Abstract
Background:
Biomarker for prognosis of stroke is urgently needed for the management of acute ischemic stroke (AIS) patients.
Objective:
To evaluate the course of inflammatory cytokines in AIS patients and its comparison with inter-alfa trypsin inhibitor heavy chain 4 (ITIH4) and outcome after AIS.
Materials and Methods:
A panel of 12 inflammatory cytokines and ITIH4 were estimated in serial blood samples collected at admission, 24 h, 48 h, 72 h, 144 h and at discharge of AIS patients (n = 5).
Results:
Out of the 12 cytokines, only interleukin (IL)-2, tumor necrosis factor-alfa (TNF-α), IL-10, IL-6, IL-1B and IL-8 were in the measurable range of the kit (10 pg/mL). We found high IL-2 at admission, which decreased (P < 0.05) in the follow-up samples. TNF-α initially increases (P < 0.05) at 24 h followed by gradual decrease (P < 0.05) after 72 h. IL-10 decreases initially (P < 0.05) till 72 h as compared with its level at admission and then increases (P < 0.05) after 144 h. Similarly, ITIH4 was down-regulated in the early 72 h followed by further increase with improvement of the patient. ITIH4 correlates with IL-10 and computed tomography scan infarct volume. Serum IL-6, IL-1B and IL-8 increased in the AIS patients, but did not show any pattern.
Conclusions:
Serial measurement of IL-10, IL-2 and TNF-α and ITIH4 may be useful for the follow-up of clinical outcome after AIS.
Keywords: Acute ischemic stroke, cytokine, inter-alfa trypsin inhibitor heavy chain 4, prognosis
Introduction
Stroke is one of the most frequent causes of death and disability worldwide. Although different mechanisms are involved in the pathogenesis of stroke, there is increasing evidence showing that inflammation accounts for its progression.[1–3]
Inflammatory molecules are those that were up-regulated, and contribute to the inflammatory process. This includes adhesion molecules, chemokines and cytokines. The intercellular adhesion molecule and vascular cell adhesion molecule-1 were reported to be expressed on endothelial cells during inflammation after transient middle cerebral artery occlusion in the rat.[4–6] Similarly, increase in monocyte chemoattractant protein-1 expression has been reported to exacerbate ischemic brain injury.[7,8] Inhibition or modulation in the level of these inflammatory molecules has shown a beneficial effect in a number of studies.[9–11]
During the last decade, extensive research has been done on discovery of biomarkers, which can help in the prognosis of stroke.[12–14] But, in most of these studies, they have compared the level of these inflammatory molecules at the time of admission with the infarct volume and outcome. There are very few reports on repeated measurements of inflammatory molecules for prediction of clinical outcome after stroke.[13,15–17]
In our previous studies, we have reported about the novel protein inter-α trypsin inhibitor heavy chain 4 (ITIH4), which correlates with the outcome of acute ischemic stroke (AIS) patients. We have also reported that ITIH4 expression was similar to that of anti-inflammatory cytokine interleukin (IL)-10.[18,19]
The objective of this preliminary study is to evaluate the course of inflammatory cytokines in AIS patients and its comparison with ITIH4 and outcome to identify potential candidate cytokine markers for follow-up of clinical outcome after AIS.
Materials and Methods
Patients
Five patients (n = 5), aged 22–76 years, admitted within 24 h of the onset of symptoms of AIS, were included in the present study. Diagnosis was based on the WHO definition of stroke, “Rapidly developing signs of focal (or global) disturbance of cerebral function lasting >24 h (unless interrupted by surgery or death) with no apparent nonvascular cause, history, neurological examination and computerized tomography (CT).” Patients with transient ischemic attack, hemorrhage, malignancies, renal or hepatic diseases or other types of injuries were excluded from the study. Neurological deficit was assessed as per the National Institute of Health Stroke Scale (NIHSS) score during the hospitalization period and functional recovery was assessed using the modified Rankin Scale (mRS) at the time of discharge. The protocol of this study was reviewed and approved by the Institutional Ethics Committee.
