Abstract
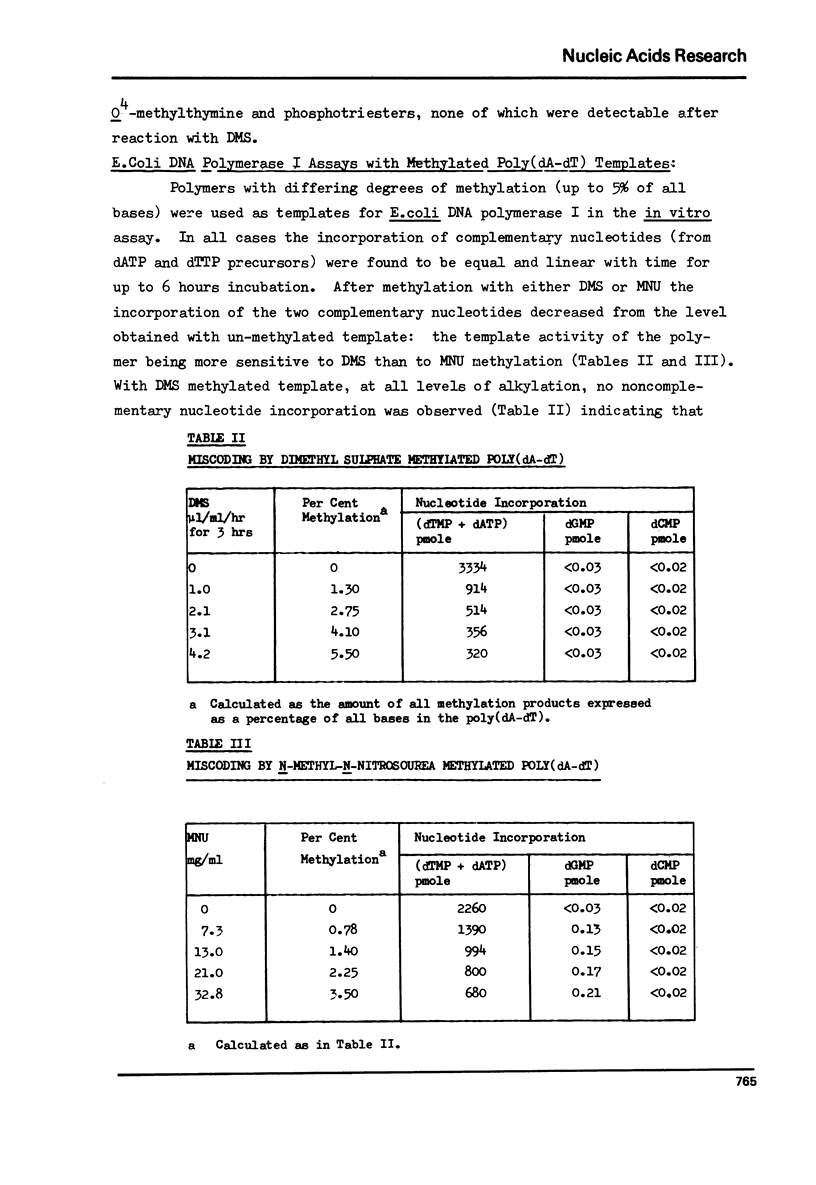
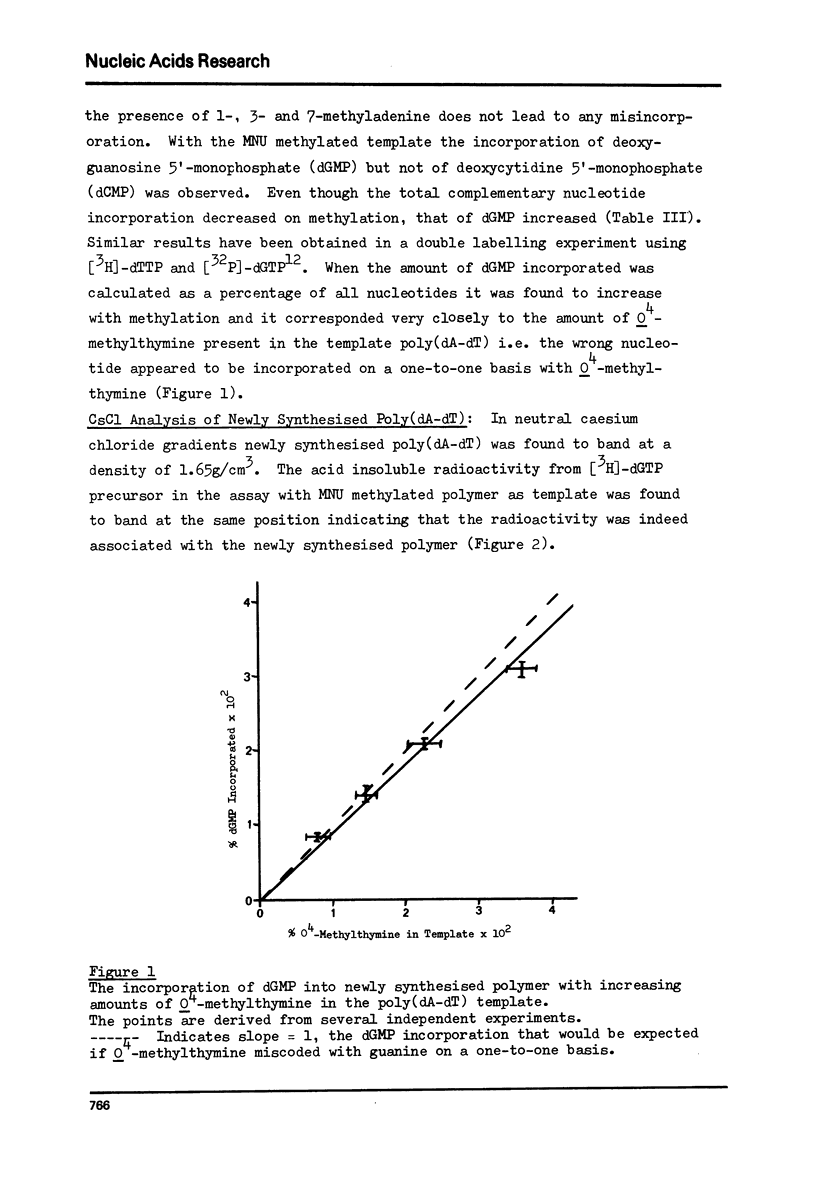
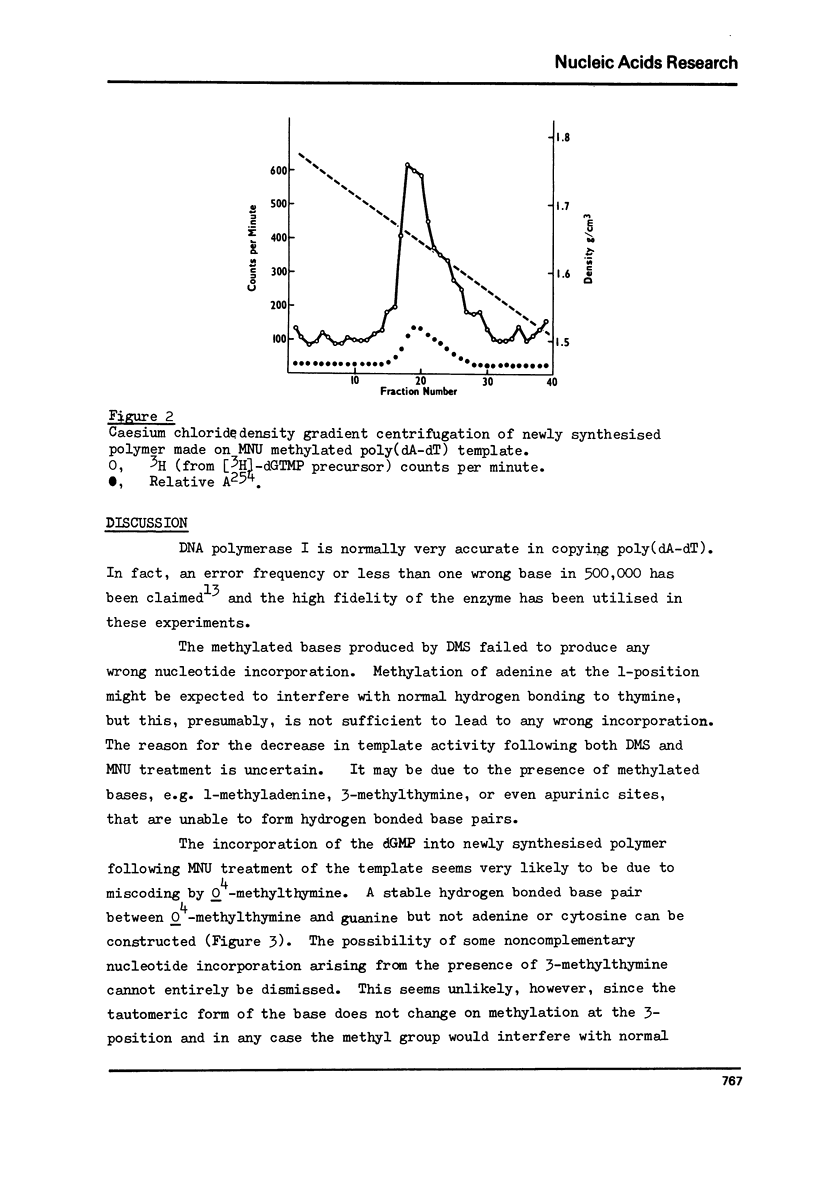
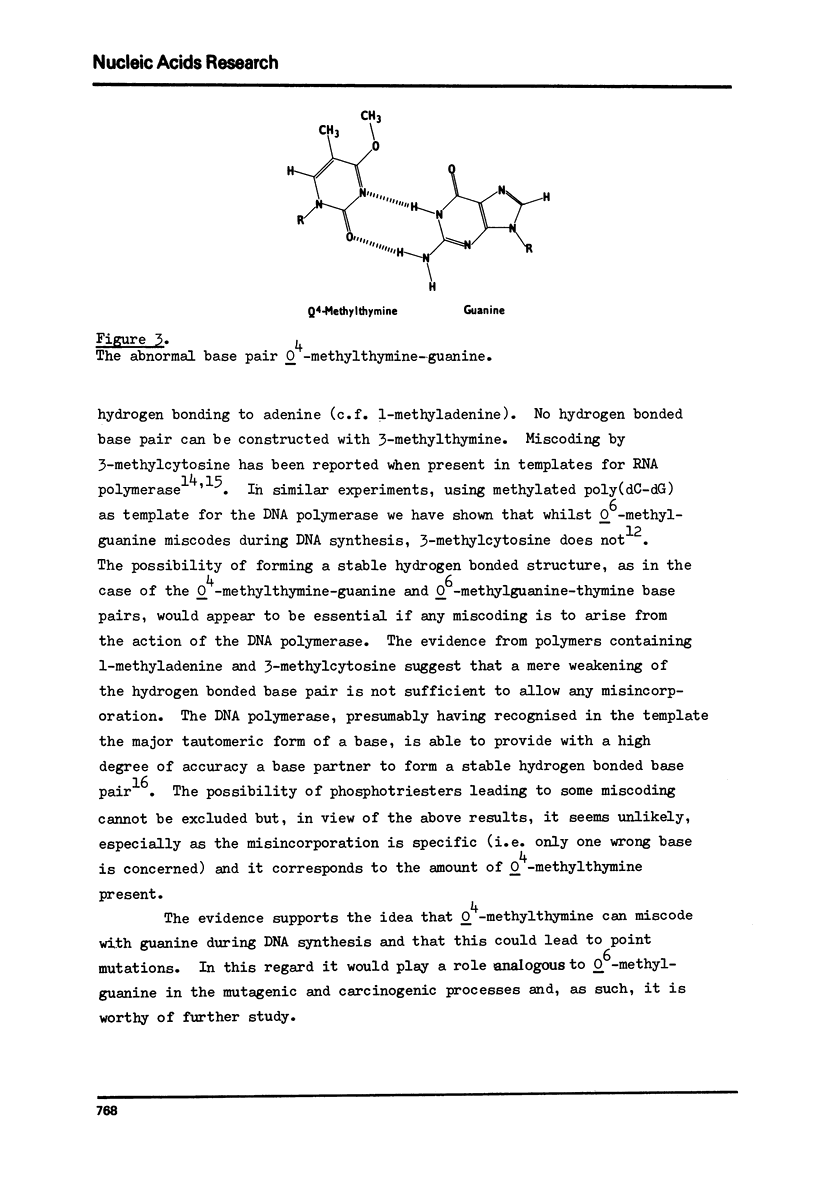
The alternating copolymer poly(dA-dT) has been methylated with either dimethyl sulphate (DMS) or N-methyl-N-nitrosourea (MNU) and the levels of the various methylation products determined. In addition to the methylated adenines formed by both methylating agents, MNU resulted also in the formation of 3-methylthymine, O4-methylthymine and phosphotriesters. The methylated polymers have been ution of complementary and non-complementary nucleotides determined. With the DMS methylated template no wrong nucleotide incorporation was detectable, but with the MNU methylated polymer the incorporation of dGMP was observed. The amount of dGMP incorporated correlated with the level of O4-methylthymine