Abstract
Locus coeruleus (LC) neurones extend noradrenergic projections throughout the neuroaxis and are involved in homeostatic functions such as pain modulation, arousal and cardio-respiratory control. To address the cellular mechanisms underlying pain modulation we have developed a patch-clamp recording technique from LC neurones in anaesthetized rats. These recordings showed LC discharge in vivo to be driven by both spontaneous membrane potential oscillations and CNQX-sensitive EPSCs opposed by bicuculine-sensitive IPSCs. Hindlimb pinch evoked a biphasic action potential response underpinned by a slow monophasic excitatory current. This approach allows detailed characterisation of the synaptic and integrative mechanisms of LC responses to naturalistic stimulation.
Introduction
The whole-cell patch-clamp recording technique (Neher & Sakmann, 1976) has become a ubiquitous method for studying the electrophysiological properties of cellular membranes and synaptic inputs. This technique has been mainly applied to in vitro preparations such as cultured cells and brain slices, contributing greatly to our understanding of the ionic mechanisms of neurone excitability, and synaptic transmission in neuronal circuits. However, the physiological significance of many neuronal and synaptic activities observed in such in vitro preparations remains to be determined, since the entire neuronal circuits are seldom preserved and it is typically not possible to apply natural or physiological stimulation.
To overcome these problems the whole-cell patch-clamp recording methodology has been extended to allow in vivo recordings from regions of the CNS in the intact animal, including spinal cord, thalamus, cerebellum, cortices and olfactory bulb. Such in vivo recordings these have provided compelling evidence of physiological circuit mechanisms (Sakmann, 2006; Furue et al. 2007). However, to date there are no reports of patch-clamp recordings from brainstem neurones in vivo despite the crucial roles of brainstem circuits in vital physiology. This is likely to be due to technical difficulties in obtaining stable recordings, since the brainstem is rather inaccessible, is heavily myelinated making imaging challenging, and is relatively mobile showing pronounced respiratory and cardiac related pulsations.
The locus coeruleus (LC) is a dense cluster of noradrenergic neurones in the dorsal pons located either side of the floor of the fourth ventricle. These noradrenergic neurones send extensive projections to most regions of the CNS. The LC is implicated in the control of many homeostatic functions including pain control (Pertovaara, 2006; Howorth et al. 2009) and circadian regulation of arousal and performance. The activity of LC neurones is thought to be driven by the integration of fast excitatory and inhibitory synaptic inputs, and modulated by a range of peptidergic inputs to the LC and peri-LC area (Aston-Jones, 2004). Subthreshold synaptic and peptidergic mechanisms in LC neurones have been studied in detail using brainstem slices and dissociated preparations (Williams et al. 1984; Ishibashi et al. 2009) but these types of study offer a limited insight into the integrated function of the LC. In this study, we developed a novel method to obtain recordings from the brainstem using the in vivo‘blind’ patch-clamp technique (Furue et al. 1999) to evaluate the physiological importance of those subthreshold responses.
Methods
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the National Institutes of Natural Sciences, were performed in accordance with the institutional guidelines for animal experiments and were consistent with the ethical guidelines of the International Association for the Study of Pain. Every effort was made to minimize the number of animals used.
Animal preparation
Sprague–Dawley rats (62 males, aged 4–8 weeks) were anaesthetized with urethane (i.p., 1.2–1.5 g kg−1). After tracheostomy, each animal was mechanically ventilated (to an end-tidal CO2 of 3–4%) to reduce active respiratory movement and positioned in a stereotaxic frame (SG-3N, Narishige, Tokyo, Japan) (Fig. 1A). Body temperature was maintained at 37°C by an infrared heating lamp and heating pad. The preparations remained in good condition for recording for more than 6 h. At the end of the experiment, the animals were killed with supplemental administration of urethane (i.p., 2.0–4.0 g kg−1).
Figure 1. In vivo patch-clamp recording from LC neurones in the brainstem.