Blood sampling
Venous blood was collected at 0 h (the time of admission) and 24 h, 48 h, 72 h and 144 h after admission. Blood was allowed to clot and, after centrifugation (1000x g, 10 min), serum was separated and stored at -20°C until used in the experiments.
ITIH4 estimation
Indirect enzyme-linked immunosorbent assays (ELISA) were performed as described by Kashyap et al.[19] In brief, 100 μL (1:100) serum samples taken from AIS patients at each time point were added to individual microtiter wells and blocked with 0.5% bovine serum albumin in phosphate-buffered saline (PBS). After washing with PBS, the polyclonal antibody against ITIH4 protein was added and plates were incubated at 37°C for 60 min. The wells were washed, followed by addition of the secondary antibody (goat anti-rabbit immunoglobulin G horseradish peroxidase; IgG-HRP) and incubation for 60 min at 37°C. After another wash, antibody reactivity was detected via the addition of 100 μL tetramethylbenzidine hydrogen peroxide (TMB/H2O2) substrate solution to the wells, which were then incubated at room temperature for about 5 min. The reaction was stopped with 100 μL of 2.5 N H2SO4, and the absorbance of each well was read at 450 nm.
Inflammatory cytokine assay
The panel of inflammatory cytokines was estimated in the follow-up serum samples collected from AIS patients using the Multi-Analyte ELISarray kit as per the manufacturer's instruction (SABiosciences Corporation, Frederick, USA). In brief, 50 μL of the assay buffer followed by 50 μL of the sample or standard were added in to respective ELISA wells. The plate was taped for 10 s and incubated for 2 h at room temperature. After incubation, the plate was washed three times with wash buffer. After washing, 50 μL of the assay buffer and 50 μL of biotin-conjugated detection antibody against corresponding cytokines and chemokines were added in the respective wells and incubated for 1 h. After incubation, the wells were again washed three times with wash buffer and 100 μL of avidin-HRP solution was added in all wells. The plate was then incubated at room temperature for 30 min. After incubation, the plate was washed again for four times and 100 μL of substrate solution was added to each well. The ELISA plate was then incubated in the dark for 15 min and 100 μL of stop solution was added and absorbance was measured at 450 nm. The concentration of the particular cytokine and chemokines were calculated with respect to their corresponding standards. The cytokine and chemokines measured in the current study includes IL-1A, IL-1B, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17A, interferon (INF)y, TNF-α and granulocyte-macrophage colony stimulating factor (GM-CSF).
Statistical analysis
The levels of ITIH4 and inflammatory cytokine at admission were compared with those of the follow-up samples by ANOVA for repeated measurement variable. The degree of association between ITIH4, inflammatory cytokine and CT scan infarct volume were analyzed using Pearson's correlation test. All the statistical analysis was performed using MedCalc (statistical software version10) and statistical significance was defined as P <0.05.
Results
All patients were admitted to the Intensive Care Unit (ICU), where the temperature was maintained at 20–25°C. The clinical characteristics of the five patients of AIS are shown in Table 1.
Table 1.
Clinical details of AIS patients
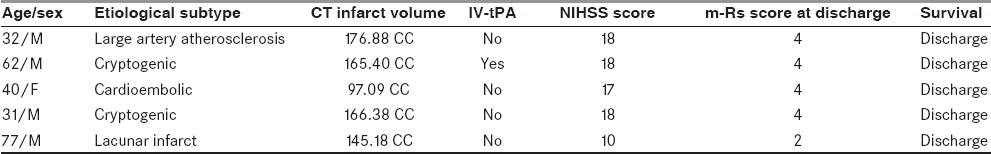
All patients received anti-platelet agents (aspirin 150 mg, clopidregel 75 mg once a day) and other supportive measures for the treatment of concurrent illnesses like hypertension. Anti-edema measures, such as mannitol 20%, 0.25–0.5 g/kg over 20 min (not exceeding a total of 2 g/1 kg of body weight in 24 h), were given with symptoms of raised intracranial pressure. One patient was thrombolysed using intravenous recombinant tissue plasminogen activator (IV-tPA); mean admission NIHSS was 16.2 (range 10–18) and mean m-Rs score at discharge was 3.6 (range 2–4).