in the template over the range of methylation studied. The results indicate that O4-methylthymine is capable of miscoding on a one-to-one basis while the products of DMS methylation (1-, 3- and 7-methyladenines), and also possibly the phosphotriesters, do not lead to any misincorporation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frei J. V., Lawley P. D. Tissue distribution and mode of DNA methylation in mice by methyl methanesulphonate and N-methyl-N' -nitro-N-nitrosoguanidine: lack of thymic lymphoma induction and low extent of methylation of target tissue DNA at 0-6 of guanine. Chem Biol Interact. 1976 Jun;13(3-4):215–222. doi: 10.1016/0009-2797(76)90075-2. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Carcinogenicity of N-methyl-N-nitrosourea: possible role of excision repair of O6-methylguanine from DNA. J Natl Cancer Inst. 1974 Dec;53(6):1839–1841. [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J., Shah S. A., Farmer P. B., Jarman M. Reaction products from N-methyl-N-nitrosourea and deoxyribonucleic acid containing thymidine residues. Synthesis and identification of a new methylation product, O4-methylthymidine. Biochem J. 1973 Sep;135(1):193–201. doi: 10.1042/bj1350193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Ludlum D. B., Magee P. N. Reaction of nitrosoureas with polycytidylate templates for ribonucleic acid polymerase. Biochem J. 1972 Jul;128(3):729–731. doi: 10.1042/bj1280729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlum D. B. Methylated polydeoxyribocytidylic acid templates for RNA polymerase. Biochim Biophys Acta. 1971 Oct;247(3):412–418. doi: 10.1016/0005-2787(71)90027-x. [DOI] [PubMed] [Google Scholar]
- Nicoll J. W., Swann P. F., Pegg A. E. Effect of dimethylnitrosamine on persistence of methylated guanines in rat liver and kidney DNA. Nature. 1975 Mar 20;254(5497):261–262. doi: 10.1038/254261a0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Person S., Phillips S., Funk F. A determination of mutagen specificity in bacteria using nonsense mutants of bacteriophage T4. J Mol Biol. 1967 Jun 28;26(3):437–447. doi: 10.1016/0022-2836(67)90314-2. [DOI] [PubMed] [Google Scholar]
- TRAUTNER T. A., SWARTZ M. N., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. X. Influence of bromouracil substitutions on replication. Proc Natl Acad Sci U S A. 1962 Mar 15;48:449–455. doi: 10.1073/pnas.48.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Complementary base pairing and the origin of substitution mutations. Nature. 1976 Sep 23;263(5575):285–289. doi: 10.1038/263285a0. [DOI] [PubMed] [Google Scholar]


