Abstract
The imaging features of 42 histopathologically confirmed cases of Gastrointestinal Stromal Tumors (GIST) were analyzed, to observe the pattern of metastasis. At presentation 22 of 42 patients (52.3%) showed metastasis. During follow-up, three more cases developed metastasis, within one year of resection. Mesentery, omentum, and liver were the most frequent sites for metastasis. Other sites that were rarely reported to be involved were increasingly recognized to show metastasis due to longer survival. The metastasis often showed attenuation and enhancement characteristics, similar to primary GIST, and frequently showed necrosis, hemorrhage, and calcification.
Keywords: Gastrointestinal stromal tumor, gastrointestinal neoplasia, metastasis, sarcomas, smooth muscle mesenchymal tumor
INTRODUCTION

Gastrointestinal stromal tumors (GIST) are a group of smooth muscle mesenchymal tumors of variable malignancy. They account for 0.1 to 3% of all gastrointestinal neoplasms and 5 – 7% of sarcomas.[1] GISTs are defined as spindle cell, epitheloid, or occasionally pleomorphic mesenchymal tumors of the gastrointestinal tract, which express the KIT protein (CD117, stem cell factor receptor) detected on immunohistochemistry. They are thought to arise from interstitial cells of Cajal — a pleuripotent mesenchymal stem cell that acts as a pacemaker for controlling motility. The tumor can arise from any part of the gastrointestinal tract, mesentery, and omentum.[2] Sometimes, the origin cannot be established, because of an extensive peritoneal spread. These tumors occur in middle-aged and older patients, with no sex predilection. The symptoms are minimal in the early stage and go unnoticed, and by the time of presentation, the tumor is often large and spreads to other organs. One-third of the GISTs are malignant and their recurrence rate is about 90%.[3] A computed tomography (CT) scan is the most valuable investigation for the initial diagnosis and staging. The management of GISTs has been revolutionized after the advent of Imatinib. Effective management requires regular imaging assessment. Positron emission tomography (PET)-CT will become the Gold Standard for assessment of response, by virtue of its unique diagnostic functional characteristics when combined with CT, which shows a picture of better quality. This study is aimed at analyzing the radiological pattern of metastasis in cases of gastrointestinal stromal tumors and to define the role of a CT scan in diagnosis and staging.
MATERIALS AND METHODS
A total of 42 histologically confirmed cases were included and the imaging findings were analyzed. Ultrasound and CT scans were done in all cases in our institute. The age of the patients ranged from 24 years to 82 years. Majority were in the 40 – 60 year age group (n = 21) with a male : female ratio of 2 : 1.
RESULTS
Table 1 shows the sites of metastasis in the 42 cases that formed the study group. The primary tumor was localized to the stomach (n = 11), small bowel (n = 18), rectum (n = 6), esophagus (n = 3), mesentery (n = 1), and retroperitoneum (n = 1). There were two cases with extensive peritoneal deposits and the origin could not be established. At presentation, 22 of the 42 (52.3%) patients had metastasis. Mesentery, omentum, and liver were the most frequent sites of metastasis. Three patients did not show metastasis at presentation, but developed metastasis after resection of the primary tumor.
Table 1.
Site of metastasis seen in our study group
DISCUSSION
Nilsson et al., reported that at least 50% of the GISTs have metastasis at presentation.[4] In a recent report from India, metastasis from GIST has been most commonly reported in the liver and peritoneal cavity. Metastasis to the lymph nodes, lungs, bone, subcutaneous tissue, and brain are rare.[5] According to Katz SC et al., liver metastasis occurs in 65% and metastasis in the peritoneum in 21 – 45% the cases.[6] In our study, 52.3% had metastasis at their first presentation and liver, mesentery, and omentum were the most affected sites.
