Abstract
Aims:
To study the effect of intravenous magnesium sulfate infusion on clinical outcome of patients of acute stroke.
Materials and Methods:
Sixty consecutive cases of acute ischemic stroke hospitalised within 24 h of an episode of stroke were taken as subjects. All subjects underwent a computed tomography head, and those found to have evidence of bleed/space-occupying lesions were excluded from the study. The subjects taken up for the study were divided into two groups of 30 subjects each. Both the groups received the standard protocol management for acute ischemic stroke. Subjects of Group 1 additionally received intravenous magnesium sulfate as initial 4 g bolus dose over 15 min followed by 16 g as slow infusion over the next 24 h. In all the subjects of the two study groups, serum magnesium levels were estimated at the time of admission (Day 0), Day 1 and Day 2 of hospitalization using an atomic absorption spectrometer.
Statistical Analysis Used:
Scandinavian stroke scores were calculated on Day 3, day of discharge and Day 28. Paired t-test was employed for comparison of stroke scores on Day 3, day of discharge and Day 28 within the same group and the unpaired t-test was used for the intergroup comparison, i.e. comparison of stroke scores of control group with corresponding stroke scores of magnesium group.
Results:
Comparison of stroke scores on Day 3 and day of discharge, on the day of discharge and Day 28 and on Day 3 and Day 28 in the magnesium group produced a t-value of 5.000 and P <0.001, which was highly significant. However, the comparison of the mean stroke scores between the magnesium and the control groups on Day 3, day of discharge and Day 28 yielded a P-value of >0.05, which was not significant.
Conclusions:
The study failed to document a statistical significant stroke recovery in spite of achieving a significant rise in serum magnesium level, more than that necessary for neuroprotection, with an intravenous magnesium sulfate regime.
Keywords: Ischemic stroke, magnesium sulfate, neuroprotection
Introduction
Stroke is defined as rapidly developing clinical signs of focal or global disturbance of cerebral functions, lasting more than 24 h, or leading to death with no apparent cause other than vascular origin.[1] The worldwide incidence of stroke is estimated to be 0.22.5 per 1000. It accounts for 2% of all hospital admissions in India.[1,2] Cerebral thrombosis is the most common type of stroke, and hemiplegia its most common somatoneurological presentation encountered clinically.
The therapeutic management for acute ischemic stroke is divided into therapies that target the vasculature and those that target the nervous system. Vascular strategies include prevention of clot propagation, recanalization and collateral enhancement. Acute neuroprotection and subacute promotion of brain plasticity are the current neural strategies. The available modalities of stroke treatment constitute a limited armamentarium to combat this dreaded disease, which has such a high impact on mortality and morbidity and is a financial burden on mankind.[3]
A variety of neuroprotective agents like glycine antagonists, calcium antagonists, free radical scavengers, etc. presumed to be intervening at one of the various steps in the mechanism of ischemic cell injury have been tried in the past but were found to be either ineffective or too toxic for man.[4] In recent times, magnesium has been found to be neuroprotective in several preclinical and clinical trials. Magnesium ions affect various cellular enzymes involved in a variety of cellular functions, e.g. cell membrane permeability, mitochondrial functions, the ionic membrane current in conducting cells, etc.[5] It can produce vasodilation in systemic and pulmonary circulation by inhibiting calcium ion influx. Magnesium also decreases platelet aggregation and increases bleeding time, thereby preventing thrombus propagation and re-occlusion of thrombosed vessels after recanalization.[6,7]
Clinical exposure with magnesium sulfate administration in preeclampsia/eclampsia, acute myocardial infarction and arrhythmias has established its safety and tolerability in human subjects beyond doubt. The present study was undertaken to evaluate the effects of intravenous magnesium sulfate on clinical outcome in patients of acute ischemic stroke.
Materials and Methods
Sixty consecutive cases of acute ischemic stroke hospitalised at Pt. B. D. Sharma, PGIMS, Rohtak within 24 h of an episode of stroke were taken as subjects for the present study. An informed consent of the subject or a close relative was taken for inclusion in the study group. The study was an open randomized trial.
Exclusion criteria
Subjects with systolic blood pressure less than 90 mmHg.
Presence of bundle branch block or atrioventricular block.
Serum creatinine >3mg%.
