Abstract
Endogenous cannabinoids (endocannabinoids, eCBs) are ubiquitous regulators of synaptic transmission in the brain, mediating numerous forms of short- and long-term plasticity, and having strong influences on synapse formation and neurogenesis. Their roles as retrograde messengers that suppress both excitatory and inhibitory transmission are well-established. Yet, despite intensive investigation, many basic aspects of the eCB system are not understood. This brief review highlights recent advances, problems that remain unresolved, and avenues for future exploration. While 2-arachidonoylglycerol (2-AG) is probably the major eCB for intercellular CB1R-dependent signalling, anandamide (AEA) has come to the forefront in several novel contexts, both as a dual endovanilloid/endocannabinoid that regulates synaptic transmission acutely and as the source of a steady eCB tone in hippocampus. Complexities in the cellular processing of 2-AG are receiving renewed attention, as they are increasingly recognized as major determinants of how 2-AG affects cells. Long-standing fundamental issues such as the synthesis pathway for AEA and the molecular mechanism(s) underlying cellular uptake and release of eCBs remain problematical.

Bradley Alger got his Ph.D. in Experimental Psychology from Harvard University in 1977 with Timothy Teyler; his thesis was on synaptic plasticity in the hippocampal slice. He took the slice to the University of California at San Francisco where he became Roger Nicoll's first postdoc, and acquired his career-long interest in the regulation of GABAergic synaptic transmission. In the early 1990s, in parallel with Alain Marty, Alger and colleagues co-discovered ‘DSI’, the first well characterized and widely accepted instance of retrograde signalling in the brain. He is Professor of Physiology and Psychiatry at the University of Maryland School of Medicine.
Introduction
Endocannabinoids, mainly N-arachidonoylethanol-amide (AEA) and 2-arachidonoylglycerol (2-AG), are the principal natural agonists of the most prevalent cannabinoid receptor in the brain, CB1R (Kano et al. 2009). Although they were discovered in the early 1990s (Devane et al. 1992; Mechoulam et al. 1995; Sugiura et al. 1995), their synaptic roles were obscure for years.
Concurrently, studies of inhibitory synaptic transmission had uncovered a phenomenon eventually called depolarization-induced-suppression inhibition (DSI) (Llano et al. 1991; Pitler & Alger, 1992). Calcium (Ca2+) influx induced by action potential firing or simple depolarization of a principal cell in cerebellum or hippocampus led to a transient suppression of incoming GABAergic synaptic inputs. DSI appeared to represent a ‘retrograde’ signalling process, i.e. an unknown ‘messenger’ generated in a postsynaptic cell travelled across the synapse in the direction counter to the normal flow of neurotransmission and transiently reduced the release of GABA from presynaptic nerve terminals. At first, the main issue was whether or not retrograde signalling actually occurred, but by the mid-1990s this was unambiguous (Alger & Pitler, 1995; Morishita & Alger, 1997). Identifying the messenger became a central concern and numerous candidates were ruled out. There were close similarities between the suppression of GABAergic inhibition caused by activation of group I mGluRs and DSI (Glitsch et al. 1996; Morishita et al. 1998; Morishita & Alger, 1999). DSI could be enhanced or occluded by mGluR agonists, increased by blocking glutamate uptake, and reduced by mGluR antagonists. Nevertheless, even high concentrations of mGluR antagonists failed to abolish DSI, and any role for glutamate remained uncertain.
On 29 March 2001, the publication of four papers from four independent groups (in three different journals) provided compelling theoretical and experimental evidence that an eCB was the long-sought retrograde messenger. The papers suddenly and forcibly brought eCBs to the attention of a broad international community of neuroscientists, settled several issues that had been bubbling for over a decade, united two disparate research fields, and established a new agenda for many laboratories. PubMed lists over 4800 references to ‘endocannabinoids’, of which roughly 4300 have appeared since that day. While credit for the explosion of interest in eCBs cannot, of course, be given exclusively to these four papers – pioneering efforts of others had discovered the fundamental neurochemical and structural elements of the eCB system, and developed the essential pharmacological tools beforehand (see reviews by Di Marzo et al. 1998; Hillard, 2000; Howlett, 2002; Piomelli, 2003; Pertwee, 2005) – their immediate and lasting impact justifies regarding March 29, 2001 as a truly ‘wonderful day’ in the history of the field.
This short review focuses narrowly on some issues that were raised in 2001 but are not yet resolved; important topics that have necessarily been omitted can be found in previous comprehensive reviews (Alger, 2002; Freund et al. 2003; Chevaleyre et al. 2006; Heifets & Castillo, 2009; Kano et al. 2009; Alger & Kim, 2011). The principles of eCB signalling have now been extended from rodent brain tissue to surgically removed human neocortical slices studied in vitro (Kovacs et al. 2012), which implies that these results will have broad significance for understanding human neurophysiology.