Correlation analyses between CT scan infract volumes and all the studied parameter levels at the time of admission shows a significant correlation only with ITIH4 (r = -0.9422; P < 0.05), as shown in Figure 1a. Similarly, when we analyzed the correlation between ITIH4 [Figure 3] and all inflammatory cytokines, we observed a positive and significant correlation only with IL-10 (r = 0.3973; P < 0.05), as shown in Figure 1b.
Figure 1.
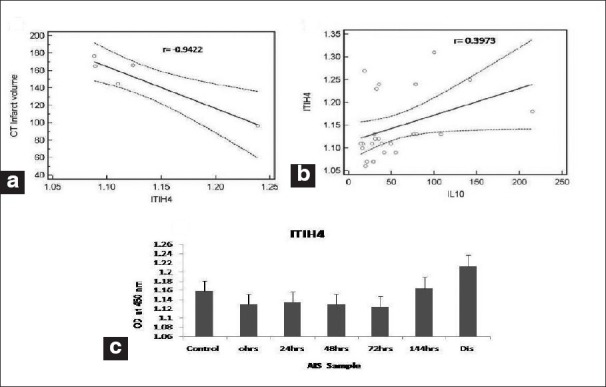
(a) Correlation (with 95% regression confidence limits) between computed tomography scan infarct volume and inter-alfa trypsin inhibitor heavy chain 4 (ITIH4) at admission in acute ischemic stroke (AIS) patients. (b) Correlation (with 95% regression confidence limits) between ITIH4 and interleukin-10 cytokine at admission in AIS patients. (c) ITIH4 levels in the follow-up sample of AIS patients
Figure 3.
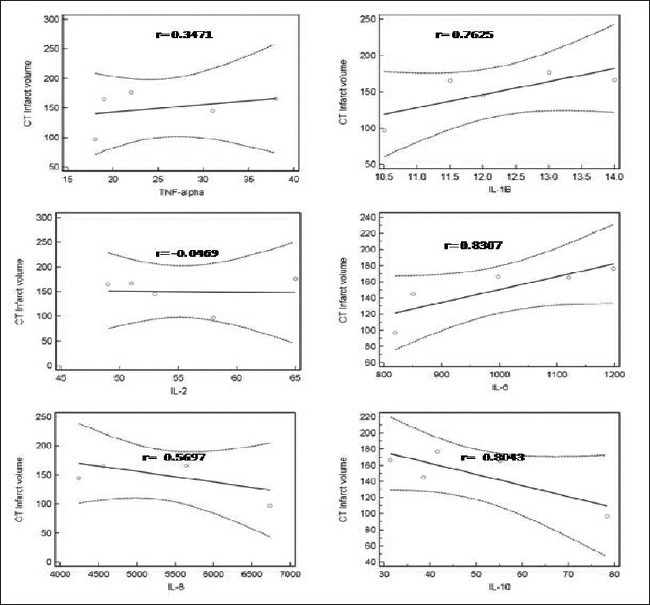
Correlation (with 95% regression confidence limits) between computed tomography scan infarct volume and inflammatory cytokine at admission in acute ischemic stroke patients
Figure 1c shows ITIH4 measured [Figure 4] in the serum sample of AIS patient collected at 0 h, 24 h, 48 h, 72 h, 144 h and at discharge. ITIH4 expression was down-regulated in AIS patient in the early 72 h and further increased with improvement of AIS patients.
Figure 4.