Any clinical condition in which there was a therapeutic indication for magnesium sulfate.
Pregnancy.
All subjects underwent a computed tomography (CT) head and those found to have evidence of bleed/space-occupying lesions were excluded from the study. The subjects taken up for the study were divided into two groups of 30 subjects each. Both the groups received the standard protocol management for acute ischemic stroke. Subjects of Group 1 additionally received intravenous magnesium sulfate as initial 4 g bolus dose over 15 min followed by 16 g as slow infusion over the next 24 h. Simultaneously, a keen watch was kept for signs and symptoms of magnesium toxicity such as loss of deep tendon reflexes, respiratory depression, prolongation of PR, QT or QRS interval or complete heart block.
In all the subjects of the two study groups, serum magnesium levels were estimated at the time of admission (Day 0), Day 1 and Day 2 of hospitalization using an atomic absorption spectrometer. All data were collected and analyzed using standard principles of statistics.
Results
All patients received standard treatment for stroke and were randomly divided into two equal groups of 30 patients each. Group 1 comprised of those subjects who additionally received magnesium sulfate infusion, while Group 2 served as the control group receiving the standard therapy for stroke only. The mean ages of Group 1 and Group 2 were 56.47 + 14.45 years and 55.93 + 15.59 years, respectively. More than 50% of the patients were above 60 years of age. The male:female ratio was 3:2 in Group 1 and 4:1 in Group 2. Hypertension (32%), hypercholesterolemia (27%), ischemic heart disease (18%) and diabetes mellitus (16%) were found to be the principal risk factors.
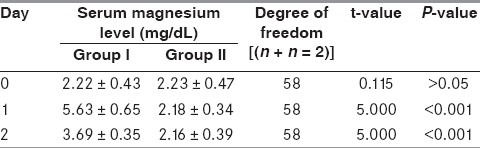
The serum magnesium levels were estimated on Day 0, 1 and 2 in the two groups [Table 1]. To study the changes in the level of magnesium in the two groups on subsequent days, paired t-test was applied. Group 1 was paired as Pair 1 (Day 0 and Day 1), Pair 2 (Day 1 and Day 2) and Pair 3 (Day 0 and Day 2), whereas Group 2 was paired as Pair 4 (Day 0 and Day 1, Pair 5 (Day 1 and Day 2) and Pair 6 (Day 0 and Day 2), respectively [Table 2]. The values of Group 1 showed a statistically significant rise in magnesium levels unlike Group 2. To study the comparison of serum magnesium levels on Days 0, 1 and 2 between the two groups, unpaired t-test was applied [Table 3]. The serum magnesium levels were comparable on Day 0 between the two groups, but thereafter on Days 1 and 2, the values of serum magnesium were statistically higher in Group 1.
Table 1.
Serum magnesium levels (mg/dL)
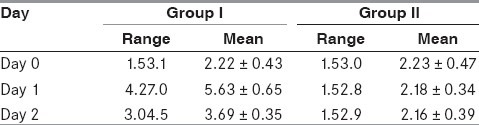
Table 2.
Comparison of serum magnesium
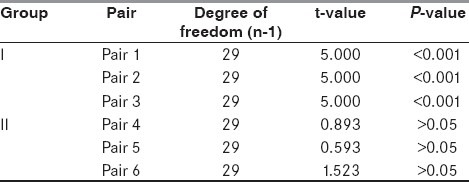
Table 3.