29 March 2001
Reasoning from prior pharmacological and anatomical studies of the actions of CB1R agonists, and the localization of CB1Rs and the degradative enzyme for AEA, fatty acid amide hydrolase (FAAH), Elphick & Egertova (2001) proposed an explicit model in which eCBs functioned mainly as retrograde messengers (see their Fig. 2). Wilson & Nicoll (2001) and Ohno-Shosaku et al. (2001) showed that DSI could be mimicked and occluded by activating CB1R, and that CB1R antagonists abolished it. Kreitzer & Regehr (2001) reported that a new phenomenon, depolarization-induced suppression of excitation, DSE, occurred at cerebellar excitatory synapses and was also mediated by the retrograde action of eCBs. DSI and DSE were initiated by increases in Ca2+ in the postsynaptic cells and associated with changes in the paired-pulse ratio (PPR) of synaptic transmitter release that were consistent with a presynaptic effect. Kreitzer & Regehr (2001) directly demonstrated a retrograde influence, recording not only the suppression of Ca2+ influx into the presynaptic terminals during DSE, but prevention of this suppression by manoeuvres confined to the postsynaptic cell.
After confirmation of the retrograde role of eCBs, the next critical development was the finding that glutamate acting via group I mGluRs could directly generate eCBs (Maejima et al. 2001; Varma et al. 2001) and enhance DSI (Varma et al. 2001) implying a synergistic effect between G protein coupled receptors (GPCRs) and Ca2+ mechanisms of eCB synthesis, and reconciling the previous mGluR data with the new results on eCBs and DSI. It was then found that stimulation of muscarinic M1/M3 receptors by acetylcholine also liberated eCBs (Kim et al. 2002; Ohno-Shosaku et al. 2003), and it is now known that numerous GPCR-coupled neurotransmitters use eCBs to deliver or fine-tune their messages to their target cells (Kano et al. 2009). The great abundance of CB1Rs in the mammalian brain (Herkenham et al. 1990) now seems less surprising than it once did.
Identity of the signalling eCBs
Which, if either, of the two main candidate eCBs mediated DSI and DSE was unknown in 2001; indirect evidence favoured 2-AG in hippocampus (e.g. Stella et al. 1997), but the model of Elphick & Egertova (2001) was based on the AEA system. Early support for the 2-AG hypothesis came from pharmacological experiments. Interference with AEA metabolism had no effect on DSI, whereas interference with cyclooxygenase-2 (COX-2), an enzyme which uses 2-AG as a substrate (Kozak et al. 2000), enhanced DSI (Kim & Alger, 2004), presumably by reducing 2-AG degradation.
The major enzymes for 2-AG synthesis are phospholipase C (PLCβ) and diacylglycerol lipase (Kano et al. 2009). Knocking out PLCβ abolished GPCR-coupled eCB effects but not DSI (DSI is a consequence of the purely Ca2+-dependent mode of eCB production) (Hashimotodani et al. 2005), indicating that at least two distinguishable biochemical pathways generate signalling-related eCBs. In the bed nucleus of the stria terminals (BNST) (Puente et al. 2011) DSE is reduced by the PLC inhibitor, so there may be regional differences in the involvement of PLC in Ca2+-dependent eCB responses. DGL has two isoforms: DGLα and DGLβ (Bisogno et al. 2003). Molecular genetic deletion studies (Gao et al. 2010; Tanimura et al. 2010; Yoshino et al. 2011) concluded that DGLα is the primary enzyme for eCB-related signalling because DSI and DSE are absent in DGLα−/− tissue, but intact in DGLβ−/− tissue. Loss or deficiency in GPCR-induced forms of eCB signalling is also observed when DGLα−/− is absent (Tanimura et al. 2010). Combined, the data bolster the conclusion that 2-AG predominantly mediates the phasic forms of intercellular eCB signalling that have been investigated to date.
Unexpectedly, AEA levels are also reduced by ∼50% in certain DGLα−/− animals (Gao et al. 2010; Tanimura et al. 2010). This suggests a more direct link between the AEA and 2-AG pathways than had been generally assumed, and renews questions about the mechanisms of AEA synthesis. It also raises the possibility that 2-AG might not be the eCB in every case in which DGLα deletion disrupts signalling. Reduction in AEA occurred in hippocampus, but not prefrontal cortex, of a different DGLα−/− line (Yoshino et al. 2011), suggesting the relationship between AEA and DGLα is region-specific. AEA is not altered in any strain of DGLβ−/− animal, suggesting that its reduction by DGLα−/− is not simply a side-effect of disturbing the general (‘housekeeping’) lipid metabolism-related functions of DGL.
