Abstract
Objectives
We tested whether an assessment of myocardial scarring by cardiac magnetic resonance (CMR) would improve risk stratification in patients evaluated for implantable cardioverter-defibrillator implantation.
Background
Current SCD risk stratification emphasizes left-ventricular ejection fraction (LVEF), however the majority of patients suffering SCD have a preserved LVEF and many with poor LVEF do not benefit from ICD prophylaxis.
Methods
One hundred thirty-seven patients undergoing evaluation for possible ICD placement were prospectively enrolled and underwent CMR assessment of LVEF and scar. The prespecified primary endpoint was death or appropriate ICD discharge for sustained ventricular tachyarrhythmia.
Results
During a median follow-up of 24 months the primary endpoint occurred in 39 patients. Whereas the rate of adverse events steadily increased with decreasing LVEF, a sharp step-up was observed for scar size >5% of LV mass (HR=5.2 [95% CI, 2.0-13.3]). On multivariable Cox proportional hazards analysis, including LVEF and electrophysiological-study results, scar size (as a continuous variable or dichotomized at 5%) was an independent predictor of adverse outcome. Among patients with LVEF >30%, those with significant scarring (>5%) had higher risk than those with minimal-or-no (≤5%) scarring (HR=6.3 [1.4-28.0]). Those with LVEF >30% and significant scarring had similar risk to patients with LVEF ≤30% (p=0.56). Among patients with LVEF ≤30%, those with significant scarring again had higher risk than those with minimal-or-no scarring (HR=3.9 [1.2-13.1]). Those with LVEF ≤30% and minimal scarring had similar risk to patients with LVEF >30% (p=0.71).
Conclusions
Myocardial scarring detected by CMR is an independent predictor of adverse outcome in patients being considered for ICD placement. In patients with LVEF >30%, significant scarring (>5% LV) identifies a high-risk cohort similar in risk to those with LVEF ≤30%. Conversely, in patients with LVEF ≤30%, minimal-or-no scarring identifies a low-risk cohort similar to those with LVEF >30%.
Keywords: cardiovascular magnetic resonance, implantable cardioverter-defibrillator, myocardial scarring
INTRODUCTION
Sudden cardiac death (SCD) is a leading cause of mortality responsible for approximately 325,000 deaths annually in the United States alone.(1) Currently, risk stratification for SCD emphasizes left-ventricular ejection fraction (LVEF), and significant LV dysfunction has become the primary basis for determining a patient's eligibility for an implantable cardioverter-defibrillator (ICD).(2-5) However, LVEF has limitations in predicting clinical events. SCD typically results from ventricular tachyarrythmias,(6) and LVEF provides an indirect measure of the arrhythmic potential. Not surprisingly, in population studies, up to 70% of patients suffering SCD have a preserved LVEF and are not identified for prophylactic ICD implantation.(7) On the other hand, in patients with poor LVEF—who are eligible for ICD prophylaxis—many do not benefit. Recent trials suggest that approximately 14 to18 patients with ventricular dysfunction need to have an ICD implanted to prevent one death.(3,5) Moreover, considering the substantial cost(8) and the potential for complications,(9) improved risk stratification to identify patients who would benefit most from ICD implantation remains an important public health challenge.
Myocardial scar tissue is known to serve as a substrate for malignant ventricular tachyarrhythmias in both ischemic(10,11) and nonischemic cardiac disorders.(12,13) Importantly, the presence and extent of scarring may not be concordant with LVEF. For instance, some patients with extensive scarring may have preserved LVEF either because the scar is not full-thickness and/or because there is hyperkinesia of remote segments.(14,15) Conversely, some patients without myocardial scarring may have severely reduced LVEF.(16,17)
We postulated that an assessment of myocardial scarring would improve risk stratification for SCD beyond that provided by LVEF. Delayed-enhancement cardiovascular magnetic resonance (DE-CMR) provides high spatial resolution images of scar tissue that directly correlate with pathology.(18,19) Additionally, DE-CMR has shown prognostic utility above and beyond common clinical and functional indices in a variety of cohorts with ischemic(20-23) or nonischemic(19,20,24) cardiac disorders. However, in most studies evaluating prognosis there were few hard events and the primary endpoint was a composite including hospitalization for heart failure. Thus, additional studies evaluating the prognostic value of DE-CMR are essential.
The present investigation was designed to directly compare the predictive value of scar to LVEF—both simultaneously assessed during the same CMR session—for adverse outcome in patients being considered for ICD implantation.
METHODS
Population and Design
We prospectively screened patients referred to the electrophysiology service and scheduled for an electrophysiology study (EPS) and/or ICD placement between July 1, 2002 and July 1, 2004. Since we wished to evaluate both patients with preserved and impaired LVEF, a broad population was chosen and only those with contraindications for CMR (prior pacemaker or defibrillator) or were under 18 years of age were excluded. The reasons for referral to the electrophysiology service were low ejection fraction meeting criteria for an ICD in 69 (50%) patients, mild LV dysfunction not meeting criteria but with palpitations, frequent premature ventricular contractions, and/or non-sustained ventricular tachycardia in 22 (16%), evaluation of wide-complex tachycardia in 25 (18%), syncope in 17 (13%), and presumed cardiac arrest in 4 (3%). Of the 137 patients that were enrolled, CMR was performed for research purposes (only this specific protocol) in 109 patients and scan results were not used to guide clinical decision-making. The remaining 28 patients were screened concurrently and in the same prospective manner but had a clinically ordered scan for the assessment of LVEF. This group was similar to the 109 scanned only for the purpose of research with respect to age, gender, prevalence of coronary artery disease (CAD), LVEF, prevalence and extent of scar, as well as clinical outcome during follow-up (all p>0.10). All patients gave written informed consent. The study was approved by the Duke Institutional Review Board.
A comprehensive medical history including CAD risk factors, heart failure functional class (NYHA), and medications at the time of CMR was obtained in all patients. Additionally, 12-lead electrocardiography (ECG) was performed a median of 2 days (interquartile range [IQR]: 1, 5 days) from CMR and interpreted blinded to clinical and CMR data. Established criteria(3) were used to categorize patients as having ischemic or nonischemic heart disease: ischemic disease was considered present if there was ≥70% stenosis of a major epicardial coronary artery on x-ray angiography,(25) history of enzymatically proven myocardial infarction, or evidence of ischemia or infarction on clinical stress-testing. The majority of patients (n=122, 89%) had previously undergone x-ray coronary angiography.
Follow-up
Information concerning arrhythmic events and mortality status were obtained at regular intervals of 6 months via: 1) telephone interview with the patient, or if deceased, with family members, 2) contact with the patient's physician(s), and 3) hospital records. Additionally, in patients with ICDs, stored electrograms were downloaded at 3-month intervals and reviewed by an electrophysiologist blinded to clinical data and CMR findings. The prespecified primary endpoint was all-cause mortality or appropriate ICD discharge for ventricular tachycardia or fibrillation.(26) There were two secondary endpoints: 1) all-cause mortality alone, 2) sudden cardiac death or appropriate ICD discharge. For the primary endpoint, all-cause rather than cardiac mortality was included (as recommended by a policy statement on endpoints for trials that include ICDs written by the North American Society for Pacing and Electrophysiology(27) since the former is objective, clinically relevant, and unbiased, which is often not the case for cardiac mortality.(28) The secondary endpoint of SCD or appropriate ICD discharge was included to explore the mechanism of adverse outcome, and SCD was defined as death within 1 hour of symptom onset, or an unobserved death in which the patient was seen and known to be doing well within 24 hours of death.(29) All event information was obtained and classified without knowledge of clinical or CMR findings.
Patients were enrolled before the recent Federal Drug Administration alerts regarding the rare occurrence of nephrogenic systemic fibrosis associated with gadolinium contrast administration.(30) Two patients had end-stage renal disease and were receiving dialysis (one hemodialysis, one peritoneal dialysis) at the time of enrollment. None of the study participants developed nephrogenic systemic fibrosis during the follow-up period.