Comparison of serum magnesium levels in both groups
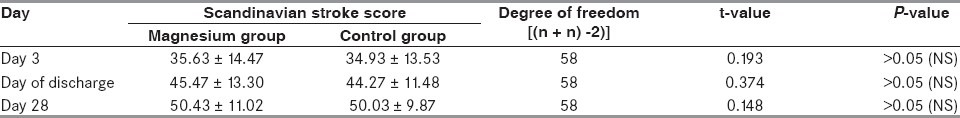
The mean Scandinavian stroke score in the control group on Day 3 was 34.93 + 13.65, on the day of discharge was 44.27 + 11.48 and on Day 28 was 50.03 + 9.87. Similarly, the stroke score in the magnesium group on Day 3 was 35.63 + 14.47, on the day of discharge was 45.47 + 13.30 and on Day 28 was 50.43 + 11.02. For comparison of stroke scores on Day 3, day of discharge and Day 28 within the same group, paired t-test was used. For intergroup comparison, i.e. comparison of stroke scores of control group with corresponding stroke scores of magnesium group, unpaired t-test was employed. A total of six pairs of stroke score values were formed for statistical computation. Pair 1 (Day 3 and day of discharge) had a t-value of 5.000 and P <0.001; Pair 2 (day of discharge and Day 28) had a t-value of 3.471 and P <0.01; and Pair 3 (Day 3 and Day 28) had a t-value of 5.000 and P <0.001 [Table 4]. Comparison of stroke scores on Day 3 and day of discharge, on the day of discharge and Day 28 and on Day 3 and Day 28 in the magnesium group produced a t-value of 5.000 and P <0.001, which was highly significant [Table 5]. However, the comparison of the mean stroke scores between the magnesium and the control groups on Day 3, day of discharge and Day 28 yielded a P-value of >0.05, which was not significant [Table 6].
Table 4.
Comparison of stroke score values in the control group
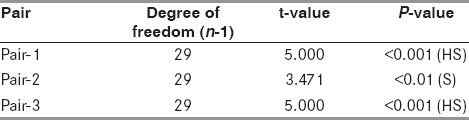
Table 5.
Comparison of stroke score values in the magnesium group
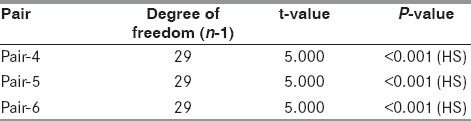
Table 6.
Comparison of corresponding stroke scores in both groups
Discussion
Neuroprotection is a relatively newer approach in the management of stroke. The cardinal feature in the concept of neuroprotection was to boost tolerance to ischemic insult such that the tissue retains viability till other defence mechanisms come into play. Calcium ions play the sheet anchor role in the mechanism of cell injury by causing the release of excitatory amino acids like glutamate (through NMDA receptors), cell membrane lipid peroxidation, free radical generation and, ultimately, cell death.[8,9] Magnesium is the second most common intracellular cation having a serum concentration of 1.72.6 mg/dL. It is believed to be nature's physiological calcium antagonist. The neuroprotective attributes of magnesium are presumed to be due to its (1) noncompetitive antagonism of NMDA receptors,[9] (2) inhibition of glutamate release,[10] (3) antagonism of all subtypes of calcium channels,[11] (4) early restoration of cellular ATP reserves and (5) buffering of intramitochondrial calcium.[12] The present study was undertaken to evaluate the effect of intravenous magnesium sulfate on the clinical outcome in patients of acute ischemic stroke.
Sixty patients of acute stroke presenting within 24 h were included as subjects in the study and were randomly divided into two equal groups of 30 each. Group 1 (magnesium group) and Group 2 (control group) were evenly matched for age and sex ratios. Subjects aged 60 years and above constituted the main bulk, an observation also reported by Park[13] and Brodericks[14] et al. In the present study, hypertension emerged as the most common comorbid condition followed by hypercholesterolemia, ischemic heart disease and diabetes. The findings are consistent with the reports of previous authors. Saha and his associates observed in their study that 20% of the subjects were hypertensive while 8% had diabetes.[15]
The dose of magnesium sulfate used was well tolerated as none of the subjects reported any adverse effects and they all remained hemodynamically stable during and after the magnesium therapy. On infusing magnesium, it was found that serum magnesium on Day 1 was more than double the value on Day 0 (t = 5.000 and P < 0.001), and it remained elevated on Day 2 in comparison with serum magnesium levels on Day 0 (t = 5.000 and P < 0.001) [Table 2]. On applying unpaired t-test to compare corresponding serum magnesium levels in the two groups, no significant difference was found between the magnesium levels on Day 0. However, a significant difference was observed on comparing the corresponding values on Day 1 (t = 5.000, P < 0.001) and on Day 2 (t = 5.000, P < 0.001) [Table 3]. This significant and sustained elevation in serum magnesium levels after magnesium infusion has also been reported by Wester et al.[16] and Muir et al.[17,18]
Many scoring systems have been proposed in the past for clinical evaluation in stroke patients. The Scandinavian scale was used for functional assessment (maximum 60 and minimum 0) in the present study due to its simplicity and practical value (annexure 1). A stroke score on the Scandinavian scale of 34.93 + 13.53, 44.27 + 11.48 and 50.03 + 9.87 was observed in the control group on Day 3, day of discharge and Day 28, respectively. On applying the paired t-test for mutual comparison (Day 3 vs. day of discharge, day of discharge vs. Day 28 and Day 28 vs. Day 3), the t-value was 5.000 (P < 0.001), 3.471 (P < 0.01) and 5.000 (P < 0.001), respectively [Table 4].