Thus the findings from the DGL−/− mice support the 2-AG hypothesis, but stimulate further questions: (1) does AEA play any role in eCB signalling? (2) how is AEA synthesized? (3) is the 2-AG system a unitary entity? (4) what functions does DGLβ serve? These questions are considered below.
Anandamide
Tanimura et al. (2010) strongly stimulated cerebellar tissue and observed no change in AEA levels in WT or DGLα−/− mice, which indirectly supported their conclusion that 2-AG is the predominant eCB involved in intercellular signalling. However, the spatial resolution of the bulk neurochemical assays would be inadequate to detect localized, input-specific AEA production in a small group of cells. Moreover, AEA levels might be increased in other brain regions (Giuffrida et al. 1999). Indeed, previous data implicated AEA rather than 2-AG in intercellular signalling in certain cases (Giuffrida et al. 1999; Azad et al. 2004; Ade & Lovinger, 2007).
Recent reports (Chavez et al. 2010; Grueter et al. 2010) conclude that AEA modifies synaptic transmission in dentate gyrus and nucleus accumbens, respectively. In both cases, AEA generated in a postsynaptic cell by mGluR5 activation induced an input-specific down-regulation of AMPA-type ionotropic glutamate receptors that was brought about by dynamin-mediated endocytosis. The end result is a form of LTD that is purely postsynaptic in induction and expression. This LTD does not result from activation of CB1R by AEA, but rather from its action as an agonist at the vanilloid receptor, TRPV1 (transient receptor potential receptor, also called VR1) (Zygmunt et al. 1999; Ross, 2003). Chavez et al. (2010) and Grueter et al. (2010) used physiological and genetic tools, including the TRPV1−/− mouse, to argue convincingly that AEA acts via TRPV1 in down-regulating AMPARs.
Importantly, Grueter et al. (2010) showed in addition that stimulus-induced AEA also affected presynaptic CB1Rs, confirming that it is a dual-function, endocannabinoid/endovanilloid agonist (Fig. 1A). It will be interesting to reinvestigate the physiologically-induced AEA actions and the effects of FAAH inhibitors in DGLα−/− animals, as a deficiency in them might mean that DGLα−/− is functionally upstream of AEA. A thorough behavioural analysis of the DGLα−/− mice should also prove rewarding.
Figure 1. New roles for anandamide (AEA).
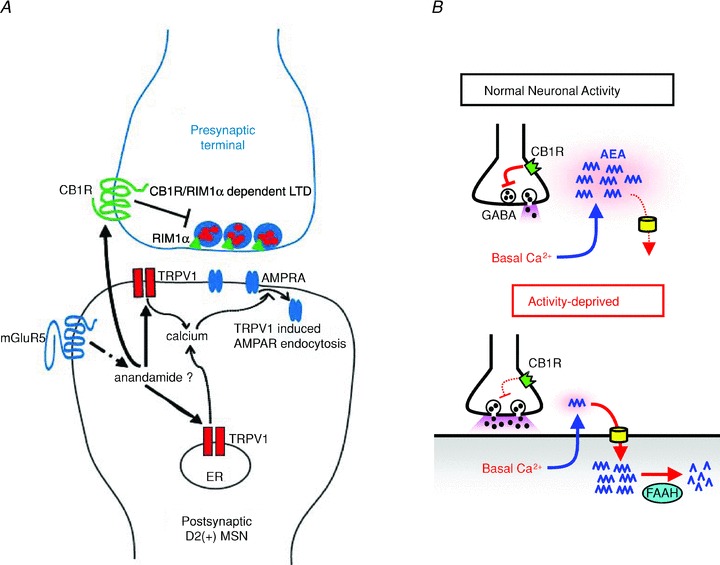
A, model showing postsynaptic generation of AEA by mGluR5 activation in D2 receptor expressing (D2+) medium spiny neurons (MSNs) in the nucleus accumbens. AEA then induces a postsynaptic form of LTD by activating postsynaptic TRPV1 channels and triggering dynamin-dependent endocytosis of AMPARs. AEA is also released as a retrograde messenger and activates CB1Rs on presynaptic glutamatergic terminals onto the MSNs. The CB1R-mediated effect leads to a presynaptic, RIM1α-dependent form of LTD, by persistently suppressing glutamate release. B, tonic suppression of GABA release from CB1R-expressing interneurons in hippocampus represents a homeostatic activity-dependent mechanism. In these cells AEA is normally released in a steady manner that is dependent on the basal, not stimulated, level of [Ca2+]i (top). Chronic inactivity of the system leads to an upregulation in the activity of uptake and degradation mechanisms, thus lowering the amount of AEA available to activate CB1R. A from Grueter et al. (2010) with permission from Nature Publishing Group. B from Kim & Alger (2010) with permission.