Cardiovascular Magnetic Resonance
Acquisition
Clinical 1.5-T scanners (Siemens Sonata or Avanto) with phased-array receiver coils and standard protocols were used.(31) Briefly, cine images were acquired in multiple short-axis (every 10 mm throughout the entire LV) and 3 long-axis views using a steady-state free precession sequence (slice thickness, 6 mm; inter-slice gap, 4 mm; TR, 3.0 ms; TE, 1.5 ms; temporal resolution, 35-40 ms; flip angle, 60°; in-plane resolution 1.7×1.4 mm). DE-CMR was performed using a segmented inversion-recovery gradient-echo sequence (slice thickness, 6 mm; inter-slice gap, 4 mm; TR, 9.5 ms; TE, 3.8 ms; flip angle, 25°; in-plane resolution 1.8x1.4 mm) 10 minutes after contrast administration (gadoversetamide, 0.15 mmol/kg) in the identical locations as cine-CMR. Inversion delay time was set to null signal from normal myocardium, and was typically 280-360 ms.
Analysis
Cine-CMR and DE-CMR images were evaluated separately masked to all patient information. Left ventricular volumes, mass, and ejection fraction were quantitatively measured from the stack of short-axis cine images using standard techniques.(32) Presence or absence of left ventricular aneurysm was noted. The presence and location of hyperenhanced tissue on DE-CMR, which was interpreted as representing scarred myocardium,(31) was determined by visual inspection using the AHA 17-segment model.(33) Regional enhancement was scored according to the spatial extent of hyperenhanced tissue within each segment (0=no hyperenhancement, 1=1-25% hyperenhanced, 2=26-50%, 3=51-75%, 4=76-100%).(14) Scar size was measured by planimetry from the stack of short-axis DE-CMR images in our CMR core laboratory by a single, blinded reader. Inter- and intraoberver agreement for scar size is routinely tested in the core laboratory for quality assurance; Bland-Altman analysis demonstrated a bias of 1.0% and -0.1%, respectively with a standard deviation of differences of 2.6% and 0.8%, respectively;(22) the intra class correlation coefficients were 0.942 and 0.982, respectively. We also assessed other morphological characteristics of scar. These included the number of separate scars, scar surface area (determined from the scar circumference on the stack of short-axis DE-CMR images(34)), and scar pattern (classified as CAD-type when subendocardial or transmural in a typical vascular distribution, or non CAD-type when mid-myocardial or epicardial(35)). The extent of the “grey-zone” (i.e. regions with partial hyperenhancement) was also determined.(36) As described previously,(36) grey zones were defined as those regions with image intensity between 2 and 3 SD above that of reference, remote myocardium, and was expressed as a percentage of LV mass.
Electrophysiologic Testing
A total of 105 (77%) patients underwent EPS within a median of 0 days (IQR 0, 3.5) of CMR. No patient experienced a change in clinical status in the time between CMR and EPS. EPS was performed using standard techniques. Briefly, programmed stimulation was performed using two drive trains followed by one to three ventricular extrastimuli that were 2 ms in duration at twice the diastolic threshold at two right ventricular sites.(37) All EPS data were reinterpreted at a later timepoint by an experienced electrophysiologist blinded to patient information and CMR findings by reviewing the intracardiac electrograms and the surface ECG stored on the commercial recording system (Prucka Cardiolab, GE Healthcare). The EPS endpoint included the induction of a sustained monomorphic ventricular tachycardia (VT), polymorphic VT, or ventricular fibrillation (VF), or completion of the protocol.(37) Similar to previous studies, a sustained ventricular arrhythmia was defined as one lasting 30 seconds or requiring termination sooner because of hemodynamic compromise, monomorphic VT was defined as a VT with a uniform beat-to-beat QRS morphology, polymorphic VT had a variable QRS morphology, and VF was defined as a rapid, disorganized rhythm without consistently identifiable complexes.(37)
Statistical Analysis
Normally distributed data are presented as the mean ± SD or, in cases where the distribution is not normal, as median and interquartile range. Two sample t tests were used to compare mean values of continuous data between two groups. Chi square tests were used to compare discrete data between groups; in those cases where the expected cell count was <5, Fisher's exact test was used. Cumulative event rates were calculated according to the Kaplan-Meier method. Differences in event rates between groups were assessed with the log-rank test without adjustment for multiple comparisons. In order to identify the baseline characteristics associated with adverse outcome, univariable Cox proportional hazards regression analysis was performed. For patients with two or more events during follow-up (several arrhythmic events or an arrhythmic event followed by death), only the time to the first event was considered per patient. Because coronary revascularization may result in procedure-related myocardial injury,(38,39) patients who underwent coronary bypass graft surgery or percutaneous coronary intervention after study enrollment were censored at the date of the procedure. Patients were to be censored on the date of heart transplantation, but none underwent heart transplantation during follow-up.
Two Cox regression multivariable models were subsequently developed. In the first, candidate variables showing a possible association with prognosis by univariable analysis (p<0.10) were considered one at a time starting with the most significant variable. Significant variables were determined by stepwise selection (and backwards elimination) at the 0.05-level of significance. In the subgroup with EPS a separate analysis was performed with monomorphic VT added as a covariate. Relative risks were expressed as hazard ratios with associated 95% confidence intervals (HR [95% CI]). In the second multivariable model, only 3 variables were included to avoid the potential for overfitting. These were NYHA functional class (the most significant clinical predictor), LVEF, and scar size>5%. Formal risk reclassification analyses were conducted with both integrated discrimination improvement (IDI) and net reclassification improvement (NRI) methods.(40) All statistical tests were two tailed and p<0.05 was regarded as significant. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Baseline Characteristics
Of the 137 enrolled patients, all successfully underwent CMR and their baseline characteristics are shown in Table 1. Briefly, the mean age was 59 years, 63% were male, about half (53%) had ischemic heart disease, and the mean LVEF was 35%. Just over 60% had NYHA functional class II or higher, and two-thirds were treated with a beta-blocker and an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. The majority (n=105) underwent EPS, and monomorphic VT was induced in 21 (20%) patients. Myocardial scar was found in 107 patients (78%) with a median scar size of 7.8% of the left ventricular mass (IQR 1.1, 15.8). Patients with ischemic heart disease were older, more often male, more likely to have diabetes, hypertension, and hypercholesterolemia, and had lower LVEF and higher prevalence of myocardial scar, compared to those without ischemic disease.
Table 1.