Liver metastasis
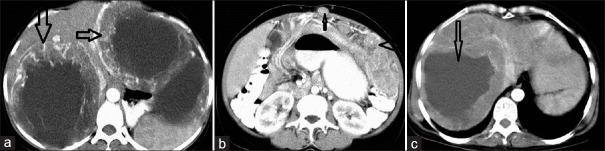
In our study, 12 (28%) cases showed liver metastasis. A heterogeneous enhancing mass was seen in five cases and a hypodense mass in two cases. In these two cases, one had dense homogenous enhancement and the other had minimal peripheral enhancement. Four cases had predominant cystic attenuation, with thin, solid, enhancing soft tissue at the periphery showing neovascularity. A small amount of bleeding was seen in one of the liver metastasis [Figure 1]. The liver is the most common site of metastasis, both at the time of presentation and during relapse. It is seen in 49 – 65% of the cases.[6,7] Different authors have described various appearances of liver metastasis. However, lesions appear similar to the primary mass in many cases. They are usually multiple, involve both lobes, and appear heterogeneous with peripheral enhancement. Low attenuation at the center may be due to necrosis and the enhancing peripheral portion represents the solid mass. Usually the tumor enhances in the late arterial or portal venous phase as it spreads through the portal vein. Neovascularity is also shown in the solid part of metastasis [Figure 2]. As some lesions enhance in the arterial phase, triple phase CT of the liver is mandatory. Sometimes, liver lesions may show hypoattenuation on a plain scan and enhance homogenously on a contrast scan. Cystic mass with fluid–fluid level and at times multilocular appearance may be seen. The variegated appearance is due to tumor necrosis, protein material, bleeding, and calcification [Figure 1]. With treatment there is rapid transition from a heterogeneously hypoattenuating pattern, with resolution of the enhancing tumor nodule and decrease in tumor vessel. The density of the hepatic metastasis decreases to 20 – 25 HU, appearing cystic [Figure 3]. The appearance of a new nodule within the mass, showing nodular or ring enhancement, indicates disease progression.
Figure 1.
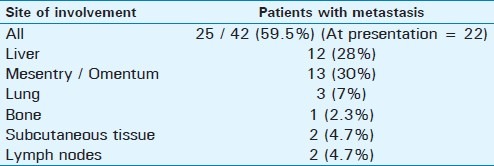
NECT of a small bowel GIST with liver metastasis in a 42-year-old male. (a) Axial scan shows two well-defined, hypodense, space-occupying lesions in the liver. There is central necrosis. A small hyperdense focus within it is suggestive of a bleed (open arrow). (b) The primary mass shows heterogeneous enhancement (thin white arrow); another heterogeneously enhancing SOL can be seen in segment VI of the liver (solid thick arrow).
Figure 2.
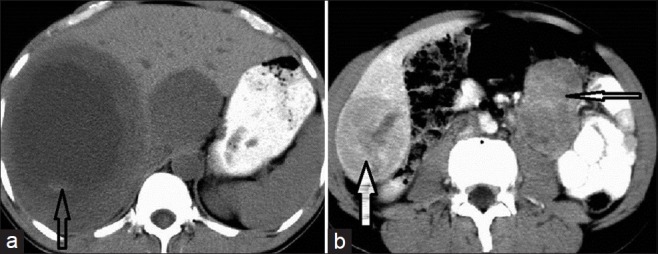
Contrast Enhanced Computed Tomography (CECT) of abdomen shows Jejunal GIST in a 32-year-old female patient. (a) CECT shows a large hypodense mass with neovascularity at the periphery in right and left lobes of the liver (white arrow) and central necrosis (open arrow) suggestive of hepatic metastasis (b) Multiple heterogeneously enhancing, space-occupying lesions (SOL) in the pouch of Douglas indenting on the rectum (open arrows) (c) Subcutaneous deposits seen in anterior abdominal wall in a subcutaneous plane (narrow arrow) and multiple peritoneal and omental deposits (broad open arrow). The lesions enhance with contrast. (d) The primary jejunal GIST shows central necrosis, peripheral neovascularity, and enhancement (broad open arrow).
Figure 3.
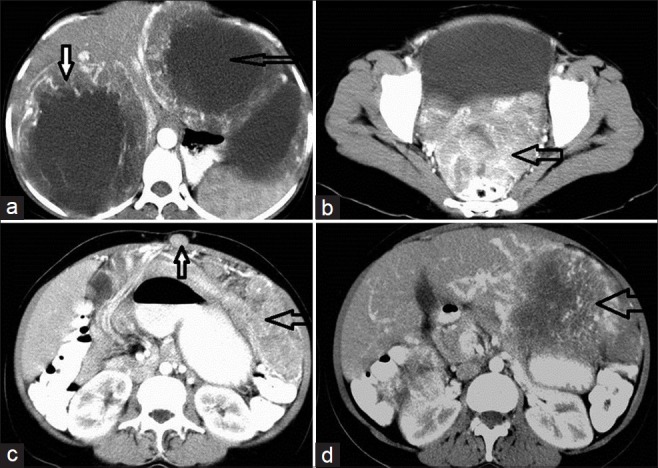
CECT of the abdomen in a 45-year-old female patient shows rectal GIST with metastasis, before and after treatment. (a) Scan before treatment shows multiple solid enhancing SOL of varying sizes scattered in both lobes of the liver (open arrows). (b) After treatment the lesions are more hypodense, similar to the cyst, which is characteristic of metastatic deposits (two solid arrows). (c) The thick white solid arrow indicates rectal GIST; the lesion appears as a solid enhancing exophytic growth from the rectum; the thinner arrow indicates deposit in the pouch of Douglas. (d) After treatment the rectal GIST disappears and no mass is visible at the primary site (arrow).