Correlation (with 95% regression confidence limits) between inter-alfa trypsin inhibitor heavy chain 4 and inflammatory cytokine in acute ischemic stroke patients
Out of the 12 cytokines, we observed that only six cytokines were in the measurable range of the kit (10 pg/mL), which included four pro-inflammatory cytokines (i.e., IL-1B, IL-2, IL-8 and TNF-α) and two anti-inflammatory cytokines (IL-10 and IL-6). TNF-α significantly increased (P < 0.05) in AIS patients at 24 h and 48 h, followed by a gradual decrease (P < 0.05) at 72 h, 144 h and at discharge [Figure 2a]. The level of IL-1B increased at 24 h (P < 0.05) after AIS followed by a significant decrease (P < 0.05) at 48 h and 72 h and again an increase (P < 0.05) at 144 h and then normalized at discharge [Figure 2b]. IL-2 was found to be high at the time admission in AIS patients, which then gradually decreased (P < 0.05) in the follow-up samples [Figure 2c]. There was no significant difference in the IL-8 level in the follow-up samples as compared with the control [Figure 2d].
Figure 2.

Temporal profile of serum levels of inflammatory cytokines: (a) tumor necrosis factor-α, (b) interleukin (IL)-1B, (c) IL-2, (d) IL-8, (e) IL-6 and (f) IL-10 in acute ischemic stroke patients at different time intervals. *P < 0.05 vs. admission; #P < 0.05 vs. 24 h
Among anti-inflammatory cytokines, IL-10 was found to be initially decreased in AIS patients and then significantly increased (P < 0.05) at 144 h and discharge [Figure 2e]. However, a significant increase (P < 0.05) in IL-6 was noted at 24 h, which remained high till 144 h, and then significantly decreased (P < 0.05) at discharge [Figure 2f].
Discussion
There is a balance between pro- and anti-inflammatory cytokines in a normal physiological state. This balance is lost after stroke.[20] In this study, we evaluated the course of inflammatory cytokines in AIS patients and compared its level with ITIH4 and outcome of AIS patients to identify potential candidate cytokines for the prediction of clinical outcome in AIS patients.
Out of the 12 cytokines, we observed that only six cytokines were in the measurable range of the kit (10 pg/mL), which included four pro-inflammatory cytokines (i.e., IL-1B, IL-2, IL-8 and TNF-α) and two anti-inflammatory cytokines (IL-10 and IL-6).
We observed a decrease in the serum level of ITIH4 after AIS, which further increased with the improvement of patients. Decrease in ITIH4 level correlates with CT scan infarct volume. Results of the current study are consistent with our earlier reports.[18,19]
IL-8 is predominantly produced within the central nervous system by damage of tissue at the site of ischemia. IL-8 mRNA was also reported to increase in peripheral blood mononuclear cell (PBMC) after stroke. The plasma level of IL-8 (chemokines, CXCL8) increased after stroke and remained elevated up to 1 month.[21] IL-1b and IL-17 were also elevate systemically after AIS.[22] IL-1b and IL-17 induced the secretion of IL-8.[23,24] We also observed a high IL-8 level in the serum of AIS patients throughout the follow-up. Thus, our results are in agreement with the earlier report. But, interestingly, in contrast to previous reports, we did not observe IL-17 in a detectable level, while IL-1B was found to be elevated only at 24 h and 144 h. This shows that IL-8 might be regulated by other mechanisms also. A recent study by Asare et al. showed that decrease in IL-1b increases the risk of stroke, and a mild increase in IL-1b is protective against stroke.[25] Thus, the observed increase in IL-1b at 24 h and 144 h in AIS patients may be due to protective immune response after AIS.
IL-10 is known to be the main down-regulator of the deleterious effect of pro-inflammatory cytokines.[26,27] IL-10 was reported to be significantly decreased after stroke as compared with the control, and again increased at the seventh day after stroke, and was associated with neurological deficit and/or stroke outcome.[9,17,20] We also observed a decrease in serum IL-10 levels after AIS, which further increased after 72 h with improvement of the AIS patient, and also correlated with ITIH4.