Baseline Patient Characteristics
| Characteristic | All patients (n=137) | Death or ICD discharge (n=39) | No Death or ICD discharge (n=98) | P | CAD (n=73) | No CAD (n=64) | P |
|---|---|---|---|---|---|---|---|
| Age (yr) | 59.2±15.1 | 61.6±15.9 | 58.2±14.9 | 0.23 | 65.3±10.9 | 52.3±16.2 | <0.0001 |
| Male gender | 86 (63%) | 26 (67%) | 60 (61%) | 0.55 | 54 (74%) | 32 (50%) | 0.004 |
| Clinical History | |||||||
| Diabetes mellitus | 32 (23%) | 13 (33%) | 19 (19%) | 0.08 | 27 (37%) | 5 (8%) | <0.0001 |
| Hypertension | 73 (53%) | 23 (59%) | 50 (51%) | 0.40 | 49 (67%) | 24 (38%) | 0.0005 |
| Cigarette Smoker | 25 (18%) | 9 (23%) | 16 (16%) | 0.36 | 16 (22%) | 9 (14%) | 0.24 |
| Hypercholesterolemia | 67 (49%) | 21 (54%) | 46 (47%) | 0.47 | 55 (75%) | 12 (19%) | <0.0001 |
| Ischemic Heart Disease | 73 (53%) | 26 (67%) | 47 (47%) | 0.04 | ... | ... | |
| Prior Revascularization | 54 (39%) | 16 (41%) | 38 (39%) | 0.81 | ... | ... | |
| CABG | 38 (70%) | 11 (69%) | 27 (71%) | 0.94 | ... | ... | |
| PCI | 16 (30%) | 5 (31%) | 11 (29%) | 0.79 | ... | ... | |
| Prior Myocardial Infarction* | 48 (35%) | 15 (38%) | 33 (34%) | 0.60 | ... | ... | |
| NYHA functional class† | 0.003 | 0.15 | |||||
| I | 51 (37%) | 7 (18%) | 44 (45%) | 21 (29%) | 30 (47%) | ||
| II | 34 (25%) | 10 (26%) | 24 (24%) | 21 (29%) | 13 (20%) | ||
| III | 45 (33%) | 17 (44%) | 28 (29%) | 26 (36%) | 19 (27%) | ||
| IV | 7 (5%) | 5 (13%) | 2 (2%) | 5 (7%) | 2 (3%) | ||
| Medications | |||||||
| ACE-Inhibitor | 75 (55%) | 22 (56%) | 53 (54%) | 0.80 | 44 (66%) | 31 (48%) | 0.16 |
| ARB | 15 (11%) | 6 (15%) | 9 (9%) | 0.24 | 9 (12%) | 6 (9%) | 0.58 |
| Antiarhythmic Class I | 2 (1%) | 1 (3%) | 1 (1%) | 0.49# | 0 (0%) | 2 (3%) | 0.12# |
| Antiarhythmic Class III | 16 (12%) | 4 (10%) | 12 (12%) | 0.78# | 8 (11%) | 8 (13%) | 0.78# |
| Antiplatelet | 96 (70%) | 30 (77%) | 66 (67%) | 0.27 | 63 (86%) | 33 (52%) | <0.0001 |
| Beta-blocker | 92 (67%) | 31 (79%) | 61 (62%) | 0.053 | 57 (78%) | 35 (55%) | 0.004 |
| Calcium-channel Blocker | 18 (13%) | 4 (10%) | 14 (14%) | 0.59# | 7 (10%) | 11 (17%) | 0.19# |
| Digitalis | 36 (26%) | 11 (28%) | 25 (26%) | 0.75 | 21 (29%) | 15 (23%) | 0.48 |
| Diuretics | 71 (52%) | 24 (62%) | 47 (48%) | 0.15 | 42 (58%) | 29 (45%) | 0.15 |
| Spironolactone | 31 (23%) | 10 (26%) | 21 (21%) | 0.60 | 18 (25%) | 13 (20%) | 0.54 |
| Statin | 66 (48%) | 19 (49%) | 47 (48%) | 0.94 | 51 (70%) | 15 (23%) | <0.0001 |
| Electrocardiogram | |||||||
| Heart rate (bpm) | 74.0±14.8 | 77.6±15.3 | 72.6±14.3 | 0.07 | 72.4±13.4 | 75.8±16.0 | 0.18 |
| QRS (ms) | 111.9±31.8 | 116.2±29.9 | 110.3±32.4 | 0.33 | 115.2±30.3 | 108.3±33.2 | 0.21 |
| Left bundle branch block | 21 (16%) | 7 (18%) | 14 (14%) | 0.57 | 11 (15%) | 10 (16%) | 0.92 |
| Right bundle branch block | 18 (13%) | 5 (13%) | 13 (13%) | 0.97 | 10 (14%) | 8 (13%) | 0.84 |
| Electrophysiologic Study § | |||||||
| Monomorphic VT | 21 (20%) | 10 (34%) | 11 (14%) | 0.02 | 15 (28%) | 6 (11%) | 0.02 |
| Polymorphic VT or VF | 22 (21%) | 5 (17%) | 17 (22%) | 0.56 | 14 (27%) | 8 (15%) | 0.14 |
| Non inducible‡ | 57 (54%) | 14 (48%) | 43 (57%) | 0.45 | 33 (44%) | 24 (45%) | 0.89 |
| CMR | |||||||
| LVEF(%) | 35.3±18.1 | 27.9±14.3 | 38.3±18.8 | 0.002 | 30.5±14.0 | 40.9±20.6 | 0.0008 |
| LV EDV (ml) | 207.7±111.0 | 246.0±155.5 | 192.4±83.5 | 0.048 | 224.0±98.9 | 183.0±90.6 | 0.13 |
| LV ESV (ml) | 147.7±110.6 | 190.0±152.2 | 130.9±84.1 | 0.03 | 165.1±96.8 | 122.1±94.2 | 0.01 |
| LV mass (g) | 196.8±71.0 | 201.2±73.1 | 194.9±70.4 | 0.64 | 204.4±63.1 | 186.8±76.4 | 0.14 |
| LV aneurysm | 12 (9%) | 6 (21%) | 6 (6%) | 0.01 | 10 (14%) | 2 (3%) | 0.03 |
| Any scar on DE-CMR | 107 (78%) | 37 (95%) | 70 (71%) | 0.003 | 70 (96%) | 37 (58%) | <0.0001 |
| Scar size (% of LV mass) | 7.8 (1.1, 15.8) | 12.9 (6.3, 19.2) | 5.2 (0.0, 14.7) | 0.002 | 13.9 (6.2-19.3) | 1.9 (0-8.5) | <0.0001 |
ARB = angiotensin receptor blocker, ACE-Inhibitor = angiotensin-converting-enzyme inhibitor, CAD = coronary artery disease, CABG = coronary artery bypass graft surgery, CMR = cardiovascular magnetic resonance imaging, DE-CMR = delayed enhancement CMR, EDV = enddiastolic volume, ESV = endsystolic volume, LVEF = left ventricular ejection fraction, NYHA = New York Heart Association, PCI = percutaneous coronary intervention, VF = ventricular fibrillation, VT = ventricular tachycardia.
10 patients with subacute infarction (<30 days of CMR study).
NYHA functional class was documented at time of hospital admission; P value pertains to the comparison between the groups with and without events, and with and without CAD in the distribution of patients according to NYHA class.
Fisher exact test (two-tailed).
Performed in 105 patients.
Includes 5 patients without structural heart disease in whom bundle branch re-entry tachycardia was induced.
Follow-up
The median follow-up time was 24 months (IQR 19.9, 29.0). No patient was lost to follow-up. 104 patients (75%) had an ICD placed, generally during the initial evaluation, 2 days (IQR 1, 7) after enrollment. The decision for ICD implantation was guided by standard consensus criteria(2,41) including LVEF and EPS results, but was at the discretion of the treating physician after discussion with the patient. The indication for ICD implantation was primary prophylaxis in 92 patients and secondary prophylaxis (sustained VT or presumed cardiac arrest) in 12. The primary endpoint of death or appropriate ICD discharge occurred in 39 (28%) patients: 19 died (5 of whom also had an ICD discharge) and 20 had ICD discharge only. Sudden cardiac death occurred in 5 patients. Four patients underwent revascularization (all percutaneous coronary interventions) at 9, 12, 12, and 21 months after enrollment, and were censored at that time.
Predictors of Adverse Events
Patient characteristics related to the primary endpoint are listed in Table 1. Patients who died or had an appropriate ICD discharge were more likely to have ischemic heart disease, worse NYHA functional class, and monomorphic VT elicited on EPS. Among the CMR parameters, patients with events had worse LVEF, larger end-diastolic and end-systolic volumes, and more frequently had a LV aneurysm. Additionally, they were more likely to have myocardial scar, and scar size as a percentage of left ventricular mass was larger compared to patients without events.
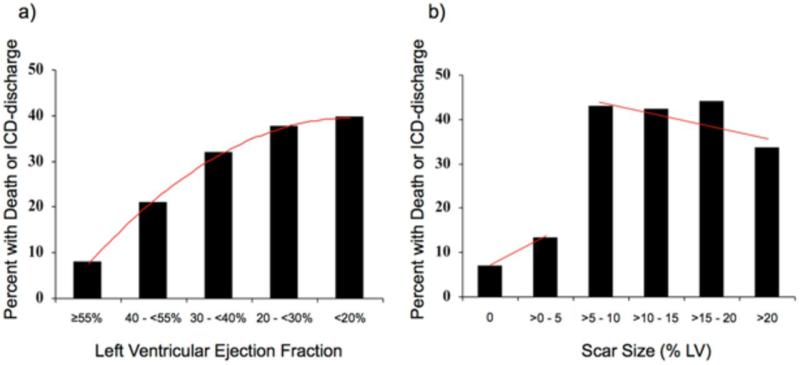
Figure 1a details the relationship between LVEF and events. For each decrement in LVEF, there was a monotonically increasing event rate. Figure 1b demonstrates a different relationship between scar size and events. There was a marked step-up in event rate in patients with a scar size exceeding 5% of LV mass (HR=5.2 [2.0-13.3], p=0.0006), without further rise with each increment in scar size. Among the 84 patients with scar >5%, 34 had events—17 died (4 of whom also had an ICD discharge) and 17 had ICD discharge only— representing an event rate of 19.6%/year and a mortality rate of 9.8%/year. Conversely, among the 53 patients with scar ≤5%, 5 had events—2 died (1 of whom also had an ICD discharge) and 3 had ICD discharge only—representing an event rate of 4.25%/year and a mortality rate of 1.7%/year. Among the 30 patients without any myocardial scar, there were 2 events (both ICD discharges, no deaths) representing a total event rate of 2.8%/year.