Peritoneal and omental spread
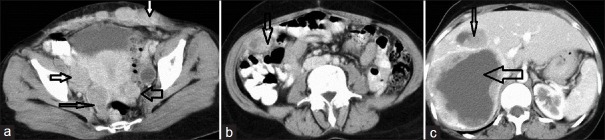
In this study we observed peritoneal deposits in 12 cases. All showed solid heterogeneous enhancement. There was no evidence of hemorrhage into the deposits in any case. In four cases the nodules were multiple and diffusely involved (omental caking). In two cases, due to extensive deposits, the origin could not be made out [Figure 4]. Ascites was observed in only one case.
Figure 4.
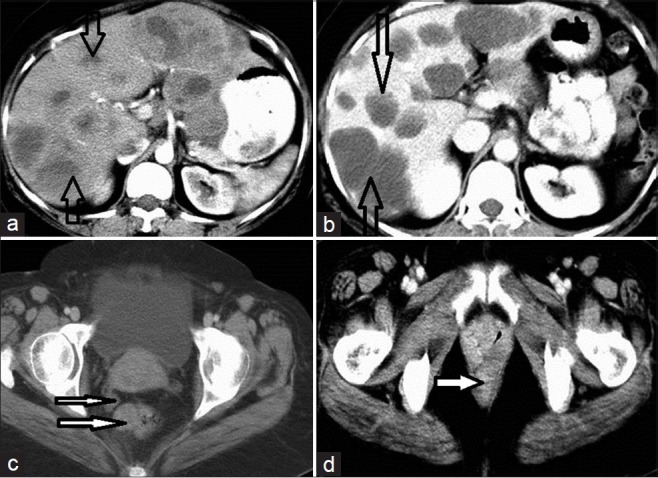
CE-axial CT scan of the abdomen and pelvis, in a 32-year-old female patient, shows extensive peritoneal metastasis from the GIST and subcutaneous deposits obscuring the origin. (a) Multiple, solid, enhancing deposits are noted on both sides of the pelvis, abutting the uterus and rectum (open arrow); the white solid arrow indicates subcutaneous deposits. (b) Multiple omental deposits (open arrows) show heterogeneous enhancement; their origin is obscure. (c) Two discrete deposits in the liver show solid (white solid narrow arrow) and cystic (open wide arrow) components on a contrast-enhanced scan.
When a tumor extends beyond the serosal tumor seedling, a peritoneal implant occurs. Most of the peritoneal spread is by the tumor seedling during surgery / biopsy. Peritoneal deposits are often large discrete masses that look like the primary tumor and can extend up to the pelvis [Figures 1 and 4]. The attenuation and enhancement pattern is similar to that of the primary lesion. A hyperattenuating enhancing mass, with areas of necrosis, hemorrhage, and cystic degeneration are seen. Tumor vessels may be seen. They may appear as large discrete masses / multiple nodules, involving the peritoneal surface and sub- peritoneal spaces, and less commonly it may be a diffuse hypervascular omental / peritoneal caking [Figure 5].[8] With treatment the tumor deposits may liquefy. Sometimes the origin of GIST cannot be established because of extensive peritoneal metastasis. Due to hypervascularity there can be GI bleeding or hemoperitoneum [Figure 6], and the size may get reduced.[9] Calcification of myxohyaline and hemorrhagic stroma may occur. Mesenteric masses grow around the vessels and rarely produce thrombosis. Ascites is rare, as the tumor deposits do not cause inflammation. Ascites is more likely attributable to the treatment rather than the tumor itself.
Figure 5.