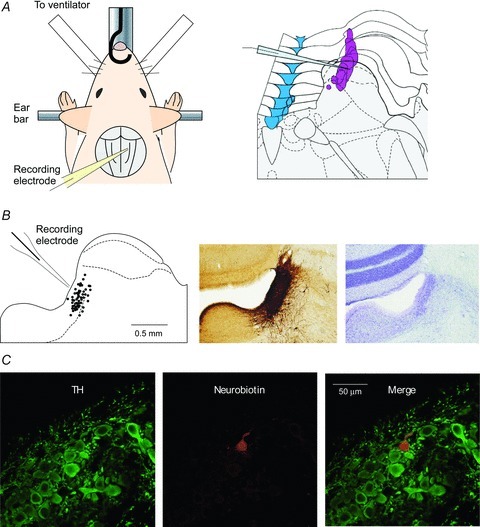
A, schematic diagrams of in vivo preparation and patch pipette insertion into the LC. The urethane-anaesthetised animal was fixed in ear bars and patch pipettes were inserted into the LC from the dorsal surface of the pons after cerebellectomy (left). The topography of the LC (mauve) was mapped from Paxinos and Watson's The Rat Brain (right). B, location of cells recorded with in vivo whole-cell patch-clamp method (left). This recording area was identified as including the LC whose location was verified by TH-immunohistochemistry and Nissl staining of coronal sections in age matched rats. C, an example of a recording from a TH-positive LC neurone (double-stained for anti-TH (green) and neurobiotin (red)).
Extracellular recordings from LC neurone
Extracellular single-unit recordings of LC neurones were performed as described previously (Pineda et al. 1993). A tungsten microelectrode (impedance, 10–12 MΩ) was stereotaxically placed into the LC through a 3 mm occipital burr hole and neurones were identified by established criteria (Pineda et al. 1993).
In vivo patch-clamp recordings from cells in the LC
To target patch electrodes to the dorsal pons we exposed the floor of the fourth ventricle through a posterior occipital craniotomy using gentle suction aspiration to remove the central portion of the cerebellum. The surface of the exposed brainstem was irrigated with Krebs solution (mm: NaCl 117, KCl 3.6, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, glucose 11, NaHCO3 25) equilibrated with 95% O2–5% CO2 at 38 ± 0.5°C at a flow rate of 5–10 ml min−1. Using this approach we were able to insert patch pipettes into the LC from the dorsal surface of the pons (Fig. 1A). The patch pipettes had a long shank with a resistance of 5–10 MΩ when filled with either of recording solutions of the following composition (mm): potassium gluconate 135, KCl 5, CaCl2 0.5, MgCl2 2, EGTA 5, ATP-Mg 5, Hepes-KOH 5, pH 7.2; or Cs2SO4 110, TEA-Cl 5, CaCl2 0.5, MgCl2 2, EGTA 5, ATP-Mg 5, Hepes-CsOH 5, pH 7.2.
The electrodes were advanced into the vicinity of the LC (3.5 mm caudal to lambda, 0.85–1.0 mm lateral to the midline) at an angle of 45 deg from the horizontal using a micromanipulator according to coordinates derived from the atlas of Paxinos and Watson (Fig. 1A and B). Cells in this region were shown to be within the LC using coronal brainstem slices of same age rats with immuno-staining for tyrosine hydroxylase (TH, Fig. 1B). Gigaohm seals (4.2 ± 2.1 GΩ, n = 37) were successfully formed using the ‘blind’ patch technique with cells at a depth of 10–250 μm from the dorsal surface of the pons. In some cases cell-attached recordings were made from the LC neurones that were identifiable by their spontaneous firing pattern and their responses to sensory stimuli as per the extracellular recordings. The whole-cell recording configuration was then obtained by applying further suction. The success rate for achieving whole-cell recording from neurones was ∼20%, presumably because a gigaohm seal could be made on either an unfavourable location on a neurone or upon a glial cell. In some instances, the recorded neurone was filled with neurobiotin (0.4%) for post hoc identification (as shown in Fig. 1C). For immunocytochemistry, the brainstem was perfusion fixed in 4% paraformaldehyde, sliced into 40 μm-thick sections and incubated overnight with rabbit anti-tyrosine hydroxylase (TH) antibody (Biomol International L.P., Exeter, UK), followed by AlexaFluor488-labelled donkey anti-rabbit secondary antibody (1:500, 2 h) and AlexaFluor555-labelled streptavidin (1:100) to identify the biocytin-filled neurone.