Of course a deficiency in AEA signalling in the DGLα−/− knock-out would not imply that DGL activation is directly required for its synthesis. Complexity of lipid metabolic pathways presents many opportunities for cross-talk that would not be directly related to signalling (Di Marzo, 2011). AEA synthesis is quite complex and can proceed via at least four distinct pathways (Di Marzo, 2011); which is the most relevant for eCB signalling is not known, and interactions among them are likely. A candidate enzyme for AEA synthesis, N-acylphosphatidylethanolamine hydrolyzing phospholipase D (NAPE-PLD), is localized to presynaptic excitatory terminals (Egertova et al. 2008; Nyilas et al. 2008), not postsynaptic structures of hippocampus or some other areas. In the BNST, 2-AG and AEA also serve different roles (Puente et al. 2011), and here it is found the different modes of cellular stimulation produce either 2-AG or AEA. Moreover, in the BNST, NAPE-PLD is found in postsynaptic dendrites and dendritic spines apposed to apparently excitatory synaptic terminals. It is expected that the synthetic enzymes for AEA production will be found in the postsynaptic cell, and hence, at least in the BNST, a mechanism for supporting AEA in intercellular signalling is in place. Interference with FAAH activities has numerous physiologically relevant actions on the nervous system and behaviour (Cravatt et al. 2001, 2004; Long et al. 2009b), and thus working out the details of AEA production in individual brain regions remains an important goal.
Tonic eCB release
Wilson & Nicoll (2001) found that an antagonist of eCB uptake suppressed IPSCs by a CB1R-dependent process. The antagonist did not act directly on CB1Rs and it was inferred that eCBs were continually being released, taken up, and recycled. Prevention of uptake evidently led to extracellular eCB accumulation and activation of presynaptic CB1Rs. These observations were taken as support for the concept of retrograde release of eCBs, as it was assumed that continual (‘tonic’) eCB secretion was related to the stimulation of (‘phasic’) eCB release by a rise in [Ca2+]i. Indeed, strongly chelating Ca2+ in the postsynaptic cell abolishes both tonic (Neu et al. 2007; Kim & Alger, 2010) and phasic eCB effects. However, the phenomena are mediated by entirely different processes.
If 2-AG generated by DGLα were released as soon as it is produced, constitutive CB1R-mediated suppression of synaptic transmission should be ubiquitous. Tonic eCB-mediated actions do occur (Losonczy et al. 2004; Hentges et al. 2005; Oliet et al. 2007; Zhu & Lovinger, 2010), but there is little evidence that 2-AG is the mediator. In fact, if 2-AG were synthesized, released, and then quickly degraded, the existence of large basal quantities of 2-AG might be difficult to explain. Instead, releasable and stored 2-AG may represent separate pools. In line with this concept, it is found that potent and selective inhibitors of 2-AG degradation enhance stimulated tissue levels of 2-AG, without affecting basal levels (Long et al. 2009a; Marrs et al. 2010).
In fact, tonic eCB actions in hippocampus appear to be mediated by AEA and not 2-AG (Kim & Alger, 2010). In organotypic hippocampal cultures, chronic inactivity caused by TTX or experimental deafferentation decreased the tonic CB1R-mediated suppression of GABA release. The effect was mediated presynaptically, but there was no change in the sensitivity of presynaptic CB1Rs to a synthetic agonist. Instead, the normal, continuous availability of an eCB was reduced. Neither DSI magnitude nor DSI kinetics were altered, suggesting that 2-AG mobilization was not responsible. In contrast, inhibitors of eCB uptake or FAAH (which does not metabolize 2-AG in intact cells) had much greater effects in the activity-deprived than normal tissue. Evidently, chronic inactivity increased AEA uptake and degradation, thereby decreasing the CB1R-mediated suppression of GABA release (Fig. 1B). While an inactivity-induced decrease in basal [Ca2+] appeared to be responsible for triggering the reduction in AEA mobilization, details have not been worked out. This novel mode of control of AEA-mediated actions also demonstrated the importance of specific microcircuit regulatory mechanisms in understanding neuronal homeostasis.
Is tonic CB1R activation mediated by AEA in other areas of the brain? What is the function of tonic eCB release and how is it regulated? Does tonic eCB release affect glutamatergic synapses as well? These questions remain to be addressed.