IL-6 and TNF-α have been reported to increase after stroke.[28] An increase in serum IL-6 and TNF-α within 24 h after AIS was also observed in the current study. The TNF-α level further decreased with an improvement in the AIS patients. IL-6 is a pleiotropic cytokine and can function as a pro-inflammatory cytokine by enhancing leukocyte recruitment by up-regulating production of chemokines and adhesion molecule expression.[29,30] IL-6 also serves as an anti-inflammatory cytokine by inhibiting TNF-α expression and inducing the expression of soluble TNF-α receptors and the IL-1R antagonist.[31,32] Similarly, a recent report by Al-Bahrani et al. demonstrates that the TNF-α level was also found to decrease in response to anti-platelet therapy.[33] Therefore, decrease in the TNF-α after 48 h in the follow-up sample of AIS patients might be due to the anti-inflammatory effect of IL-10, reported anti-inflammatory activity of IL-6 or as a result of anti-platelet therapy, as treatment regiments for AIS also include the anti-platelet drug. These could be the possible explanation for further decrease in TNF-α in AIS patients after 48 h.
In an earlier study, it was shown that the IL-2 level decreases with the increase in anti-inflammatory cytokine IL-10.[17] Results of the current study also show that IL-2 decreases after increase in the anti-inflammatory response. Pro-inflammatory cytokines IL-1A, IL-12, IL-17A, INF-y, GM-CSF and anti-inflammatory cytokine IL-4 were not detected in the serum samples. This shows that, possibly, there may be less involvement of these cytokines in poststroke inflammatory reaction.
Conclusion
Results of the current study suggest that serial measurement of IL-2, IL-10, TNF-α and ITIH4 may be useful for the follow-up of the clinical outcome after AIS. Although serum levels of IL-6, IL-1B and IL-8 increased in AIS patients, they did not show any pattern; thus, they were not useful for prognosis. Another study with selected cytokines and high number of patients is needed to confirm the results.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: New opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–34. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–3. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samson Y, Lapergue B, Hosseini H. [Inflammation and ischaemic stroke: Current status and future perspectives] Rev Neurol (Paris) 2005;161:1177–82. doi: 10.1016/s0035-3787(05)85190-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhang RL, Chopp M, Li Y, Zaloga C, Jiang N, Jones ML, et al. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–51. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo Y, Onodera H, Shiga Y, Shozuhara H, Ninomiya M, Kihara T, et al. Role of cell adhesion molecules in brain injury after transient middle cerebral artery occlusion in the rat. Brain Res. 1994;656:344–52. doi: 10.1016/0006-8993(94)91478-8. [DOI] [PubMed] [Google Scholar]
- 6.Connolly ES, Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, et al. Cerebral protection in homozygous nullICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesionin the pathogenesis of stroke. J Clin Invest. 1996;97:209–16. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocytechemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22:308–17. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, et al. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23:748–55. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- 9.Basic Kes V, Simundic AM, Nikolac N, Topic E, Demarin V. Pro-inflammatory and anti-inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcome. Clin Biochem. 2008;41:1330–4. doi: 10.1016/j.clinbiochem.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 10.Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–40. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto T, Ikeda K, Mukaida N, Harada A, Matsumoto Y, Yamashita J, et al. Prevention of cerebral edema and infarct in cerebral reperfusion injury by an antibody to interleukin-8. Lab Invest. 1997;77:119–25. [PubMed] [Google Scholar]