Cutaneous sensory stimulation
Mechanical nociceptive stimulation was applied to the ipsilateral or contralateral hindlimb by pinching the skin with toothed forceps by hand (using a force that was determined as noxious when applied to the experimenters own fingertip) as we have reported previously (Furue et al. 2007). Innocuous touch stimulation was applied to the contralateral hind paw by brushing the skin.
Drug application
Drugs were dissolved in Krebs solution and applied to the dorsal pontine surface via the perfusate. The drugs used were 6-cyano-7-nitroquinoxalone-2, 3-dione (CNQX) – a selective AMPA receptor antagonist (Sigma, St. Louis, MO, USA) – and bicuculline – a selective GABAA receptor antagonist (Sigma).
Statistical analysis
All data are expressed as means ± SD. Statistical significance was determined as P < 0.05 using one way analysis of variance or Student's two-tailed t test. In all cases, n refers to the number of neurones studied.
Results
Responses of the LC neurones to sensory stimuli recorded with extracellular and cell-attached recordings
We first used the conventional extracellular technique to record spontaneous firing and stimulus evoked responses of the LC. These neurones showed a stable spontaneous discharge with an average frequency of 7.4 ± 3.3 Hz (n = 10). When noxious pinch stimulation was applied to the contralateral hindlimb, the firing frequency characteristically transiently increased and then decreased (Fig. 2A). Patch-clamp cell-attached recordings (at a holding potential of −70 mV) showed spontaneous action currents (Fig. 2B). The frequency of spontaneous firing was 6.6 ± 4.0 Hz (n = 16) and the neurones showed a biphasic excitation–inhibition response to pinch stimulation applied to the contralateral (but not ipsilateral) hindpaw (Fig. 2B, n = 12). The response profile and time course were similar to those recorded with extracellular recordings (Fig. 2C). Innocuous touch stimulation applied to the contralateral hindlimb also showed a similar biphasic response (the lowest trace in Fig. 2B). In seven cells tested, the amplitude of maximum frequency for 1–2 s during pinch stimulation was higher than that during touch stimulation (pinch, 172 ± 48% of control; touch, 131 ± 11% of control; P < 0.05) in the same neurones.
Figure 2. In vivo recordings of AP discharge in LC neurones.
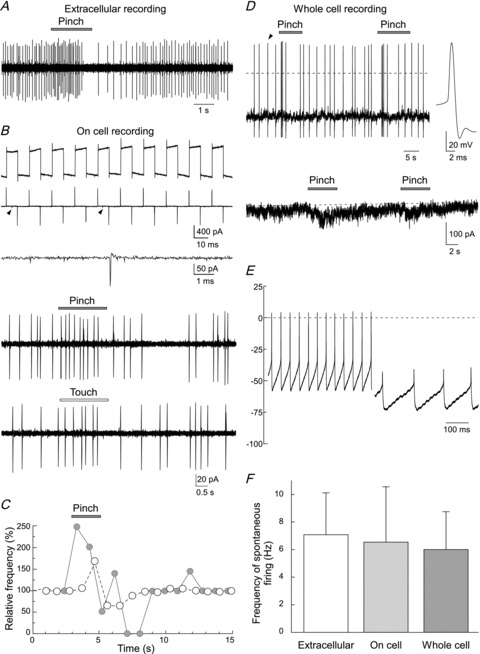
A, extracellular recording of AP discharge in response to cutaneous pinch stimulation applied to the contralateral hindlimb showing a characteristic biphasic response with excitation followed by inhibition. B, cell-attached patch recording – currents in response to voltage-step pulses (5 mV) were negligible after a giga-ohm seal formation (top trace before and middle trace after seal formation). Spontaneous AP discharge was typically observed (indicated by arrowheads, shown on trace expanded below). These cell-attached LC recordings also showed a biphasic response to contralateral pinch and touch stimulation. These two sensory responses were obtained from the same neurone. C, comparison of the time courses of AP discharge in response to pinch stimulation for the neurones in A (open circles) and B (filled circles). D, an example of in vivo whole-cell current clamp recording. Pinch stimulation elicited APs followed by an inhibition. Note hyperpolarization was not detected during the inhibitory phase. An AP is shown on an expanded time base (right trace). In voltage clamp the pinch was seen to elicit a monophasic slow inward current (Vhold−90 mV). Noise in this trace was relatively higher than those in traces in Fig. 3. This is due to the presence of our hand in the Faraday cage for sensory stimulation. E, tonic activity in an LC neurone at rest was driven by underlying intrinsic membrane potential oscillations (right trace, revealed by the injection of hyperpolarising current) whose frequency was slowed by increasing hyperpolarisation. F, summary graph showing the frequency of spontaneous APs in LC neurones recorded with in vivo extracellular, cell-attached and whole cell recording techniques. There were no significant differences in AP discharge between the three recording methods (P > 0.05, one way ANOVA).