Figure 1. Event Rate Depending on LVEF and Scar Size.
The percentage of patients with the primary endpoint of death or appropriate ICD discharge is shown according to different levels of left ventricular ejection fraction (Panel a) and scar size (Panel b). For ejection fraction, the trendline (red line) shows a positive slope over the entire range, indicating that event rate monotonically increases with decreasing LVEF. In contrast, a marked step-up in event rate is noted for scar size greater than 5% of left ventricular mass, which however does not rise further with increasing scar size.
In Table 2 the hazard ratios for the significant clinical and CMR predictors of adverse events are shown. For the primary endpoint of death or ICD discharge, multivariable analysis demonstrated that NYHA functional class (HR=1.7 [1.2-2.4], p=0.003) and scar size >5% (HR=4.6 [1.8-11.8], p=0.002) were the only independent predictors. Scar size >5% remained an independent predictor for the secondary endpoints of SCD or ICD discharge, and all-cause mortality. Notably, although LVEF was a significant univariable predictor of adverse events (primary and both secondary endpoints), it was not an independent predictor on multivariable analysis, either as a continuous variable or using a cutoff of 30% or 35%. Multivariable analysis excluding the 28 patients with a clinically ordered scan demonstrated the same independent predictors as in the entire population. For the primary endpoint, NYHA functional class (HR=1.9 [1.3-2.9], p=0.002) and scar size >5% (HR=4.9 [1.7-14.0], p=0.003), again were the only independent predictors.
Table 2.
Clinical and CMR Predictors of Time to Event
| Parameter | Death or ICD discharge | SCD or ICD discharge | Death | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * Only parameters with p < 0.10 for one or more endpoints are shown | Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | ||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| All (n=137) | ||||||||||||
| Clinical | ||||||||||||
| Male gender | ... | ... | ... | ... | ... | ... | ... | ... | 3.43 (1.00-11.80) | 0.05 | ... | ... |
| Ischemic Heart Disease | 1.98 (1.01-3.86) | 0.05 | ... | ... | ... | ... | ... | ... | 3.76 (1.25-11.33) | 0.02 | ... | ... |
| Diabetes mellitus | 1.81 (0.93-3.53) | 0.08 | ... | ... | ... | ... | ... | ... | 3.51 (1.43-8.66) | 0.006 | ... | ... |
| NYHA functional class | 1.81 (1.29-2.56) | 0.0007 | 1.70 (1.19-2.41) | 0.003 | 1.50 (1.01-2.21) | 0.042 | ... | ... | 2.86 (1.65-4.96) | 0.0002 | 2.19 (1.29-3.70) | 0.004 |
| Beta-blocker | 2.12 (0.97-4.62) | 0.06 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... |
| Heart rate (bpm) | 1.017 (0.997-1.038) | 0.10 | ... | ... | ... | ... | ... | ... | 1.05 (1.02-1.08) | 0.0004 | 1.05 (1.02-1.08) | 0.002 |
| QRS (ms) | ... | ... | ... | ... | ... | ... | ... | ... | 1.01 (1.00-1.02) | 0.09 | ... | ... |
| CMR | ||||||||||||
| LVEF (%) | 0.971 (0.952-0.991) | 0.005 | ... | ... | 0.977 (0.955-0.999) | 0.04 | ... | ... | 0.957 (0.927-0.989) | 0.009 | ... | ... |
| LVEF ≤30% | 2.17(1.13-4.17) | 0.02 | ... | ... | 1.73 (0.83-3.63) | 0.15 | ... | ... | 2.56 (0.97-6.73) | 0.06 | ... | ... |
| LVEF ≤35% | 2.65 (1.26-5.60) | 0.01 | ... | ... | 2.11 (0.93-4.78) | 0.07 | ... | ... | 4.16 (1.21-14.28) | 0.02 | ... | ... |
| LV EDV (ml) | 1.003 (1.001-1.006) | 0.03 | ... | ... | 1.003 (1.000-1.006) | 0.06 | ... | ... | 1.006 (1.003-1.009) | 0.0004 | ... | ... |
| LV ESV (ml) | 1.003 (1.001-1.006) | 0.01 | ... | ... | 1.003 (1.001-1.006) | 0.04 | ... | ... | 1.006 (1.003-1.009) | 0.0002 | ... | ... |
| LV mass (g) | ... | ... | ... | ... | ... | ... | ... | ... | 1.005 (0.999-1.010) | 0.09 | ... | ... |
| LV aneurysm | ... | ... | ... | ... | 2.65 (1.08-6.51) | 0.03 | ... | ... | ... | ... | ... | ... |
| Any scar on DE-CMR | 6.15 (1.48-25.5) | 0.01 | ... | ... | 4.50 (1.07-18.9) | 0.04 | ... | ... | NA# | NA# | ... | ... |
| Scar size (% LV mass) | 1.038 (1.008-1.069) | 0.01 | ... | ... | 1.038 (1.003-1.074) | 0.03 | ... | ... | ... | ... | ... | ... |
| Scar size > 5% | 5.18 (2.02-13.3) | 0.0006 | 4.59 (1.79-11.8) | 0.002 | 4.76 (1.65-13.7) | 0.004 | 4.76 (1.65-13.7) | 0.004 | 5.89 (1.36-25.5) | 0.02 | 8.75 (1.89-41.0) | 0.006 |
|
Electrophysiology Study (EPS) Subgroup (n=105) | ||||||||||||
| Clinical | ||||||||||||
| Ischemic Heart Disease | 2.20 (1.02-4.73) | 0.04 | ... | ... | ... | ... | ... | ... | 3.85 (1.06-13.99) | 0.04 | ... | ... |
| Cigarette Smoker | ... | ... | ... | ... | 2.23 (0.91-5.46) | 0.08 | ... | ... | ... | ... | ... | ... |
| Diabetes mellitus | ... | ... | ... | ... | ... | ... | ... | ... | 2.94 (0.99-8.76) | 0.05 | ... | ... |
| NYHA functional class | 1.66 (1.11-2.50) | 0.01 | 1.53 (1.00-2.33) | 0.05 | ... | ... | ... | ... | 2.32 (1.23-4.43) | 0.009 | ... | ... |
| Heart rate (bpm) | 1.02 (1.00-1.04) | 0.05 | ... | ... | ... | ... | ... | ... | 1.05 (1.02-1.09) | 0.0006 | 1.06 (1.03-1.10) | 0.0006 |
| QRS (ms) | 1.011 (1.000-1.021) | 0.05 | ... | ... | ... | ... | ... | ... | 1.018 (1.003-1.033) | 0.02 | ... | ... |
| LBBB | ... | ... | ... | ... | ... | ... | ... | ... | 3.14 (0.96-10.24) | 0.06 | ... | ... |
| EPS | ||||||||||||
| Monomorphic VT | 2.47 (1.15-5.30) | 0.02 | ... | ... | 3.26 (1.39-7.63) | 0.007 | ... | ... | ... | ... | ... | ... |
| CMR | ||||||||||||
| LVEF(%) | 0.972 (0.950-0.994) | 0.01 | ... | ... | 0.977 (0.950-1.002) | 0.07 | ... | ... | 0.965 (0.930-1.000) | 0.05 | ... | ... |
| LVEF ≤30% | 2.25 (1.06-4.78) | 0.03 | ... | ... | 2.00 (0.86-4.69) | 0.11 | ... | ... | 2.06 (0.67-6.26) | 0.21 | ... | ... |
| LVEF ≤35% | 2.31 (1.02-5.22) | 0.04 | ... | ... | 1.90 (0.77-4.66) | 0.16 | ... | ... | 2.86 (0.79-10.41) | 0.11 | ... | ... |
| LV EDV (ml) | ... | ... | ... | ... | ... | ... | ... | ... | 1.004 (1.001-1.008) | 0.01 | ... | ... |
| LV ESV (ml) | 1.003 (1.000-1.006) | 0.08 | ... | ... | ... | ... | ... | ... | 1.005 (1.001-1.008) | 0.01 | ... | ... |
| LV aneurysm | ... | ... | ... | ... | ... | ... | ... | ... | NA† | NA† | ... | ... |
| Any scar on DE-CMR | 6.37 (1.51-26.8) | 0.01 | ... | ... | 4.72 (1.10-20.18) | 0.04 | ... | ... | NA# | NA# | ... | ... |
| Scar size (% LV mass) | 1.034 (1.001-1.067) | 0.04 | ... | ... | 1.033 (0.996-1.071) | 0.08 | ... | ... | ... | ... | ... | ... |
| Scar size > 5% | 5.16(1.97-13.6) | 0.0009 | 4.36 (1.65-11.6) | 0.003 | 4.83 (1.63-14.3) | 0.004 | 4.83 (1.63-14.3) | 0.004 | 5.28 (1.17-23.8) | 0.03 | 5.81 (1.26-26.8) | 0.02 |
Only variables with p<0.10 by univariable analysis were considered for inclusion in the multivariable model.
