), it is possible that ET + RESV increased PGC1-α and mitochondrial functions in an AMPK-dependent manner. While this is an attractive hypothesis, further studies are required to prove this empirically.
The gene expression profile of the heart also revealed an improved capacity for the synthesis and storage of fatty acids in ET + RESV rats compared to ET rats. While other studies demonstrated that exercise alone increased the expression and activities of Dgat and Scd (Petridou et al. 2005; Bergman et al. 2010; Dobrzyn et al. 2010), we detected even higher levels of these transcripts in the hearts of ET + RESV rats compared to ET alone (Table 4). While we do not have data to explain the physiological effects of these changes, we propose that the synthesis of TGs by the heart may act as a buffer against the lipotoxicity of fatty acids released by adipose tissues during exercise. Furthermore, recent evidence has indicated that most of the fatty acids that are oxidized by the heart are derived from the intramyocardial storage pool of TG (Haemmerle et al. 2006; Schoiswohl et al. 2010). Therefore, RESV appears to stimulate a gene expression profile that adapts the tissue for the efficient metabolism and oxidation of fatty acids during exercise. In accordance with our findings, it was recently shown that RESV improves mitochondrial function and promotes fatty acid utilization in both soleus and cardiac muscle from rats with heart failure (Rimbaud et al. 2011). In that model, prevention of the loss of fatty acid oxidation contributed to improved cardiac performance which is consistent with improved performance in non-diseased and exercise trained rats observed in our study. Together, these findings suggest that at least one additional protective/beneficial effect of RESV in the heart is enhanced fatty acid metabolism that is at least partly mediated by certain signal transduction pathways and increased expression of specific metabolic enzymes.
Lastly, as a marker of the degree of endurance training (Goodpaster et al. 2001; Dumortier et al. 2003), we also determined that RESV improved insulin sensitivity in ET rats (Fig. 4A and B). Based on this as well as the fact that we also observed enhanced fatty acid oxidation in ET + RESV rats compared to ET rats (Fig. 4E), we speculate that increased oxidation of fat observed during the addition of RESV to ET may not only contribute to improving exercise performance, but also prevent insulin resistance in pre-diabetic individuals undergoing moderate exercise. This is an exciting finding as adherence to vigorous exercise programmes is poor in humans, but moderate exercise accompanied by RESV may prove to be of equal benefit in terms of improved glucose performance and insulin sensitivity. This is particularly relevant in individuals with chronic diseases such as diabetes, hyperlipidaemia and heart disease where it may be difficult to achieve an ideal level of exercise intensity.
Taken together, our data show that RESV optimizes fatty acid metabolism, which may contribute to the increased contractile force response of skeletal muscles and improved parameters of cardiac structure and function. As such, these RESV-induced adaptations are likely to contribute to the improved endurance capacity of ET rats and we conclude from these findings that dietary supplementation of RESV during exercise improves exercise performance beyond exercise alone. This strategy may have clinical utility in many situations where improved physical performance needs to be augmented due to the patient's inability to perform intense exercise.
Acknowledgments
The authors acknowledge the expert technical assistance of Donna Beker, Jamie Boisvenue, Sandra Kelly, Grant Masson, Brandi Sidlick and Neil Tyreman of the University of Alberta, and Sari Yakubovich of the St Boniface Research Centre. This research was supported by grants from the Canadian Institutes of Health Research (CIHR) to J.R.B.D. and from the Canadian Foundation for Innovation to M.P.C. V.W.D. was supported by post-doctoral fellowships from the Alberta Heritage Foundation for Medical Research (AHFMR) and the Heart and Stroke Foundation of Canada (HSFC). J.R.B.D. is an AHFMR Senior Scholar. All authors have approved the final submission.
Glossary
Abbreviations
- AMPK
AMP-activated protein kinase
- ET
exercise training
- GTT
glucose tolerance test
- HR
heart rate
- ITT
insulin tolerance test
- IVRT
isovolumic relaxation time
- LV
left ventricular
- LVDP
LV developed pressure
- LVED
LV end diastolic
- LVES
LV end systolic
- LVPW
LV posterior wall
- PSP
peak systolic pressure
- RER
respiratory exchange ratio
- RESV
resveratrol
- TA
tibialis anterior
- TG
triacylglycerol
Author contributions
V.W.D. designed and performed the experiments, collected and analysed the data and wrote the manuscript. K.E.J. and M.P.C. collected and interpreted data, contributed to discussion and reviewed/edited the manuscript. R.S.S. performed experiments and collected data. M.H. and T.G. contributed to discussion and reviewed/edited the manuscript. J.R.B.D. designed the experiments, contributed to discussion and wrote the manuscript. The authors have no disclosures.