Whole-cell voltage and current recordings of LC activity and responses to sensory stimulation
Stable whole-cell patch-clamp recordings were made from 63 LC neurones in vivo. All of these neurones exhibited spontaneous action potential (AP) discharge with an average frequency of 6.0 ± 2.7 Hz (n = 23). There was no significant difference in the spontaneous frequency of AP obtained from extracellular, cell-attached and whole-cell recordings (Fig. 2F), suggesting that tight seal formation and subsequent dialysis with pipette solution does not fundamentally alter their excitability. The resting membrane potential of the LC neurones was −55.4 ± 8.3 mV and the input resistance was 215.9 ± 56.4 MΩ. The injection of hyperpolarising current showed that some of the LC neurones exhibited large spontaneous oscillations in membrane potential whose frequency was altered by the degree of hyperpolarization (Fig. 2E). Pinch stimulation elicited a biphasic AP response without obvious prolonged depolarization or hyperpolarization (upper trace in Fig. 2D). We could also record the membrane currents under voltage-clamp conditions during pinch stimulation. Interestingly, the voltage clamp recordings of the response to pinch at a holding potential of −70 mV did not demonstrate the presence of fast EPSCs, but rather a slow, monophasic, inward current was observed. Outward currents were not detected (lower trace in Fig. 2D).
Recordings of excitatory and inhibitory synaptic currents
When the caesium-containing pipette solution was used, EPSCs and IPSCs could be isolated under voltage-clamp conditions at holding potentials of −70 and 0 mV, respectively. Although recordings with potassium gluconate solution also showed EPSCs, the recordings with the caesium solution had a higher signal to noise ratio; this is due to the blockade of potassium channels. As shown in Fig. 3A, LC neurones exhibited spontaneous EPSCs with an amplitude of 14.7 ± 5.7 pA and a frequency of 3.7 ± 2.8 Hz (n = 21). Analysis of the EPSC time course (Fig. 3A) showed the presence of populations with fast (half-width: 0–4 ms) and slow (half-width: >4 ms) kinetics, perhaps consistent with a distinct cellular locations or differential receptor subunit compositions. Local application of CNQX (20 μm) to the surface of the pons completely inhibited EPSCs within 1 min, followed by recovery on washout within 5 min (left traces in Fig. 3C and D). The amplitude and frequency of spontaneous IPSCs were 13.6 ± 2.0 pA and 9.0 ± 3.8 Hz (n = 7) (Fig. 3B). Bicuculline (20 μm) applied to the surface of the brainstem also completely and reversibly suppressed IPSCs (right traces in Fig. 3C and D). Even when caesium solution was used, pinch stimulation applied to the contralateral but not ipsilateral hindlimb elicited a similar slow current (23.6 ± 4.7 pA) to that recorded with potassium gluconate solution without eliciting any fast EPSCs in most (5 out of 7) cells examined.
Figure 3. In vivo excitatory and inhibitory synaptic currents in LC neurones.