In the magnesium group (Group 1), recovery scores observed on Day 3, day of discharge and Day 28 were 35.63 + 14.47, 45.47 + 13.30 and 50.43 + 11.02, respectively. On comparison of these values with paired t-test, the corresponding t-value for each pair was 5.000 and P <0.001 [Table 5].
Unpaired t-test was applied to compare the corresponding stroke score (i.e., on Day 3, day of discharge and Day 28) in the two groups. The resultant t-values were 0.193, 0.374 and 0.149, respectively, demonstrating no significant correlation. Muir et al. in a 3-month follow-up study found a similar response to magnesium therapy when compared with placebo.[17] However, they found fewer early deaths in the magnesium group as compared with the placebo group. Survival curve analysis indicated slightly early recovery in the magnesium group (P = 0.066 by long rank test). In a second study by Muir et al.[18] no significant difference in clinical outcome was seen with magnesium therapy. Lampl et al.[19] in their randomized, placebo-controlled, double-blind trial to study the protective effect of magnesium sulfate infusion reported at the end of 1-month follow-up that the Barthel index was nonsignificantly higher while the Rankin disability score was lower (marginally significant) in the magnesium group. They finally arrived at the conclusion that intravenous magnesium sulfate had a significant positive effect on the outcome in stroke patients, but further larger trials were needed to confirm this. Therefore, the marginal stroke recovery at 1-month follow-up was in accordance with the studies available in the literature.
But, Saver et al.[20] in a small pilot FAST-MAG trial demonstrated good functional outcome at 3 months after early administration of magnesium sulfate. The Intravenous Magnesium Efficacy in Stroke (IMAGES)[21] trial recruited more than 2200 subjects within 12 h of stroke onset. The final results of the IMAGES trial demonstrated that randomised treatment with magnesium sulfate did not reduce the death and disability in stroke patients as compared with placebo. Further in-depth analysis of the IMAGES data by Aslanyan et al.[22] suggested a positive correlation between magnesium and lacunar syndrome, which was not ascribed to confounding factors like severity, time of treatment, blood pressure and other baseline factors. Moreover, the beneficial effect was found to be unrelated to the time from stroke onset to infusion of magnesium.
The present study failed to document a statistical significant stroke recovery in spite of achieving a significant rise in serum magnesium level, more than that necessary for neuroprotection with an intravenous magnesium sulfate regime. Therefore, in spite of the beneficial effects of magnesium therapy on the histological and functional outcome on cerebral ischemia in animal models, no beneficial effects on the functional outcome of stroke were accrued in man except in case of lacunar syndromes. Further clinical trials are necessary to determine the benefits, the dose, duration and timing of magnesium therapy for reducing the morbidity and mortality in lacunar syndromes.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: Results of a WHO collaborative study. Bull World Health Organ. 1980;58:113–30. [PMC free article] [PubMed] [Google Scholar]
- 2.Dalal PM. Strokes (CVD) in India. Jpn Circ J. 1982;46:621–4. [Google Scholar]
- 3.Hankey GJ, Jamrozik KD, Braudhurst RJ, Forbes S, Anderson CS. Long term disability after first ever stroke and related prognostic factors in the Perth community stroke study, 1989-90. Stroke. 2002;33:1034–1040. doi: 10.1161/01.str.0000012515.66889.24. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M. Neuroprotection of acute ischaemic stroke: Where are we? Neuroscientist. 1999;5:392–401. [Google Scholar]