Not available because no patient in the EPS subgroup who died had an LV aneurysm.
Not available because all patients who died had scar tissue.
When scar size was included as a continuous (% LV mass) rather than dichotomous variable, it remained an independent predictor of the primary endpoint, death or ICD discharge (HR=1.03 [1.01-1.07], p=0.03) and the secondary endpoint, SCD or ICD discharge (HR=1.04 [1.01-1.07], p=0.03).
An analysis of the subgroup of patients undergoing EPS (n=105) was performed after including inducible monomorphic VT as a covariate (Table 2). Scar size >5% was an independent predictor for all endpoints. Inducible monomorphic VT was a significant univariable predictor of death or ICD discharge, and SCD or ICD discharge but not all-cause death. On multivariable analysis, inducible monomorphic VT was not an independent predictor of the primary or secondary endpoints.
In a separate multivariable modeling approach, only three variables (NYHA class, LVEF, and scar size >5%) were included to avoid the potential for overfitting. In this model, scar size >5% was the strongest predictor of the primary endpoint (HR=4.5 [1.7-11.6], p=0.002). While NYHA class was an independent predictor (HR=1.6 [1.0-2.4], p=0.04), LVEF was not (HR=0.1 [0.97-1.20], p=0.58).
To assess the incremental prognostic value of the scar data over NYHA class and over LVEF, we performed a risk reclassification analysis for the primary endpoint. The IDI showed significant reclassification when adding scar data to the model with NYHA class (IDI=0.134, p=0.0004) and when adding scar data to the model with LVEF (IDI=0.111, p=0.003). The NRI was calculated using 4 risk categories (0-20%, 20-40%, 40-60%, and >60%), and also showed significant reclassification when adding scar data to the model with NYHA class (NRI=41%, p=0.03) and to the model with LVEF (NRI=32%, p=0.049).
Improved Risk Stratification in LVEF subgroups
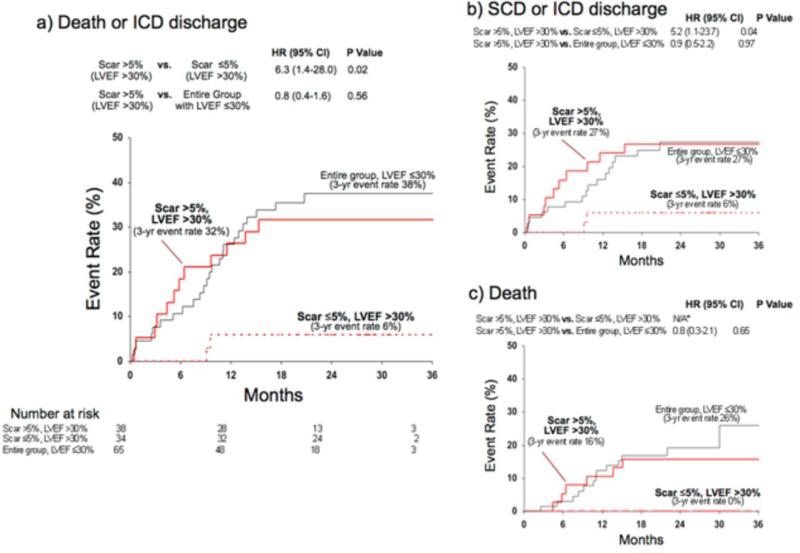
Survival analysis in subgroups with >30% and LVEF ≤30%(5,42) are shown in Figures 2 and 3, respectively. Among patients with LVEF >30%, those with significant scarring (>5%) had higher incidence of death or ICD discharge compared to those with minimal-or-no (≤5%) scarring (HR=6.3 [1.4-28.0], p=0.02; Figure 2a). Despite an LVEF >30%, the high-risk subcohort with scar >5% had a similar event rate to the entire group with LVEF ≤30% (HR=0.8 [0.4-1.6], p=0.56). Similar relationships were observed for the secondary endpoints (Figure 2b and c).
Figure 2. Kaplan-Meier Estimates of Adverse Events in Patients With LVEF >30%.
In patients with LVEF >30%, those with significant scarring (>5% of LV mass) had a higher event rate than those with minimal-or-no scarring (≤5%) for both the primary (panel a) and the two secondary endpoints (panels b, c). Those with LVEF >30% and significant scarring had similar event rate to the entire group of patients with LVEF ≤30%. * For the secondary endpoint of death alone, the hazard ratio between scar >5%, LVEF >30% and scar ≤5%, LVEF >30% cannot be calculated because there were no deaths in the latter group.
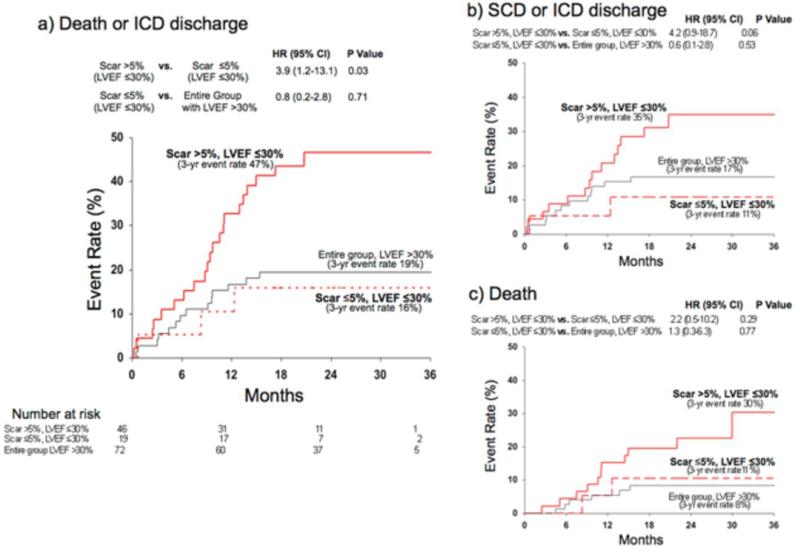
Figure 3. Kaplan-Meier Estimates of Adverse Events in Patients With LVEF ≤30%.
In patients with LVEF ≤30%, those with significant scarring (>5% of LV mass) had a higher event rate than those with minimal-or-no scarring (≤5%) for both the primary (panel a) and the two secondary endpoints (panels b, c). Those with LVEF ≤30% and minimal-or-no scarring had similar event rate to the entire group of patients with LVEF >30%.
Among patients with LVEF ≤30%, again those with scar >5% had higher incidence of death or ICD discharge compared to those with scar ≤5% (HR=3.9 [1.2-13.1, p=0.03]; Figure 3a). Similar trends were found for the secondary endpoints, but these did not reach statistical significance (Figure 3b and c). Despite an LVEF ≤30%, the low-risk subcohort with scar ≤5% had a similar event rate (for all 3 endpoints) to the entire group with LVEF >30%.