The ‘on-demand’ model of eCB production and pools of 2-AG
A fundamental tenet in eCB signalling is that eCBs are produced ‘on-demand’, i.e. synthesized only when needed in response to a stimulus (Marsicano et al. 2003). A simple model therefore predicts that there will be tight coupling between eCB synthesis and release – eCBs will be released for signalling as soon as they are synthesized – since these lipophilic molecules cannot be stored in vesicles. Supporting evidence for the model includes bulk neurochemical measurements of increases in eCBs when tissue is appropriately stimulated (i.e. depolarizing conditions, Ca2+ influx, etc.). On the other hand, high levels of 2-AG are detected in nominally unstimulated cells, and 2-AG has non-signalling-related roles (Piomelli, 2003; Di Marzo, 2011). Hence stimulus-induced increases in eCB levels might not be specifically related to signalling. The observations of tonic eCB release also pose a challenge for the on-demand model, since it is not obvious in most case what ‘demand’ (i.e. what stimulus) is driving the persistent eCB production.
Basal tissue levels of 2-AG were only reduced by 80% in DGLα−/− mice (Gao et al. 2010; Tanimura et al. 2010). Probably DGLβ mainly accounts for the DGLα-independent pool of 2-AG (Gao et al. 2010; Yoshino et al. 2011). In any case, the existence of a basal pool of 2-AG in the DGLα−/− mouse indicates that significant amounts of 2-AG exist in tissue that is not explicitly stimulated. Moreover, the data imply that the remaining 20% of the 2-AG in DGLα−/− mice was not releasable, arguing first that synthesis and release of 2-AG are not coupled processes, and second that retention of 2-AG by some mechanism within cells is possible. The subcellular sites of production of basal and signalling 2-AG may be physically separated.
Nomura et al. (2011) report that the predominant degradative enzyme for 2-AG, monoacylglyceride lipase (MAGL), is the major source of arachidonic acid for the synthesis of inflammatory prostaglandins in the brain, and not, as had been thought, the phospholipase A2 pathway. MAGL acts as a nodal switching point; when it is active, 2-AG is degraded, and the copious amounts of arachidonic acid produced enter the prostaglandin synthesis system. When MAGL is inhibited, 2-AG levels go up, and prostaglandin levels and the associated neuroinflammation are reduced. These fascinating new results underscore the importance of 2-AG in non-eCB related functions. It is not yet clear how MAGL activity is regulated. It will also be important to identify the sources of the 2-AG that contribute to prostaglandin synthesis and unravel the relationship between the prostaglandin and endocannabinoid systems. Again, the DGLα−/− mice should be very useful in this regard.
Separate pools of 2-AG for physiological signalling
Absence of 2-AG-mediated eCB effects in DGLα−/− mice does not establish that there is only one functional signalling pool of 2-AG. Indeed, marked differences exist between Ca2+-dependent and GPCR-dependent, e.g. mGluR-activated, eCB processes: DSI is entirely Ca2+ dependent, whereas eCBmGluR is much less sensitive to Ca2+. Conversely, eCBmGluR is strictly dependent on PLCβ while DSI is (generally) PLC independent. Such data may suggest the existence of multiple signalling-related 2-AG pools, although this issue has received little attention. Assuming that the results from the DGLα−/− mice mean that 2-AG is involved in most Ca2+- and GPCR-dependent eCB-signalling processes, and that signalling-2-AG exists within a cell in an undifferentiated pool, inhibiting DGLα should prevent all 2-AG mediated effects simultaneously. Alternatively, differential sensitivity of the responses to the action of pharmacological inhibitors of DGL would suggest the existence of multiple, operationally definable pools of 2-AG. Recently, it has been found that pharmacological inhibitors of DGL, either internally or externally applied, can abolish DSI while having little or no effect on eCBmGluR (Zhang et al. 2011). In addition, after a DGL inhibitor had abolished DSI, stimulation of eCBmGluR revealed a decline in its magnitude, as expected. Surprisingly, though, the rate with which eCBmGluR declined was not simply a function of time since the DGL inhibitor took effect. Rather, the decrease in eCBmGluR was use dependent – it occurred more rapidly if eCBmGluR responses were repeatedly elicited over the same interval of time. Together, the results are compatible with a model in which 2-AG is not always produced and released on demand from a single source immediately following DGL activation, but can in part go into a preformed pool which can be tapped for release during eCBmGluR.
The relatively large size of the basal DGLα-sensitive 2-AG pool suggests that either DGLα is intrinsically active in quiescent cells, or that spontaneous neuronal activity stimulates it. It should be noted that the low levels of spontaneous neuronal activity normally recorded in vitro could magnify the degree of experimentally stimulated endocannabinoid synthesis. The higher levels of spontaneous activity in vivo might generate higher basal levels, and stimulus-induced elevations of 2-AG might then be less apparent in vivo than in vitro.