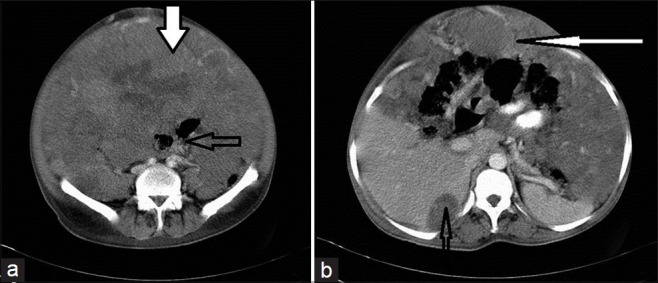
CECT of abdomen shows extensive peritoneal dissemination in a 45-year-old male. (a) Scan shows the entire peritoneal cavity is filled with solid enhancing SOL (thick solid arrow), displacing and compressing the mesentery and bowel loops (open arrow). (b) Scan shows scalloping of the liver, caused by a peritoneal deposit (thin open arrow); Also shows omental caking (thin white arrow) that mimics pseudomyxoma peritonei.
Figure 6.
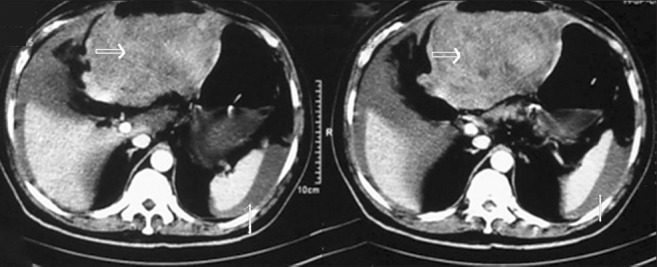
CECT of the abdomen shows Gastric GIST with hemoperitoneum in a 70-year-old male patient. The solid thick arrows that point to the primary lesion show heterogeneous enhancement and the other thin arrows point to the high density fluid within the peritoneal cavity, indicating hemorrhage.
Lymph node metastasis
In two of our cases (one a case of rectal GIST and the other a small bowel GIST) there were lymph node metastases in the inguinal region; one appeared as a solid heterogeneously enhancing mass similar to the primary tumor [Figure 7] and the other was hypodense with peripheral enhancement.
Figure 7.
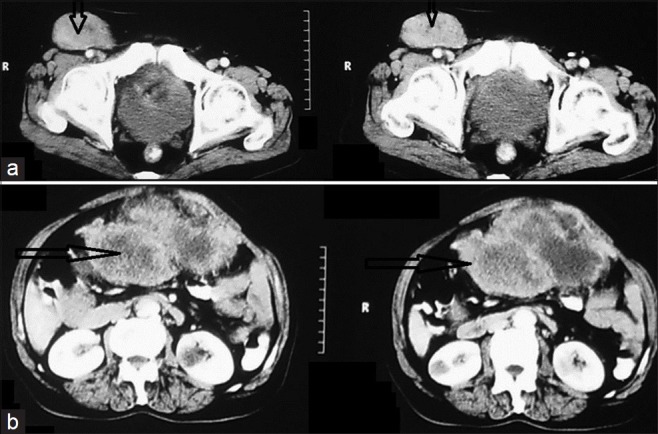
CECT of the abdomen and pelvis shows small bowel GIST with lymph node metastasis, in a 56-year-old male (open arrow). (a) Scan shows a large heterogeneously enhancing lymph node in right inguinal region. (b) Scan shows a large heterogeneously enhancing mass with areas of necrosis in the upper jejunum (open arrow), which is the primary GIST.
The prevalence of lymph node (LN) metastasis is reported to occur in 1.1 to 3.4% of the cases.[10] After the tumor cells invade the liver they have a higher probability to spread to the peripheral blood and migrate to the peripheral lymph nodes. Zhang Q reported a case of gastric GIST, with metastasis in the inguinal lymph nodes.[11] In children and young adults, especially when Carney's triad is present, the LN metastasis is common compared to sporadic GIST (29% vs. 2%). The cause of this behavior is not fully understood. In some studies the expression of the lymphatic endothelial marker D2-40 / podoplanin in carcinoma cells and the presence of intra-tumoral lymphatics have been correlated with tumor invasiveness and LN metastasis.[12] Metastatic LN may appear as a primary mass with similar characteristics of attenuation and enhancement, and sometimes it may be hypoechoic [Figure 7]. When LN metastasis is predominant, other conditions like adenocarcinoma and lymphoma of the bowel should be thought of as differential diagnosis.