Survival analysis using an LVEF cutoff of 35% (rather than 30%) demonstrated similar findings. Despite an LVEF >35%, those patients with scar >5% had a similar event rate to the entire group with LVEF ≤35% (HR=0.6 [0.3-1.5], p=0.29). Conversely, among patients with an LVEF ≤35%, the subgroup of patients with scar ≤5% had a similar event rate to the entire group with LVEF >35% (HR=0.8 [0.2-2.8], p=0.69). Figure 4 illustrates typical CMR images in patients with various levels of myocardial scarring and left ventricular function.
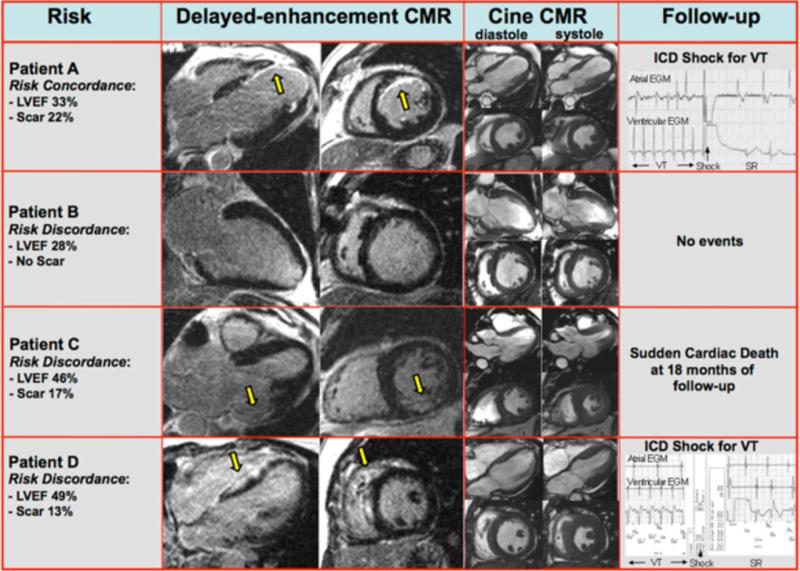
Figure 4. Typical CMR Images In Patients With Various Levels Of Myocardial Scarring And Left Ventricular Function.
Example CMR images are shown in patients with concordance (Patient A) and discordance (Patient B, C, and D) in the assessment of risk as determined by LVEF and myocardial scarring (yellow arrows). The last column reports findings during follow-up including the ICD electrograms when available. Patient A had poor LVEF and substantial scarring. This patient, who had both parameters concordant for high risk, had an ICD discharge for ventricular tachycardia during the first month of follow-up. Conversely, Patient B had poor LVEF but no myocardial scar, representing discordance in risk between these two parameters. This patient received an ICD based on LVEF criteria, however had no adverse events during follow-up (29 months). Patients C and D represent examples of those with significantly higher LVEF (46% and 49%, respectively) in whom there was discordance in risk in that substantial scarring was found. Patient C had CAD-type scarring (involves LV subendocardium), whereas patient D had non-CAD-type scarring (spares the LV subendocardium). Both patients had events during follow-up (patient C: sudden cardiac death 18 months after study enrollment; patient D: appropriate ICD discharge to terminate VT at one month and again at 10 months of follow-up).
Scar Morphology and Events
A number of characteristics of scar morphology were evaluated, and their relationships to outcome are shown in Table 3. Many parameters were associated with adverse outcome (primary and both secondary endpoints) on an unadjusted basis. However, a multivariable analysis including only scar morphology covariates, demonstrated that scar size >5% (p=0.03, HR=3.1 [1.1, 8.6]) and the number of separate scars (p=0.02, HR=1.7 [1.1, 2.5]) were independent predictors of the primary endpoint.
Table 3.
Relationship of Scar Morphology Variables With Time to Event
| Parameter | Death or ICD discharge | SCD or ICD discharge | Death | |||
|---|---|---|---|---|---|---|
| Univariable | Univariable | Univariable | ||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Any scar | 6.15 (1.48-25.53) | 0.01 | 4.50 (1.07-18.89) | 0.04 | NA# | NA# |
| CAD type* | 5.69 (1.33-24.39) | 0.02 | 4.02 (0.91-17.76) | 0.07 | NA# | NA# |
| Non-CAD type† | 4.71 (1.02-21.82) | 0.05 | 3.23 (0.65-16.02) | 0.15 | NA# | NA# |
| Scar size (% of LV mass) | 1.04 (1.01-1.07) | 0.01 | 1.04 (1.00-1.07) | 0.03 | 1.03 (0.99-1.08) | 0.2 |
| Scar size >5% | 5.18 (2.02-13.25) | 0.0006 | 4.76 (1.66-13.69) | 0.004 | 5.89 (1.36-25.48) | 0.02 |
| Scar surface area (cm2) | 1.005 (1.001-1.008) | 0.008 | 1.005 (1.001-1.009) | 0.03 | 1.006 (1.001-1.011) | 0.03 |
| Number of separate scars | 2.07 (1.46-2.94) | <0.0001 | 1.91 (1.28-2.87) | 0.002 | 3.11 (1.83-5.28) | <0.0001 |
| Largest separate scar size (% of LV mass) | 1.03 (1.00-1.06) | 0.09 | 1.03 (1.00-1.07) | 0.08 | 1.01 (0.96-1.06) | 0.7 |
| Largest separate surface area (cm2) | 1.004 (1.000-1.008) | 0.06 | 1.005 (1.000-1.009) | 0.06 | 1.003 (0.997-1.009) | 0.4 |
| Segments with transmural scar (>75%) | 1.06 (0.85-1.32) | 0.6 | 1.11 (0.87-1.41) | 0.4 | 0.80 (0.51-1.26) | 0.3 |
| Segments with transmural scar (>50%) | 1.07 (0.93-1.25) | 0.4 | 1.10 (0.93-1.29) | 0.3 | 0.90 (0.69-1.17) | 0.4 |
| Segments with non-transmural scar (1-50%) | 1.13 (1.04-1.22) | 0.005 | 1.10 (1.00-1.22) | 0.05 | 1.18 (1.05-1.32) | 0.007 |
| Segments with non-transmural scar (26-75%) | 1.17 (1.04-1.31) | 0.01 | 1.17 (1.02-1.34) | 0.02 | 1.07 (0.89-1.29) | 0.5 |
| Grey zone (% of LV mass) | 1.011 (0.994-1.027) | 0.20 | 1.003 (0.980-1.026) | 0.81 | 1.019 (1.000-1.039) | 0.052 |
CAD-type scar required involvement of the subendocardium. Hazard ratios were calculated excluding patients with non-CAD type scar.
Isolated midwall or epicardial scar was considered Non-CAD type. Hazard ratios were calculated excluding patients with CAD type scar.
Not available because all patients who died had scar tissue.
DISCUSSION
The main finding of this study is that myocardial scarring detected by DE-CMR strongly predicts death or appropriate ICD discharge for sustained ventricular arrhythmia in patients undergoing evaluation for possible ICD placement. On multivariable analysis, which included LVEF and electrophysiological study results, scar size was an independent predictor of adverse outcome when considered as a continuous variable or dichotomized at 5% of LV mass. For the latter, the hazard ratio was 4.6 [1.8-11.8] in all patients and 4.4 [1.7-11.6] in patients undergoing EPS (n=105). Furthermore, scar size >5% was an independent predictor of both secondary endpoints, SCD or ICD discharge and all-cause mortality alone.