Regulation of eCB mobilization
There is a 15-fold increase in basal AEA levels in FAAH−/− mice compared to WT mice (Cravatt et al. 2001). Azad et al. (2004) reported that iLTD is enhanced in the FAAH−/− mice. If AEA were simply released as soon as it is produced, as required by the on-demand model, then it should cause a tonic suppression of CB1R+ synapses, rather than an increase in iLTD. To account for their results, Azad et al. (2004) propose that release of AEA might be a dynamically regulated step. If eCBs are not necessarily released as soon as they are synthesized, then what mechanisms could govern their release? It is not yet clear how the lipophilic eCBs cross the plasma membranes of the cells where they are produced to get to their target receptors on presynaptic terminals. There is some evidence that the eCB transporter participates in certain forms of eCB-dependent LTD (Bender et al. 2006; Adermark et al. 2009) and iLTD (Ronesi et al. 2004; Edwards et al. 2008), because intracellular application of a transporter blocker prevents the induction of eCB-LTD or eCB-iLTD. Perhaps the transporter, normally thought to govern eCB uptake, can operate in the ‘reverse’ mode and help promote the sustained release of eCBs from the cell that is required for eCB-LTD (Chevaleyre & Castillo, 2003; Ronesi et al. 2004). DSI and short-term eCB effects are not altered by intracellular transporter inhibitors, however (Edwards et al. 2008), so transporter activity is not obligatory for eCB release.
Fu et al. (2011) have discovered a FAAH-1 splice variant that drives AEA transport in neurons (FAAH-like AEA transporter, FLAT). Isolated as a cDNA product of the Faah gene, FLAT can bind AEA, and is partly cytosolic, but lacks catalytic activity. When expressed in HEK293 cells, FLAT facilitates [3H]AEA accumulation that is inhibited by conventional agents that block the AEA transport function. Of potential therapeutic importance was the isolation of a small molecule inhibitor of FLAT (ARN272) that was apparently specific and had analgesic effects in behavioural pain models. Interestingly, FLAT also facilitates the release of endogenous AEA from cells, which could be compatible with the suggestion that in certain circumstances eCBs may be released by reversed transport. It will be intriguing to learn if ARN272 influences intercellular eCB-mediated signalling. Nevertheless, FLAT does not affect 2-AG movements, and the mystery of 2-AG translocation remains.
It is worth noting that, although induction of long-term forms of eCB-dependent synaptic plasticity requires activation of CB1R lasting for several minutes, this factor alone is insufficient for the long-term effects. Neither application of the CB1R agonist WIN55212-2 (Ronesi et al. 2004) nor evoking DSI repeatedly for a 10 min period, which causes a sustained synaptic suppression brought about by the natural eCB (Edwards et al. 2006), induces LTD. Rather, other factors, e.g. presynaptic stimulation, co-activation of neurotransmitter receptors, etc., appear to be essential for the transition from short-term to long-term forms of eCB-dependent plasticity. There is no consensus as to what these factors are and they undoubtedly vary across brain regions (see Kano et al. 2009, for a detailed review).
Spread of eCB effect
How far away from their site of mobilization can eCBs activate CB1Rs?Wilson & Nicoll (2001) reported recording from two neighbouring pyramidal cells and, upon inducing DSI of sIPSCs in one cell, observed DSI in both cells (although less in the passive cell) provided that the cells were sufficiently close together – the data show essentially no spread of the eCB effect if the separation was >15 μm. However, the individual cells were not labelled and their proximity was judged by the distance between the tips of the two recording electrodes. A typical CA1 pyramidal cell is 15–20 μm in diameter; therefore if the pipettes were positioned anywhere except the very edges of the cells closest to each other, the spread of effect would be less than 15 μm. If each pipette tip were in the centre of each cell, eCBs might have to travel no more than the width of the extracellular space, ≤0.15 μm, or perhaps less since the CB1R-expressing axons may be ∼0.05 μm in diameter and are located in the gaps between pyramidal cell somata. It is estimated that eCBs spread ∼10 μm along a dendrite (Chevaleyre & Castillo, 2004). Moreover the degree of heterosynaptic spread of eCBs from synapses where they are produced to other neighbouring synapses on the same cell is dependent on stimulus strength (Maejima et al. 2001; Brown et al. 2003; Brenowitz & Regehr, 2005). However, eCBs are lipophilic molecules that can diffuse laterally within the lipid bilayer, so this value does not reflect the ability of eCBs to diffuse across extracellular space. Gerdemann et al. (2002) and Kreitzer & Regehr (2002) found that when uptake of eCBs was retarded, either by AM404 or low temperatures, respectively, then eCB effects could reach from a stimulated cell to a nearby unstimulated cell. In the same vein, Galante & Marty (2004) demonstrated that generation of eCBs by loading one cell with GTPγS (which stimulates vigorous and continual eCB production), caused CB1R-dependent suppression of IPSCs recorded in a nearby unstimulated cell. Thus, if eCB uptake is reduced or mobilization is markedly enhanced, spread of eCBs to neighbouring cells can occur; the extracellular distance travelled by eCBs under more normal conditions remains unresolved. Rather than a small cloud of liberated eCBs enveloping a cell soma, it may be more accurate to imagine a thin ‘skin’ of eCBs covering the cell near sites of eCB mobilization.
