Abstract
Background:
Vitex negundo L. (Verbenaceae) is a hardy plant widely distributed in the Indian subcontinent and used for treatment of a wide spectrum of health disorders in traditional and folk medicine, some of which have been experimentally validated. In present study, we aimed to investigate the anti-inflammatory effects of V. negundo in carrageenan-induced paw edema in rats, and to investigate the probable mechanism of anti-inflammatory action.
Materials and Methods:
Paw edema was produced by injecting 1% solution of carrageenan, and the paw volume was measured before and after carrageenan injection up to 5 h. V. negundo leaf oil was extracted using a Clevenger apparatus and administered by a trans-dermal route to Wistar rats and the percentage of inhibition of inflammation was observed using a Plethysmometer by comparing a compound aerosol-based formulation with 1 mg diclofinac diethylamine BP and 7 mg methyl salicylate IP/kg body weight served as a standard drug whereas paraffin oil served as the placebo group. After withdrawing of blood, serum was separated and cyclooxygenase (COX)-1 and COX-2 inhibitory activities were measured by the enzyme immuno assay (EIA) method by using a COX inhibitor screening assay kit.
Results and Discussion:
V. negundo leaf oil significantly (P < 0.05) reduced the carrageenan-induced paw edema as compared to the placebo group (paraffin oil) and 1 mg diclofinac diethylamine BP and 7 mg methyl salicylate IP showed the maximum inhibition of paw edema as compared to the V. negundo leaf oil treated group and the control group. Also in the present study V. negundo leaf oil showed significantly (P < 0.05) inhibits COX-1 pathways rather than COX-2 pathways as compared to the V. negundo leaf oil treated group.
Conclusion:
It is suggested that the V. negundo leaf oil is a potent anti-inflammatory agent and acts via inhibition of COX-2 without much interfering COX-1 pathways.
Keywords: Anti-inflammatory, cyclooxygenase-2 inhibitors, Vitex negundo
INTRODUCTION
Vitex negundo Linn (Verbenaceae) (VN) is a woody and aromatic shrub. It commonly bears tri- or penta-foliate leaves on quadrangular branches, which give rise to bluish-purple colored flowers in branched tomentose cymes. It thrives in humid places or along water courses in wastelands and mixed open forests and has been reported to occur in Afghanistan, India, Pakistan, Sri Lanka, Thailand, Malaysia, eastern Africa, and Madagascar. It is grown commercially as a crop in parts of Asia, Europe, North America, and the West Indies.[1] Leaves of V. negundo contain hydroxy-3,6,7,3′,4′-pentamethoxyflavone,[2] 6′-p-hydroxybenzoyl mussaenosidic acid,[3] 2′-p-hydroxybenzoyl mussaenosidic acid,[4] 5,3′-dihydroxy-7,8,4′-trimethoxyflavanone,[5] 5,3′-dihydroxy-6,7,4′- trimethoxy flavanone,[6] etc.
Leaf extracts of V. negundo reported as an anti-oxidant[7] which decreases the levels of superoxide dismutase, catalase, and glutathione peroxidase in Freund's adjuvant-induced arthritis-rats.[8] Roots of V. negundo inhibits a number of enzymes actions e.g. lipoxygenase, butyryl-cholinesterase,[9] α-chymotrypsin,[10] xanthine-oxidase,[11] and tyrosinase.[12] Administration of V. negundo extracts potentiated the effect of commonly used anti-inflammatory drugs such as ibuprofen and phenylbutazone;[13] analgesics such as meperidine, aspirin, morphine, and pethidine.[14] In the view of agonistic activity of V. negundo, the present investigation designed to investigate the effects of V. negundo leaf oil as an anti-inflammatory agent and to investigate the probable mechanism.
MATERIALS AND METHODS
Animals and housing conditions
Male Wistar rats weighing 200–250 g were procured from Laboratory Animal Resources, Division of Pharmaceutical Technology, Defence Research Laboratory, Tezpur, India. The animals were maintained under temperature-controlled rooms at an animal house with 12 h alternating light and dark cycles and given adequate nutrition and water ad libitum. All animal experimental protocols were performed according to the “Principles of Laboratory Animal care” (NIH publication 85–23, revised 1985) and approved by Institutional Use and Care Committee.
Study design
Inflammation was produced as per the method described by Leblanc et al.[15] Briefly, the male Wistar rats were fasted for 16 h and paw edema was produced by injecting 200 μl of 1% solution of carrageenan in saline into the left hind paw. After 15 min observing the swelling in left hind paw the following treatment were followed at the inflammation site: Group I (n = 6) was applied aerosol-based formulation equivalent to 1 mg diclofinac diethylamine BP and 7 mg methyl salicylate IP/kg body weight (control group); Groups II, III, and IV (n = 6) were applied V. negundo leaf oil on the inflammation site equivalent to 200 μl, 1000 μl, and 2000 μl diluted with paraffin oil (treated group); Group V (n = 6), were applied equivalent to 1 ml/kg paraffin oil on the inflammation site (placebo control group). The paw volume was measured before and after carrageenan injection up to 5 h, using a water displacement plethysmometer (Orchid Scientific, Nashik, India). The swelling ratio (% swelling) was expressed as the percentage of the increase in the paw volume before carrageenan injection.[16] After 1 h, 3 h, and 5 h blood was withdrawn from the tail vein and separated serum and were stored at –20 °C.