Scar tissue is believed to be a fundamental component of the anatomical substrate for lethal ventricular arrhythmias.(10-13,43) In the setting of coronary disease, electrical mapping studies have revealed that reentrant VT usually originates from the subendocardial surface of infarcted myocardium, adjacent to dense scar.(11,43,44) In the setting of nonischemic cardiomyopathy, scar is less common and some characteristics are different (less confluence, less endocardial involvement)(17,45,46) but again VT appears primarily the result of myocardial reentry associated with scar.(13,47) In both settings, histological analysis of myocardial specimens have shown that regions that are crucially involved in the reentry circuit consist of isolated bundles of surviving myocytes interwoven with strands of fibrous scar tissue—the consequence of which is nonuniform anisotropic conduction and other electrophysiological abnormalities that can result in VT.(11,12,43)
The potential relevance of scar as detected by DE-CMR was initially investigated by comparisons with electrophysiological testing. Bello et al.(34) observed that infarct scar size (or surface area) was a better predictor of inducible monomorphic VT on EPS than LVEF in patients with coronary disease. Similarly, Nazarian et al.(48) demonstrated that DE-CMR assessment of scar distribution was the strongest predictor of inducible VT in patients with nonischemic cardiomyopathy. More recently, in patients referred for radiofrequency ablation of VT or symptomatic premature ventricular complexes, Bogun et al.(46) reported that DE-CMR diagnosed scar in all patients with history of sustained VT; and when a critical site of VT was identified, it occurred within areas of scar in all cases. Moreover, the location of scar was a reliable guide to catheter ablation—for predominantly endocardial scar, an endocardial approach was necessary, for epicardial scar, an epicardial approach was needed, and for mid-wall intramural scar, ablation was uniformly ineffective. Thus, these data present compelling evidence that DE-CMR identified scar is associated with ventricular arrhythmias, and offer mechanistic insight into why scar assessment may be better at predicting prognosis than LVEF or indices of LV morphology.
The results of the present study corroborate and extend those of earlier reports investigating the prognostic significance of scarring identified by DE-CMR.(19,21,22,24,49,50) These studies have consistently demonstrated the additive value of scar (or infarct) assessment for predicting adverse outcome. However, most studies had few hard endpoints(19,23,24) and/or were retrospective evaluations of patients who had undergone clinically ordered CMR in which scan results were used to determine patient management.(21,49,50) In the current study, all patients were prospectively enrolled prior to CMR and in most (80%) CMR was performed only for research purposes and scan results were not used to guide clinical decision-making. The overall crude mortality rate of 6.8%/year was similar to that in comparable populations at risk for arrhythmia,(3,5) and 39 patients reached the prespecified primary endpoint of death or appropriate ICD discharge. Although still relatively small, the number of events compares favorably with recently published prospective CMR studies by Wu,(23) Assomull,(19) and Wu et al.(24) that involved 18, 23, and 15 events, respectively overall, and 2, 10, and 7 events after excluding hospitalization events.
The present study is the first to directly compare CMR scar assessment with invasive EPS for predicting prognosis. EPS has distinct advantages over LVEF in that the actual induction of VT directly establishes the presence of an arrhythmic substrate, is more specific for predicting an arrhythmic death,(51) and a risk stratification strategy involving EPS has higher efficiency (fewer ICDs needed /life saved) than one focused primarily on LVEF.(52) Nonetheless, a negative EP study is not reassuring and does not indicate low likelihood for arrhythmic death especially in patients with low LVEF or with nonischemic cardiomyopathy.(37,52,53) In our relatively broad patient population we observed that inducibilty of VT on EPS was a significant univariable predictor of adverse events, but on multivariable analysis DE-CMR scar assessment was superior to EPS in predicting both primary and secondary endpoints.
Clinical Implications
Although significant left ventricular dysfunction identifies a cohort at particularly high risk for SCD, it is a well-recognized paradox that most patients that die suddenly have less severe dysfunction. For instance, the Maastricht prospective registry found that 81% of patients experiencing SCD had an LVEF >30% before the event.(54) Likewise, the Oregon sudden death study reported that 70% of patients suffering SCD had an LVEF >35% before the event. The present investigation was not a community-wide population study, but it is notable that of the 72 patients that would have been considered low-risk solely from an LVEF perspective (LVEF >30%), 14 died or had an appropriate ICD discharge during followup. Importantly, scar >5% on DE-CMR classified 12 of these 14 as high- rather than low-risk individuals. DE-CMR images of two of these patients are shown in Figure 4 (Patient C and D).
On the other hand, it is also recognized that among patients that meet the current definition of high-risk LVEF (≤30%-35%), the majority will not derive any benefit from ICD implantation, since 14-18 patients with high-risk LVEF need to have an ICD implanted to prevent one death.(3,5) In the present study 65 patients had LVEF ≤30%, among whom 25 died or had an appropriate ICD discharge during followup. However, those with scar ≤5% had a 3-year event rate that was below or similar to that of the entire group with low-risk LVEF (Figure 3a).
Eligibility for ICD implantation is based primarily on the presence of LV dysfunction, since LVEF is considered the strongest independent predictor of SCD among traditional clinical markers.(55) However, our data corroborate prior investigations reporting LVEF lacks both sensitivity and specificity in predicting clinical events.(55) Although preliminary, our findings highlight the potential of scar assessment by DE-CMR to improve the sensitivity of risk stratification by identifying patients with relatively preserved LVEF who nevertheless are at considerable risk for poor outcome. Because most SCD occurs in patients with preserved LVEF substantial effort is justified in evaluating new noninvasive risk stratification strategies in this group.(55) Our results also suggest that DE-CMR scar assessment may be useful in identifying patients with low LVEF who may not benefit from ICD therapy. This hypothesis requires extensive further testing but seems warranted given the substantial cost of ICD therapy and the potential for harm, from unnecessary shocks, procedural complications, manufacturer recalls, and possible proarrhythmia.(56)
Study Limitations
There are limitations in using ICD discharges—even after classification as appropriate or not based on stored electrograms—as a surrogate for SCD.(57) However, our findings were similar when using all-cause mortality as the endpoint (Table 2) and we believe the main associations between scar and adverse outcome in the study are unlikely to be spurious.
We compared scar with LVEF, EPS, QRS duration, and many other clinical indices, but several others with high potential for improving risk stratification, such as T-wave alternans and heart rate variability, were not tested. A systematic comparison between scar and these other risk metrics was beyond the scope of the present study but would be an important area of future research.
An exploratory analysis of scar morphology (Table 3) suggests other characteristics besides size may be important for risk stratification, such as the number of separate scars, but this will require prospective testing to fully explore their significance. There are several ways to quantitatively assess the grey zone, and it is possible that a different analysis method than the one used in the present study may have provided different results. Likewise, there may be different thresholds in scar size to optimally stratify risk when considering CAD and non-CAD patients separately. Interestingly, similar to the results of the present study, we note that Assomull et al.(19) found that scar >4.8% was the optimal threshold to predict outcome in patients with nonischemic cardiomyopathy, and Kwong et al.(21) observed a sharp step-up in risk with even a small amount of scarring in patients with coronary disease. It is speculative, but these results are consistent with experimental investigations that have suggested that a “critical mass” of scar is necessary for reentrant VT to occur.(58,59)
Finally, an important limitation is that the conclusions are based on a limited number of events (the primary endpoint occurred in 39 patients), and this raises the possibility of overfitted multivariable models; larger studies are vital to confirm these findings.
Conclusions
In patients undergoing evaluation for possible ICD implantation, myocardial scarring detected by DE-CMR predicts worse outcome. Even in patients with LVEF >30% considered low-risk from an LVEF perspective, significant scarring (>5%) identifies a cohort with a high rate of adverse events and one similar in risk to those with LVEF ≤30%. Additionally, in patients with LVEF ≤30%, minimal-or-no scarring identifies a cohort with lower risk similar those with LVEF >30%. The findings suggest that DE-CMR scar assessment is superior to LVEF for risk stratification, and justify future studies prospectively testing whether patient management guided by CMR findings can improve patient outcome.
Acknowledgments
Funding Sources:
Funding for the research was provided in part by National Institutes of Health grant 2RO1-HL64726 (RJK).
ABBREVIATIONS
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance
- DE-CMR
delayed-enhancement cardiovascular magnetic resonance
- EPS
electrophysiology study
- ICD
implantable cardioverter-defibrillator
- LVEF
left-ventricular ejection fraction
- SCD
sudden cardiac death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests Disclosures:
Raymond Kim and Robert Judd are inventors of a US patent on Delayed Enhancement MRI, which is owned by Northwestern University. There are no other conflicts of interest or financial relationships to disclose.