Yet if eCBs can move even small distances extracellularly under normal conditions, another puzzle arises: filling a given CA1 pyramidal cell with BAPTA prevents tonic eCB-mediated suppression of the GABA inputs to that cell (Neu et al. 2007; Kim & Alger, 2010). If released eCBs can move freely about and contact CB1Rs on other cells, this result is not predicted; preventing tonic eCB release from any given cell should not prevent the release of eCBs from surrounding cells. It appears that the eCBs responsible for the tonic effects recorded in a cell originate entirely within that cell. On the other hand, perhaps the 2-AG responsible for phasic eCB effects can reach more distant targets than can AEA (which may often be tonically released). The problem of the extracellular movements of eCBs deserves further attention.
DGLβ and neurogenesis
Depolarizing stimulation did not increase basal 2-AG levels in cerebellum, hippocampus, or striatum of two strains of DGLβ−/− mice (Tanimura et al. 2010; Yoshino et al. 2011). In contrast, basal levels of 2-AG were decreased in a whole-brain preparation from a different DGLβ−/− strain (Gao et al. 2010). It could be that DGLβ only increases 2-AG in regions other than those tested by Tanimura et al. (2010) and Yoshino et al. (2011), although it may be of interest that the double KO – DGLαβ−/−– mice brain regions had consistently though not significantly lower levels of 2-AG than did those of the DGLα−/− animals (Yoshino et al. 2011).
In any event, the possibility that DGLβ−/− also contributes to the pool of 2-AG in brain (it is the main source of 2-AG in peripheral tissues, Gao et al. 2010) raises the question of whether DGLβ has any role in the CNS. Neurogenesis in the adult hippocampus is important for several behavioural functions (Deng et al. 2010), and DGL activation is implicated in adult neurogenesis in the SVZ and dentate gyrus (Goncalves et al. 2008). eCBs acting via CB2Rs are important for adult neurogenesis in the SVZ and dentate gyrus Proliferation in the SVZ is inhibited by blocking CB2R, rather than CB1R. Deletion of DGLα markedly reduces the number of proliferating cells in the SVZ and in dentate gyrus (Gao et al. 2010), implicating 2-AG in the process. In dentate gyrus, however, DGLβ as well as DGLα contributes to neurogenesis, since reduced proliferation is seen in both KO lines. If 2-AG produced by DGLβ participates in neurogenesis, why does it not also contribute to intercellular signalling? It seems that both isoforms contribute to the same pool of 2-AG for neurogenesis, but not for signalling. If both DGLα and DGLβ contribute to the same non-signalling-related 2-AG pool, it would suggest that DGLα produces 2-AG for three different functions associated with three different pools. How such hypothetical separate pools could remain separate is unknown, however.
Conclusions
Good progress continues to be made in understanding the eCB systems. 2-AG seems firmly established as ‘the’ signalling eCB for most stimulated (‘phasic’) intercellular signalling. AEA is not out of the picture, though, and its distinct roles are becoming clearer. New molecular-genetic and pharmacological tools are helping to elucidate non-signalling functions of eCBs.
Success in answering a number of basic questions about the eCB systems remains elusive. A list of refractory problems must include the issues related to transmembrane movements of eCBs. What are the molecular mechanisms of uptake and release? Is eCB ‘release’ a regulated, active process? Do AEA and 2-AG share a common transmembrane translocation mechanism? Other major questions that were not anticipated by the publications on 29 March, 2001, and are still largely open include: What are the effects of DGLα knock-out on areas of the brain, or processes, in which AEA is thought to play a prominent role? How can the high sensitivity of the inhibitory synapses to CB1R-dependent suppression be reconciled with the general sparseness of the 2-AG metabolic machinery in their neighbourhood? What processes convert the short-term eCB actions (mainly DSI, DSE) to the long-term forms of synaptic depression (LTDs)? What does DGLβ do? Such questions will continue to stimulate research into the rich field of the endocannabinoid systems and will no doubt make for other remarkable days in the future.
Acknowledgments
Work in the author's laboratory has been supported by NIH RO1 MH077277 and RO1 DA014625.