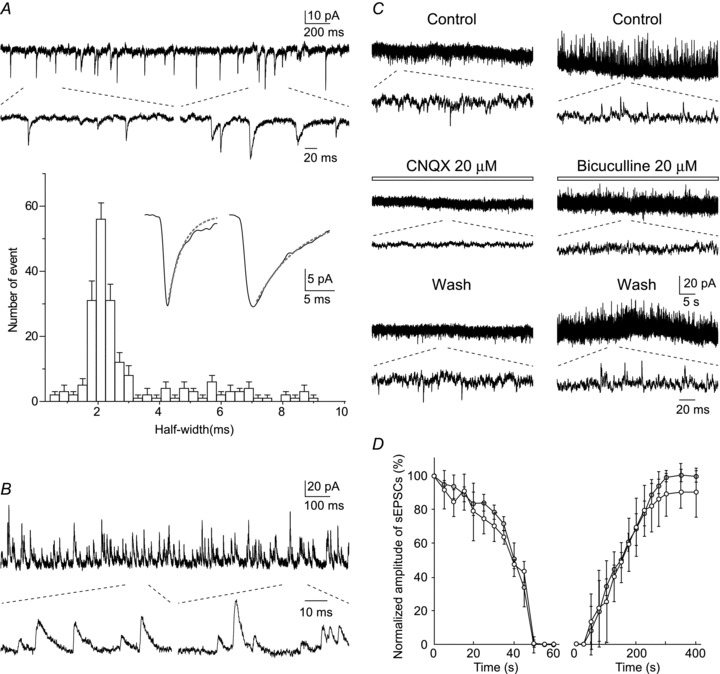
A, spontaneous EPSCs were clearly resolved (Vhold−70 mV) and showed different kinetics manifesting as a wide range of half-widths. The histogram shows one dominant population and a long tail of longer duration events. Insets in the histogram show the averaged traces of 10 spontaneous EPSCs having fast (half-width <2.5 ms) and slow (half-width >4 ms) kinetics. Dotted lines show the decay phase of the fast (τ, 2.4 ms) and slow (τ, 7.4 ms) EPSCs fitted with a single exponential. B, spontaneous IPSCs (Vhold 0 mV) resolved as outward currents (shown on an expanded time base below). C, effect of drugs applied to the dorsal surface of the pons. CNQX (20 μm) and bicuculline (20 μm) reversibly suppressed spontaneous EPSCs and IPSCs, respectively. D, time courses of the averaged amplitude of spontaneous EPSCs (open circle) and IPSCs (filled circle) following application of and recovery from CNQX and bicuculline, respectively.
Discussion
In the present study, we report an in vivo whole-cell patch-clamp recording technique from rat LC neurones which enables us to record at high fidelity the membrane potentials (including sub-threshold mechanisms), underlying synaptic currents (EPSCs and IPSCs) and gives good access for pharmacological investigation. This method is therefore useful to quantitatively elucidate which neurotransmitters are involved in the physiological responses of LC neurones, which are known to receive and respond to a variety of fast and slow transmitters (Aston-Jones, 2004). We have been able to show for the first time that adult LC neurones in vivo show spontaneous membrane potential oscillations like those attributed to gap junctional coupling from in vitro studies (Ishimatsu & Williams, 1996). We also show that LC neurones receive spontaneous small EPSCs (at a frequency lower than their spontaneous AP discharge rate) which are opposed by a higher frequency of incoming IPSCs.
The LC is believed to play an important role for pain modulation. The LC receives projections from periaqueductal grey in the midbrain which integrate inputs from limbic forebrain and diencephalon including the anterior cingulate, insular cortex and amygdala with ascending nociceptive spinal inputs. Direct spinal projections are also found within the LC (Basbaum & Fields, 1978). An illustration of the potential advantages inherent in our approach is the observation that the cutaneous pinch-evoked, biphasic firing of LC neurones was not accompanied by large depolarization or hyperpolarization. Voltage-clamp recordings from the same neurones demonstrated that a transient slow inward current was evoked by pinch and fast EPSCs were not evoked, suggesting that G-protein-coupled pathway may be involved in the excitatory phase of the response. It also suggests that the inhibitory phase is a consequence of the intrinsic properties of the LC neurones (which are known to become relatively refractory following a period of high frequency activation mediated both by an increased calcium activated potassium conductance and also feedback autoinhibition mediated by α2 receptors) rather than a fast inhibitory synaptic input. However, given that LC neurones are known from in vitro studies to be gap junction coupled into clusters (Ishimatsu & Williams, 1996), it is conceivable that the LC neurons exhibiting the slow inward current response to pinch are coupled to neighbouring LC neurones that receive noxious afferent fast synaptic inputs. Further experiments are now possible using perfusion of glutamatergic antagonists/gap junction blockers or intracellular application of G-protein signalling uncouplers to distinguish between these possibilities.