- 5.Peter O, Fenster P. Should magnesium be part of routine therapy for acute myocardial infarction? Am Heart J. 1992;124:1113–8. doi: 10.1016/0002-8703(92)91011-o. [DOI] [PubMed] [Google Scholar]
- 6.Durlah J. Magnesium deficiency thrombus. Lancet. 1967;1:1362. [Google Scholar]
- 7.Ramussen HS, Larsen OG, Meior K. Haemodynamic effects of intravenously administered magnesium sulphate in patients with ischaemic heart disease. Clin Cardiol. 1998;11:824–8. doi: 10.1002/clc.4960111205. [DOI] [PubMed] [Google Scholar]
- 8.White BC, Weigenstein JG, Winegar CD. Brain ischaemic anoxia, mechanism of injury. JAMA. 1984;251:1586–9. [PubMed] [Google Scholar]
- 9.Cheung JY, Bonventra JV, Malis CD, Leaf A. Calcium in ischaemic injury. N Engl J Med. 1986;314:1670–6. doi: 10.1056/NEJM198606263142604. [DOI] [PubMed] [Google Scholar]
- 10.Smith DA, Connick JH, Stone TW. Effects of changing extracellular levels of magnesium on spontaneous activity of glutamate release in the mouse neocortical slice. Br J Pharmacol. 1989;97:475–82. doi: 10.1111/j.1476-5381.1989.tb11975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iseri LT, French JH. Magnesium: Nature's physiologic calcium blocker. Am Heart J. 1984;108:188–93. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 12.Favaron M, Bernardi P. Tissue specific modulation of the mitochondrial calcium uniporter by magnesium ions. FEBS Left. 1985;183:260–4. doi: 10.1016/0014-5793(85)80789-4. [DOI] [PubMed] [Google Scholar]
- 13.Park k. Park's Text Book of Preventive and Social Medicine. 16th ed. Jabalpur: M/s Bansari Dass Bhanot; 2000. Epidemiology of Chronic non communicable diseases and conditions; pp. 280–1. [Google Scholar]
- 14.Broderick J, Brott T, Kothari R, Miller R, Khouri J, Pancioli A, et al. The greater Cincinnati/Northern kentucky stroke study: Preliminary first ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–21. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 15.Saha SP, Bhattacharya S, Das SK, Maity B, Roy T, Raut DK. Epidemiological study of neurological disorders in rural population in eastern India. J Indian Med Assoc. 2003;101:1–5. [PubMed] [Google Scholar]
- 16.Wester PO, Asplund K, Eriksson S, Hagg E, Lithner F, Strand T. Infusion of magnesium sulphate in patients with acute brain infarction. Acta Neurol Scand. 1984;70:143. doi: 10.1111/j.1600-0404.1984.tb07776.x. [DOI] [PubMed] [Google Scholar]
- 17.Muir KW, Lees KR. A randomized double blind placebo controlled pilot trial of intravenous magnesium sulphate in acute stroke. Stroke. 1995;26:1183–8. doi: 10.1161/01.str.26.7.1183. [DOI] [PubMed] [Google Scholar]
- 18.Muir KW, Lees KR. Dose optimization of intravenous magnesium sulphate after acute stroke. Stroke. 1998;29:918–23. doi: 10.1161/01.str.29.5.918. [DOI] [PubMed] [Google Scholar]
- 19.Lampl Y, Gilad R, Geva D, Esthel Y, Sadeh M. Intravenous magnesium sulphate in acute stroke: A randomized double blind study. Clin Neuropharmacol. 2001;24:11–5. doi: 10.1097/00002826-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Saver JL, Kidwel CS, Leary MC. Results of the Field Administration of Stroke Treatment Magnesium (FAST-MAG) pilot trial: A study of prehospital neuroprotective therapy. Stroke. 2002;33:353. doi: 10.1161/01.STR.0000124458.98123.52. [DOI] [PubMed] [Google Scholar]
- 21.Muir KW, Lees KR, Ford I, Davis S. Intravenous Magnesium Efficacy in Stroke (IMAGES) Study Investigators.Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): Randomised controlled trial. Lancet. 2004;363:439–45. doi: 10.1016/S0140-6736(04)15490-1. [DOI] [PubMed] [Google Scholar]
- 22.Aslanyan S, Weir CJ, Muir KW, Lees KR. IMAGES Study Investigators. Magnesium for treatment of acute lacunar stroke syndromes: Further analysis of the IMAGES trial. Stroke. 2007;38:1269–73. doi: 10.1161/01.STR.0000259628.94421.09. [DOI] [PubMed] [Google Scholar]