References
- Ade KK, Lovinger DM. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. J Neurosci. 2007;27:2403–2409. doi: 10.1523/JNEUROSCI.2916-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci. 2009;29:32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304–315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger BE, Pitler TA. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends Neurosci. 1995;18:333–340. doi: 10.1016/0166-2236(95)93923-l. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams E-J, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–431. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses. A novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH, Lichtman AH. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci U S A. 2004;101:10821–10826. doi: 10.1073/pnas.0401292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci. 2011;14:9–15. doi: 10.1038/nn.2720. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Kim J, Alger BE. Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol. 2006;95:67–75. doi: 10.1152/jn.00813.2005. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Zhang L, Alger BE. Metaplastic control of the endocannabinoid system at inhibitory synapses in hippocampus. Proc Natl Acad Sci U S A. 2008;105:8142–8147. doi: 10.1073/pnas.0803558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Simon GM, Cravatt BF, Elphick MR. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: A new perspective on N-acylethanolamines as neural signaling molecules. J Comp Neurol. 2008;506:604–615. doi: 10.1002/cne.21568. [DOI] [PubMed] [Google Scholar]
- Elphick MR, Egertova M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2001;356:381–408. doi: 10.1098/rstb.2000.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, Bandiera T, Reggiani A, Mor M, Cavalli A, Piomelli D. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci. 2011;15:64–69. doi: 10.1038/nn.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Galante M, Diana MA. Group I metabotropic glutamate receptors inhibit GABA release at interneuron-Purkinje cell synapses through endocannabinoid production. The Journal of Neuroscience. 2004;24:4865–4874. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang M-Y, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA, Doherty P. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nature Neuroscience. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Glitsch M, Llano I, Marty A. Glutamate as a candidate retrograde messenger at interneurone–Purkinje cell synapses of rat cerebellum. J Physiol. 1996;497:531–537. doi: 10.1113/jphysiol.1996.sp021786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves MB, Suetterlin P, Yip P, Molina-Holgado F, Walker DJ, Oudin MJ, Zentar MP, Pollard S, Yanez-Munoz RJ, Williams G, Walsh FS, Pangalos MN, Doherty P. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci. 2008;38:526–536. doi: 10.1016/j.mcn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin H-S, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000;61:3–18. doi: 10.1016/s0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses. Nat Neurosci. 2010;13:592–600. doi: 10.1038/nn.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs FE, Knop T, Urbanski MJ, Freiman I, Freiman TM, Feuerstein TJ, Zentner J, Szabo B. Exogenous and endogenous cannabinoids suppress inhibitory neurotransmission in the human neocortex. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2011.262. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism 8. Chem Biol. 2009a;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009b;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Biro AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci U S A. 2004;101:1362–1367. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hasimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS, Woodruff G, Fung S, Lafourcade M, Alexander JP, Long JZ, Li W, Xu C, Moller T, Mackie K, Manzoni OJ, Cravatt BF, Stella N. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Morishita W, Alger BE. Sr2+ supports depolarization-induced suppression of inhibition and provides new evidence for a presynaptic expression mechanism in rat hippocampal slices. J Physiol. 1997;505:307–317. doi: 10.1111/j.1469-7793.1997.307bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita W, Alger BE. Evidence for endogenous excitatory amino acids as mediators in DSI of GABAAergic transmission in hippocampal CA1. J Neurophysiol. 1999;82:2556–2564. doi: 10.1152/jn.1999.82.5.2556. [DOI] [PubMed] [Google Scholar]
- Morishita W, Kirov SA, Alger BE. Evidence for metabotropic glutamate receptor activation in the induction of depolarization-induced suppression of inhibition in hippocampal CA1. J Neurosci. 1998;18:4870–4882. doi: 10.1523/JNEUROSCI.18-13-04870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at CCK-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyilas R, Dudok B, Urban GM, Mackie K, Watanabe M, Cravatt BF, Freund TF, Katona I. Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J Neurosci. 2008;28:1058–1063. doi: 10.1523/JNEUROSCI.5102-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Oliet SHR, Baimoukhametova DV, Piet R, Bains JS. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci. 2007;27:1325–1333. doi: 10.1523/JNEUROSCI.2676-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L, Grandes P, Manzoni OJ. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci. 2011;14:1542–1547. doi: 10.1038/nn.2974. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylgylcerol – a possible endogenous cannabinoid receptor-ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase α mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yoshino H, Miyamae T, Hansen G, Zambrowicz B, Flynn M, Pedicord D, Blat Y, Westphal RS, Zaczek R, Lewis DA, Gonzalez-Burgos G. Postsynaptic diacylglycerol lipase mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J Physiol. 2011;589:4857–4884. doi: 10.1113/jphysiol.2011.212225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang M, Bisogno T, Di Marzo V, Alger BE. Endocannabinoids generated by Ca or by metabotropic glutamate receptors appear to arise from different pools of diacylglycerol lipase. PLoS ONE. 2011;6:e16305. doi: 10.1371/journal.pone.0016305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Developmental alteration of endocannabinoid retrograde signaling in the hippocampus. J Neurophysiol. 2010;103:1123–1129. doi: 10.1152/jn.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