Author's present address
V. W. Dolinsky: Manitoba Institute of Child Health, University of Manitoba, Winnipeg, MB, Canada.
References
- Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol. 2003;88:99–107. doi: 10.1113/eph8802505. [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Pugh TD, Prolla TA, Weindruch R. Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Exp Gerontol. 2008;43:859–866. doi: 10.1016/j.exger.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. J Appl Physiol. 2010;108:1134–1141. doi: 10.1152/japplphysiol.00684.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers KT, Leone TC, Sambandam N, Kovacs A, Wagg CS, Lopaschuk GD, Finck BN, Kelly DP. Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response. J Biol Chem. 2011;286:11155–11162. doi: 10.1074/jbc.M110.217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008;283:24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie WW. Rapid separation and quantification of lipid classes by high performance liquid chromatography and mass (light-scattering) detection. J Lipid Res. 1985;26:507–512. [PubMed] [Google Scholar]
- Coven DL, Hu X, Cong L, Bergeron R, Shulman GI, Hardie DG, Young LH. Physiological role of AMP-activated protein kinase in the heart: graded activation during exercise. Am J Physiol Endocrinol Metab. 2003;285:E629–636. doi: 10.1152/ajpendo.00171.2003. [DOI] [PubMed] [Google Scholar]
- Dobrzyn P, Pyrkowska A, Jazurek M, Szymanski K, Langfort J, Dobrzyn A. Endurance training-induced accumulation of muscle triglycerides is coupled to upregulation of stearoyl-CoA desaturase 1. J Appl Physiol. 2010;109:1653–1661. doi: 10.1152/japplphysiol.00598.2010. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Dyck JR. Role of AMP-activated protein kinase in healthy and diseased hearts. Am J Physiol Heart Circ Physiol. 2006;291:H2557–2569. doi: 10.1152/ajpheart.00329.2006. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Dyck JR. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim Biophys Acta. 2011;1812:1477–1489. doi: 10.1016/j.bbadis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, Des Rosiers C, Walsh K, Davidge ST, Dyck JR. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56:412–421. doi: 10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Rueda-Clausen CF, Morton JS, Davidge ST, Dyck JR. Continued postnatal administration of resveratrol prevents diet-induced metabolic syndrome in rat offspring born growth restricted. Diabetes. 2011;60:2274–2284. doi: 10.2337/db11-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier M, Brandou F, Perez-Martin A, Fedou C, Mercier J, Brun JF. Low intensity endurance exercise targeted for lipid oxidation improves body composition and insulin sensitivity in patients with the metabolic syndrome. Diabetes Metab. 2003;29:509–518. doi: 10.1016/s1262-3636(07)70065-4. [DOI] [PubMed] [Google Scholar]
- Dyck JR, Cheng JF, Stanley WC, Barr R, Chandler MP, Brown S, Wallace D, Arrhenius T, Harmon C, Yang G, Nadzan AM, Lopaschuk GD. Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circulation research. 2004;94:e78–84. doi: 10.1161/01.RES.0000129255.19569.8f. [DOI] [PubMed] [Google Scholar]
- Fenning A, Harrison G, Dwyer D, Rose’Meyer R, Brown L. Cardiac adaptation to endurance exercise in rats. Mol Cell Biochem. 2003;251:51–59. doi: 10.1007/978-1-4419-9238-3_8. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Foster C, Costill DL, Daniels JT, Fink WJ. Skeletal muscle enzyme activity, fiber composition and VO2 max in relation to distance running performance. Eur J Appl Physiol. 1978;39:73–80. doi: 10.1007/BF00421711. [DOI] [PubMed] [Google Scholar]
- Gallo M, Gordon T, Tyreman N, Shu Y, Putman CT. Reliability of isolated isometric function measures in rat muscles composed of different fibre types. Exp Physiol. 2004;89:583–592. doi: 10.1113/expphysiol.2004.027680. [DOI] [PubMed] [Google Scholar]