- 12.Miyakis S, Georgakopoulos P, Kiagia M, Papadopoulou O, Pefanis A, Gonis A, et al. Serial serum procalcitonin changes in the prognosis of acute stroke. Clin Chim Acta. 2004;350:237–9. doi: 10.1016/j.cccn.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Yip HK, Sun CK, Chang LT, Chen MC, Liou CW. Time course and prognostic value of plasma levels of N-terminal pro-brain natriuretic peptide in patients after ischemic stroke. Circ J. 2006;70:447–52. doi: 10.1253/circj.70.447. [DOI] [PubMed] [Google Scholar]
- 14.Wunderlich MT, Lins H, Skalej M, Wallesch CW, Goertler M. Neuron-specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long-term outcome in acute ischemic stroke. Clin Neurol Neurosurg. 2006;108:558–63. doi: 10.1016/j.clineuro.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: An independent prognostic factor. Stroke. 2001;32:917–24. doi: 10.1161/01.str.32.4.917. [DOI] [PubMed] [Google Scholar]
- 16.Winbeck K, Poppert H, Etgen T, Conrad B, Sander D. Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke. 2002;33:2459–64. doi: 10.1161/01.str.0000029828.51413.82. [DOI] [PubMed] [Google Scholar]
- 17.Nayak AR, Kashyap RS, Purohit HJ, Kabra D, Taori GM, Daginawala HF. Evaluation of the inflammatory response in sera from acute ischemic stroke patients by measurement of IL-2 and IL-10. Inflamm Res. 2009;58:687–91. doi: 10.1007/s00011-009-0036-4. [DOI] [PubMed] [Google Scholar]
- 18.Kashyap RS, Kabra DP, Nayak AR, Mishra RN, Deshpande SK, Karandikar P, et al. Protein electrophoretogram in serum of acute ischemic stroke patients and its correlation with S-100BBand neuron specific enolase level: A pilot study. Ann Neurosci. 2006;13:36–40. [Google Scholar]
- 19.Kashyap RS, Nayak AR, Deshpande PS, Kabra D, Purohit HJ, Taori GM, et al. Inter-alpha-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clin Chim Acta. 2009;402:160–3. doi: 10.1016/j.cca.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Perini F, Morra M, Alecci M, Galloni E, Marchi M, Toso V. Temporal profile of serum anti-inflammatory and pro-inflammatory interleukins in acute ischemic stroke patients. Neurol Sci. 2001;22:289–96. doi: 10.1007/s10072-001-8170-y. [DOI] [PubMed] [Google Scholar]
- 21.Kostulas N, Kivisäkk P, Huang Y, Matusevicius D, Kostulas V, Link H. Ischemic stroke is associated with a systemic increase of blood mononuclear cells expressing interleukin-8 mRNA. Stroke. 1998;29:462–6. doi: 10.1161/01.str.29.2.462. [DOI] [PubMed] [Google Scholar]
- 22.Kostulas N, Pelidou SH, Kivisäkk P, Kostulas V, Link H. Increased IL-1beta, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke. 1999;30:2174–9. doi: 10.1161/01.str.30.10.2174. [DOI] [PubMed] [Google Scholar]
- 23.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strieter RM, Kunkel SL, Showell HJ, Remick DG, Phan SH, Ward PA, et al. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989;243:1467–9. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- 25.Asare K, Gee BE, Stiles JK, Wilson NO, Driss A, Quarshie A, et al. Plasma interleukin-1beta concentration is associated with stroke in sickle cell disease. Cytokine. 2010;49:39–44. doi: 10.1016/j.cyto.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–32. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Morita Y, Takizawa S, Kamiguchi H, Uesugi T, Kawada H, Takagi S. Administration of hematopoietic cytokines increases the expression of anti-inflammatory cytokine (IL-10) mRNA in the subacute phase after stroke. Neurosci Res. 2007;58:356–60. doi: 10.1016/j.neures.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–75. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 29.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 30.Modur V, Li Y, Zimmerman GA, Prescott SM, McIntyre TM. Retrograde inflammatory signaling from neutrophils to endothelial cells by soluble interleukin-6 receptor alpha. J Clin Invest. 1997;100:2752–6. doi: 10.1172/JCI119821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benveniste EN, Tang LP, Law RM. Differential regulation of astrocyte TNF-alpha expression by the cytokines TGF-beta, IL-6 and IL-10. Int J Dev Neurosci. 1995;13:341–9. doi: 10.1016/0736-5748(94)00061-7. [DOI] [PubMed] [Google Scholar]
- 32.Aderka D, Le JM, Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989;143:3517–23. [PubMed] [Google Scholar]
- 33.Al-Bahrani A, Taha S, Shaath H, Bakhiet M. TNF-alpha and IL-8 in acute stroke and the modulation of these cytokines by antiplatelet agents. Curr Neurovasc Res. 2007;4:31–7. doi: 10.2174/156720207779940716. [DOI] [PubMed] [Google Scholar]