Lung and bone metastasis
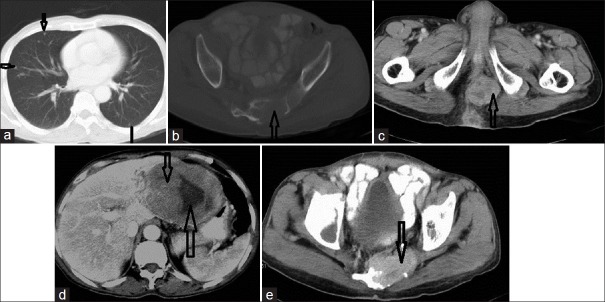
We had only one case of metastasis to the sacroiliac bone and in three cases the deposits were seen in the lung. The bone deposit was a lytic lesion with large enhancing soft tissue [Figure 8]. In patients with lung metastasis, multiple nodules along with deposits in the liver / peritoneum were noted [Figures 8 and 9].
Figure 8.
CECT of the chest (lung window), CT pelvis in bone window, CT pelvis in soft tissue window, CT upper abdomen in soft tissue window, and CT pelvis, in that order, show anorectal GIST with spread to the lung, bone, peritoneum, and liver, in a 60-year-old male patient. (a) Section through the chest in a lung window shows small multiple lung nodules represented by multiple arrows; most of these are sub-pleural. (b) The open arrow shows an expansile lesion in lower sacrum – the cortex is disrupted both on the inner and outer aspects suggestive of metastasis. (c) Scan shows a solid heterogeneously enhancing exophytic growth on the right lateral wall of the rectum obliterating the lumen, suggestive of primary mass (open arrow). (d) In the same patient, scan shows liver metastasis in the left lobe (thick open arrow); neovascularity is seen at the periphery (thin open arrow). (e) Soft tissue window for sacral metastasis shows enhancement of the soft tissue in the lytic area (arrow).
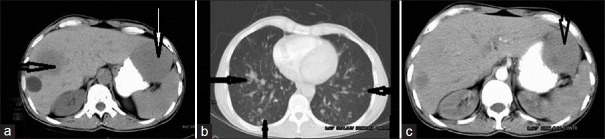
Figure 9.
CECT of the abdomen and thorax in a 61-year-old male shows gastric GIST presenting with hepatic and pulmonary metastases. (a) Non-enhanced CT shows hepatic metastasis and the primary mass (white arrow). Note that both the primary and secondary lesions are of similar density (thin arrow). (b) Scan shows multiple lung metastases (arrows). (c) Large solid enhancing SOL from the stomach lesion is predominantly exophytic.
Metastasis to the lung and bones is rare and occurs late in the course of the disease.[13] Isolated bone metastasis is rare and most of the time bone metastasis is associated with liver and peritoneal spread. Incidence of bone metastasis is about 5%.[14] Bone metastasis is seen mostly in the spine and pelvis [Figure 8]. Skull metastasis was reported in a case of rectal GIST in a recent report.[15] It was a lytic lesion like in our case. A Jain and colleagues reported a case of mesenteric GIST in a 55-year-old male, who had undergone jejunostomy three years earlier and presented with a palpable lesion in the scalp.[13] He had huge hepatomegaly as well. Investigations proved bone and hepatic metastases. Lung metastasis accounts for 7.1% of all lesions. Lung lesions are rare; but have been increasingly reported in recent years due to longer survival of patients following treatment [Figures 8 and 9]. Multiple pulmonary metastases from the jejunum, in a 75-year-old male patient, was reported by Gurer et al., in a recent study.[16]
Cutaneous deposits
Subcutaneous deposits were seen in two of our cases; one was a jejunal GIST and the other primary site could not be observed due to extensive peritoneal deposits. They were solid masses with heterogeneous enhancement [Figure 10].
Figure 10.
Liver, peritoneal, and subcutaneous deposits in a 32-year-old female patient from a jejunal GIST; Follow-up scan revealed reduced vascularity and attenuation of the hepatic metastasis. (a) Pre-treatment liver metastasis scan shows intense vascularity (two arrows). (b) Scan shows subcutaneous deposits as a solid enhancing mass (small arrows); multiple peritoneal deposits (broad open arrow). (c) Scan post-treatment shows a marked reduction in vascularity and the lesion appears more cystic (thin open arrow).