- Goldberg DI, Khoo JC. Activation of myocardial neutral triglyceride lipase and neutral cholesterol esterase by cAMP-dependent protein kinase. J Biol Chem. 1985;260:5879–5882. [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- Goodwin GW, Taegtmeyer H. Improved energy homeostasis of the heart in the metabolic state of exercise. Am J Physiol Heart Circ Physiol. 2000;279:H1490–1501. doi: 10.1152/ajpheart.2000.279.4.H1490. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- Hoerter J, Gonzalez-Barroso MD, Couplan E, Mateo P, Gelly C, Cassard-Doulcier AM, Diolez P, Bouillaud F. Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage. Circulation. 2004;110:528–533. doi: 10.1161/01.CIR.0000137824.30476.0E. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Weibel ER. Limits for oxygen and substrate transport in mammals. J Exp Biol. 1998;201:1051–1064. doi: 10.1242/jeb.201.8.1051. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, Condorelli G, Ellingsen O. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol. 2008;214:316–321. doi: 10.1002/jcp.21197. [DOI] [PubMed] [Google Scholar]
- Koonen DP, Sung MM, Kao CK, Dolinsky VW, Koves TR, Ilkayeva O, Jacobs RL, Vance DE, Light PE, Muoio DM, Febbraio M, Dyck JR. Alterations in skeletal muscle fatty acid handling predisposes middle-aged mice to diet-induced insulin resistance. Diabetes. 2010;59:1366–1375. doi: 10.2337/db09-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Mayers JR, Iliff BW, Swoap SJ. Resveratrol treatment in mice does not elicit the bradycardia and hypothermia associated with calorie restriction. FASEB J. 2009;23:1032–1040. doi: 10.1096/fj.08-115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momken I, Stevens L, Bergouignan A, Desplanches D, Rudwill F, Chery I, Zahariev A, Zahn S, Stein TP, Sebedio JL, Pujos-Guillot E, Falempin M, Simon C, Coxam V, Andrianjafiniony T, Gauquelin-Koch G, Picquet F, Blanc S. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011;25:3646–3660. doi: 10.1096/fj.10-177295. [DOI] [PubMed] [Google Scholar]
- Murakami T, Nakazawa M, Nakanishi T, Momma K. End-systolic wall stress is a major determinant of postoperative left ventricular dysfunction in patients with congenital mitral regurgitation. Cardiol Young. 2002;12:236–239. doi: 10.1017/s1047951102000525. [DOI] [PubMed] [Google Scholar]
- Murase T, Haramizu S, Ota N, Hase T. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology. 2009;10:423–434. doi: 10.1007/s10522-008-9177-z. [DOI] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Arad M, Xing Y, Fujii N, Pomerleau J, Ahmad F, Berul CI, Seidman JG, Tian R, Goodyear LJ. Functional role of AMP-activated protein kinase in the heart during exercise. FEBS Lett. 2005;579:2045–2050. doi: 10.1016/j.febslet.2005.02.052. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol. 2003;94:631–641. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- Petridou A, Nikolaidis MG, Matsakas A, Schulz T, Michna H, Mougios V. Effect of exercise training on the fatty acid composition of lipid classes in rat liver, skeletal muscle, and adipose tissue. Eur J Appl Physiol. 2005;94:84–92. doi: 10.1007/s00421-004-1294-z. [DOI] [PubMed] [Google Scholar]
- Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Dieguez C, Gualillo O, Gonzalez-Juanatey JR, Lago F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–5169. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- Rimbaud S, Ruiz M, Piquereau J, Mateo P, Fortin D, Veksler V, Garnier A, Ventura-Clapier R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS One. 2011;6:e26391. doi: 10.1371/journal.pone.0026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoiswohl G, Schweiger M, Schreiber R, Gorkiewicz G, Preiss-Landl K, Taschler U, Zierler KA, Radner FP, Eichmann TO, Kienesberger PC, Eder S, Lass A, Haemmerle G, Alsted TJ, Kiens B, Hoefler G, Zechner R, Zimmermann R. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J Lipid Res. 2010;51:490–499. doi: 10.1194/jlr.M001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriet LL, Gibala MJ. Nutritional strategies to influence adaptations to training. J Sports Sci. 2004;22:127–141. doi: 10.1080/0264041031000140608. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]