REFERENCES
- 1.American Heart Association . Heart Disease and Stroke Statistics - 2007 Update. American Heart Association; Dallas, Texas: 2007. [Google Scholar]
- 2.Centers for Medicaid and Medicare Services Decision Memo for Implantable Defibrillators (CAG-00157R3). Centers for Medicaid and Medicare Services. 2007 [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Myerburg RJ, Mitrani R, Interian A, Bassett AL, Simmons J, Castellanos A. Life-Threatening Ventricular Arrhythmias: The Link Between Epidemiology and Pathophysiology. In: Zipes D, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. Vol. 2000. W.B. Saunders Company; Philadelphia: 2000. pp. 521–530. [Google Scholar]
- 7.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–6. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 8.Groeneveld PW, Matta MA, Suh JJ, Heidenreich PA, Shea JA. Costs and quality-of-life effects of implantable cardioverter-defibrillators. Am J Cardiol. 2006;98:1409–15. doi: 10.1016/j.amjcard.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 9.Gradaus R, Block M, Brachmann J, et al. Mortality, morbidity, and complications in 3344 patients with implantable cardioverter defibrillators: results fron the German ICD Registry EURID. Pacing Clin Electrophysiol. 2003;26:1511–8. doi: 10.1046/j.1460-9592.2003.t01-1-00219.x. [DOI] [PubMed] [Google Scholar]
- 10.Richards DA, Blake GJ, Spear JF, Moore EN. Electrophysiologic substrate for ventricular tachycardia: correlation of properties in vivo and in vitro. Circulation. 1984;69:369–81. doi: 10.1161/01.cir.69.2.369. [DOI] [PubMed] [Google Scholar]
- 11.de Bakker JM, van Capelle FJ, Janse MJ, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77:589–606. doi: 10.1161/01.cir.77.3.589. [DOI] [PubMed] [Google Scholar]
- 12.Anderson KP, Walker R, Urie P, Ershler PR, Lux RL, Karwandee SV. Myocardial electrical propagation in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;92:122–40. doi: 10.1172/JCI116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soejima K, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004;43:1834–42. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357:21–8. doi: 10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 15.Mahrholdt H, Wagner A, Parker M, et al. Relationship of contractile function to transmural extent of infarction in patients with chronic coronary artery disease. J Am Coll Cardiol. 2003;42:505–12. doi: 10.1016/s0735-1097(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 16.Bello D, Shah DJ, Farah GM, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation. 2003;108:1945–53. doi: 10.1161/01.CIR.0000095029.57483.60. [DOI] [PubMed] [Google Scholar]
- 17.McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 18.Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 19.Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 20.Iles L, Pfluger H, Lefkovits L, et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. Journal of the American College of Cardiology. 2011;57:821–8. doi: 10.1016/j.jacc.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 21.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–43. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 22.Kim HW, Klem I, Shah DJ, et al. Unrecognized non-Q-wave myocardial infarction: prevalence and prognostic significance in patients with suspected coronary disease. PLoS medicine. 2009;6:e1000057. doi: 10.1371/journal.pmed.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu E, Ortiz JT, Tejedor P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart (British Cardiac Society) 2008;94:730–6. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
- 24.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–21. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation. 2003;107:149–48. doi: 10.1161/01.cir.0000047041.66447.29. [DOI] [PubMed] [Google Scholar]
- 26.Bloomfield DM, Bigger JT, Steinman RC, et al. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456–63. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Kim SG, Fogoros RN, Furman S, Connolly SJ, Kuck KH, Moss AJ. Standardized reporting of ICD patient outcome: the report of a North American Society of Pacing and Electrophysiology Policy Conference, February 9-10, 1993. Pacing Clin Electrophysiol. 1993;16:1358–62. doi: 10.1111/j.1540-8159.1993.tb01728.x. [DOI] [PubMed] [Google Scholar]
- 28.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? Journal of the American College of Cardiology. 1999;34:618–20. doi: 10.1016/s0735-1097(99)00250-8. [DOI] [PubMed] [Google Scholar]
- 29.Hinkle LE, Jr., Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–64. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 30.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–62. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 31.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 32.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 33.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 34.Bello D, Fieno DS, Kim RJ, et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104–8. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 35.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. European heart journal. 2005;26:1461–74. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 36.Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–9. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 37.Daubert JP, Zareba W, Hall WJ, et al. Predictive value of ventricular arrhythmia inducibility for subsequent ventricular tachycardia or ventricular fibrillation in Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2006;47:98–107. doi: 10.1016/j.jacc.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 38.Ricciardi MJ, Wu E, Davidson CJ, et al. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–3. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- 39.Selvanayagam JB, Petersen SE, Francis JM, et al. Effects of off-pump versus on-pump coronary surgery on reversible and irreversible myocardial injury: a randomized trial using cardiovascular magnetic resonance imaging and biochemical markers. Circulation. 2004;109:345–50. doi: 10.1161/01.CIR.0000109489.71945.BD. [DOI] [PubMed] [Google Scholar]
- 40.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 41.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). J Am Coll Cardiol. 2002;40:1703–19. doi: 10.1016/s0735-1097(02)02528-7. [DOI] [PubMed] [Google Scholar]
- 42.Grimm W, Christ M, Bach J, Muller HH, Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883–91. doi: 10.1161/01.CIR.0000100721.52503.85. [DOI] [PubMed] [Google Scholar]
- 43.Fenoglio JJ, Jr., Pham TD, Harken AH, Horowitz LN, Josephson ME, Wit AL. Recurrent sustained ventricular tachycardia: structure and ultrastructure of subendocardial regions in which tachycardia originates. Circulation. 1983;68:518–33. doi: 10.1161/01.cir.68.3.518. [DOI] [PubMed] [Google Scholar]
- 44.Horowitz LN, Harken AH, Kastor JA, Josephson ME. Ventricular resection guided by epicardial and endocardial mapping for treatment of recurrent ventricular tachycardia. The New England journal of medicine. 1980;302:589–93. doi: 10.1056/NEJM198003133021101. [DOI] [PubMed] [Google Scholar]
- 45.Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol. 1987;60:1340–55. doi: 10.1016/0002-9149(87)90618-7. [DOI] [PubMed] [Google Scholar]
- 46.Bogun FM, Desjardins B, Good E, et al. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. Journal of the American College of Cardiology. 2009;53:1138–45. doi: 10.1016/j.jacc.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704–10. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 48.Nazarian S, Bluemke DA, Lardo AC, et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–5. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon DH, Halley CM, Carrigan TP, et al. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: a delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc Imaging. 2009;2:34–44. doi: 10.1016/j.jcmg.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Cheong BY, Muthupillai R, Wilson JM, et al. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–76. doi: 10.1161/CIRCULATIONAHA.109.852517. [DOI] [PubMed] [Google Scholar]
- 51.Buxton AE, Lee KL, Hafley GE, et al. Relation of ejection fraction and inducible ventricular tachycardia to mode of death in patients with coronary artery disease: an analysis of patients enrolled in the multicenter unsustained tachycardia trial. Circulation. 2002;106:2466–72. doi: 10.1161/01.cir.0000037224.15873.83. [DOI] [PubMed] [Google Scholar]
- 52.Costantini O, Hohnloser SH, Kirk MM, et al. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. Journal of the American College of Cardiology. 2009;53:471–9. doi: 10.1016/j.jacc.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 53.Brilakis ES, Friedman PA, Maounis TN, et al. Programmed ventricular stimulation in patients with idiopathic dilated cardiomyopathy and syncope receiving implantable cardioverter-defibrillators: a case series and a systematic review of the literature. Int J Cardiol. 2005;98:395–401. doi: 10.1016/j.ijcard.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. European heart journal. 2003;24:1204–9. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 55.Goldberger JJ, Cain ME, Hohnloser SH, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation. 2008;118:1497–1518. [PubMed] [Google Scholar]
- 56.Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. Journal of the American College of Cardiology. 2008;52:1111–21. doi: 10.1016/j.jacc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 57.Ellenbogen KA, Levine JH, Berger RD, et al. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113:776–82. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]
- 58.de Bakker JM, van Capelle FJ, Janse MJ, et al. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915–26. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 59.Jones-Collins BA, Patterson RE. Quantitative measurement of electrical instability as a function of myocardial infarct size in the dog. Am J Cardiol. 1981;48:858–63. doi: 10.1016/0002-9149(81)90350-7. [DOI] [PubMed] [Google Scholar]