We anticipate that our recording approach to the brainstem will allow the function of the LC and other ponto-medullary structures to be probed in unprecedented detail and will facilitate mechanistic investigation of the key circuits involved in the regulation of sensory, autonomic and motor control.
Acknowledgments
We would like to thank Dr Shin'Ichiro Satake and Daisuke Uta for technical advice on electrophysiological recordings to D.S. and Ms Hiromi Ishihara for histological support. This work was supported by grants from the programs Grants-in-Aid for Scientific Research (H.F. and K.I.) of the Ministry of Education, Science, Sports and Culture of Japan, and by the National Research Foundation of Korea grant funded by the Korea government (2011-0030737). A.E.P. is a Wellcome Trust Senior Clinical fellow.
Glossary
Abbreviations
- AP
action potential
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- LC
locus coeruleus
- TH
tyrosine hydroxylase
Author contributions
This work was conducted in the laboratory of K.I. at the National Institute for Physiological Sciences, Japan. All authors contributed to the conception and design of the experiments as well as to the collection, analysis and interpretation of data. H.F., D.S. and A.E.P. developed the in vivo patch-recording technique from LC neurones. D.K. provided the histology. D.S., H.F and A.E.P. drafted the manuscript and all authors approve the final version of the manuscript.
References
- Aston-Jones G. Locus Ceruleus, A5 and A7 Noradrenergic Cell Groups. In: Paxinos G, editor. The Rat Nervous System. 3rd edn. San Diego, London: Elsevier Academic Press; 2004. pp. 259–294. [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Furue H, Katafuchi T, Yoshimura M. In vivo patch-clamp technique. In: Walz W, editor. Patch-Clamp Analysis Advanced Techniques. 2nd edn. Totowa: Humana Press; 2007. pp. 229–251. [Google Scholar]
- Furue H, Narikawa K, Kumamoto E, Yoshimura M. Responsiveness of rat substantia gelatinosa neurones to mechanical but not thermal stimuli revealed by in vivo patch-clamp recording. J Physiol. 1999;521:529–535. doi: 10.1111/j.1469-7793.1999.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howorth PW, Thornton SR, O’Brien V, Smith WD, Nikiforova N, Teschemacher AG, Pickering AE. Retrograde viral vector-mediated inhibition of pontospinal noradrenergic neurons causes hyperalgesia in rats. J Neurosci. 2009;29:12855–12864. doi: 10.1523/JNEUROSCI.1699-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi H, Nakahata Y, Eto K, Nabekura J. Excitation of locus coeruleus noradrenergic neurons by thyrotropin-releasing hormone. J Physiol. 2009;587:5709–5722. doi: 10.1113/jphysiol.2009.181420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimatsu M, Williams JT. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. J Neurosci. 1996;16:5196–5204. doi: 10.1523/JNEUROSCI.16-16-05196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976;260:799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Pineda J, Ugedo L, Garcia-Sevilla JA. Stimulatory effects of clonidine, cirazoline and rilmenidine on locus coeruleus noradrenergic neurones: possible involvement of imidazoline-preferring receptors. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:134–140. doi: 10.1007/BF00164789. [DOI] [PubMed] [Google Scholar]
- Sakmann B. Patch pipettes are more useful than initially thought: simultaneous pre- and postsynaptic recording from mammalian CNS synapses in vitro and in vivo. Pflugers Arch. 2006;453:249–259. doi: 10.1007/s00424-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]