Cutaneous deposits occur in about 1% of the advanced GISTs and are mostly associated with an abdominal laparotomy scar. The deposit characteristics are similar to the primary mass [Figures 1 and 10]. Kroep et al., recently reported a 69-year old female patient with GIST, who was resistant to Imatinib and presented with a subcutaneous nodule in the right arm. Histopathology confirmed the metastasis in her case.[17]
CONCLUSION
A CT scan is the modality of choice for diagnosis, staging, and follow-up. The characteristics of the primary mass as well as the spread to other sites and the response to chemotherapy are well studied by imaging modalities. The mesentery, omentum, and liver are the most frequent sites for metastasis. Other sites that have been rarely reported to be involved in the past are increasingly recognized to show metastasis, due to longer survival of the patients in the current era of better chemotherapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/43/99177
REFERENCES
- 1.Dematteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors; recurrence pattern and prognostic factors for survival. Ann Surg. 2000;231:51–8. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. GIST - definition, clinical, histological, immunohistochemical and molecular-genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 3.Daghter R, Cohen M, Williams G, Rothmann M, Gobburu J, Robbie G, et al. Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumours. Clin Cancer Res. 2002;8:3034–8. [PubMed] [Google Scholar]
- 4.Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, et al. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course and prognostication in pre-imatinib mesylate era: A population based study in western Sweden. Cancer. 2005;103:821–9. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 5.Sripathi S, Rajagopal KV, Srivasthava RK, Ayachit A. CT features, mimics and atypical presentations of gastrointestinal stromal tumor (GIST) Indian J Radiol Imaging. 2011;21:176–81. doi: 10.4103/0971-3026.85364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz SC, Dematteo RP. GISTS and leiomyosarcoma. J Surg Oncol. 2008;97:350–9. doi: 10.1002/jso.20970. [DOI] [PubMed] [Google Scholar]
- 7.Baker ME, Pelley R. Hepatic metastases.Basic principle and implication for radiologists. Radiology. 1998;197:329–7. doi: 10.1148/radiology.197.2.7480672. [DOI] [PubMed] [Google Scholar]
- 8.Levy AD, Rematti HE. GIST radiologic features with pathological correlation. Radiographics. 2003;23:283–304. doi: 10.1148/rg.232025146. [DOI] [PubMed] [Google Scholar]
- 9.King DM. The radiology of gastrointestinal Stromal tumors (GIST) Cancer Imaging. 2005;5:150–6. doi: 10.1102/1470-7330.2005.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tateishi U, Hasegawa T, Satake M, Moriyama N. Gastrointestinal stromal tumors; creation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr. 2003;134:656–9. doi: 10.1097/00004728-200309000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Yu JW, Yang WL, Liu XS, Yu JR. Gastrointestinal stromal tumor of stomach with inguinal lymph node metastasis; a case report. World J Gastroenterol. 2010;16:1808–10. doi: 10.3748/wjg.v16.i14.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agaimy A, Carney JA. Lymphatics and D2-40 / podoplanin expression in gastrointestinal tumors of stomach with and without LN metastasis-an immunohisto-chemical study with special reference to the Carney's triad. J Clin Pathol. 2010;63:229–34. doi: 10.1136/jcp.2009.074062. [DOI] [PubMed] [Google Scholar]
- 13.Jain A, Dubashi B, Mangaladevi, Chandra SS, Halanaik D. Mesenteric gastrointestinal stromal tumor with bone metastases. Indian J Cancer. 2011;48:383–4. doi: 10.4103/0019-509X.84934. [DOI] [PubMed] [Google Scholar]
- 14.Queck R, George S. Gastrointestinal stromal tumor: A clinical review. Hematol Oncol Clin North Am. 2009;23:69–78. doi: 10.1016/j.hoc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Armaiz IG, Trufero JM, Pazocid RA, Felipo F, Lecumberri MJ, Calderero V. Skull metastasis from rectal gastrointestinal stromal tumors. Clin Transl Oncol. 2009;11:625–7. doi: 10.1007/s12094-009-0415-x. [DOI] [PubMed] [Google Scholar]
- 16.Gurer A, Gokufi S, Demrbafi S, Ersoy E, Aydin R. Gastrointestinal stromal tumor with pulmonary metastasis: Report of a case. Turk J Med Sci. 2008;38:273–5. [Google Scholar]
- 17.Kroep JR, Bovee JV, Van der Molen AJ, Hogendoorn PC, Gelderblom H. Extra-abdominal subcutaneous metastasis of a gastrointestinal stromal tumor: Report of a case and a review of the literature. J Cutan Pathol. 2009;36:565–9. doi: 10.1111/j.1600-0560.2008.01067.x. [DOI] [PubMed] [Google Scholar]