Cyclooxygenase inhibitory activity
The cyclooxygenase (COX) inhibitor screening assay directly measures PGF2α produced by stannous chloride (SnCl2) reduction of COX-derived prostaglandin (PGH2) produced in the COX reaction. All procedures were performed as indicated in the assay kit (Uscn Life Science Inc. China).
RESULTS
Anti-inflammatory effects of V. negundo on carrageenan-induced edema in rat hind paws
After injecting 500 μl of 1% carrageenan into the hind paw, the paw edema of the control rats was increased along with the time course and the peak edema was observed after 3 h of injecting.
The effectiveness of V. negundo was in dose-dependent manner. At the dose of 500 μl/kg, V. negundo leaf oil significantly (P < 0.05) decreased the edema as compared to the placebo group whereas 1 mg diclofinac diethylamine BP and 7 mg methyl salicylate IP decreased the maximum edema to 29% of swelling as compared to the placebo group [Figure 1].
Figure 1.
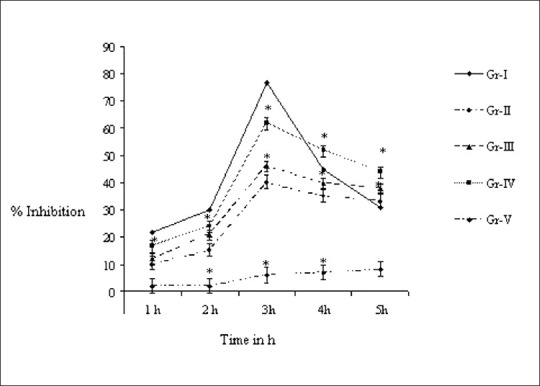
Effect of Vitex negundo leaf oil on paw edema. Results are expressed as mean ± SD (n = 6). *Statistically different (P < 0.05) from control rats. Expressed as group (I) (control group, n = 6) treated with 1 mg diclofinac diethylamine BP and 7 mg methyl salicylate IP/kg; Groups (II, III, and IV) (treated group, n = 6): V. negundo leaf oil equivalent to 200 μl, 1000 μl, and 2000 μl diluted with paraffin oil; Group V (placebo control group, n = 6) treated with equivalent 1 ml/kg paraffin oil
Effect of V. negundo on inhibition of the COX-1 and COX-2 activities
COX inhibitory activities of V. negundo leaf oil were measured by using a COX inhibitor screening assay kit. The effectiveness of V. negundo on inhibiting of COX-1 and COX-2 oil also showed in a dose-dependent manner.
V. negundo leaf oil (500 μl/kg) significantly (P < 0.05) reduced COX-2 and COX-1 activities as compared to the placebo group whereas diclofenac spray showed maximum inhibition of COX-1 and COX-2 activity [Figure 2].
Figure 2.
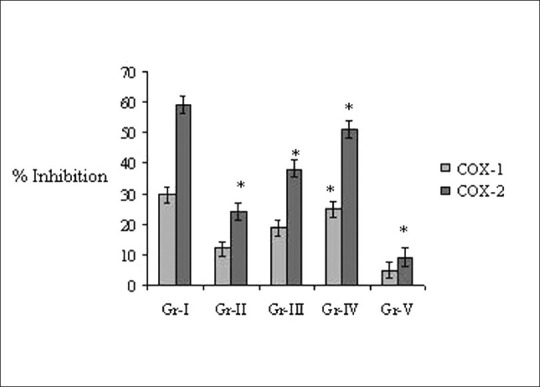
Effect of Vitex negundo on inhibition of cyclooxygenase. Results are expressed as mean ± SD (n = 6). *Statistically different (P < 0.05) from control rats. Expressed as COX-1: Cyclooxygenase-1; COX-2: Cyclooxygenase-2; Group (I) (control group, n = 6) treated with 1 mg diclofinac diethylamine BP and 7 mg methyl salicylate IP/kg; Groups (II, III, and IV) (treated group, n = 6): V. negundo leaf oil equivalent to 200 μl, 1000 μl, and 2000 μl diluted with paraffin oil; Group V (placebo control group, n = 6) treated with equivalent 1 ml/kg paraffin oil
DISCUSSION
Carrageenan-induced inflammation in the rat paw represents a classical model of edema formation and hyperalgesia, which has been extensively used in the development of nonsteroidal anti-inflammatory drugs and selective COX-2 inhibitors.[17,18]
COX is involved in the regulation of day-to-day cellular and metabolic activities such as maintaining stomach lining integrity, regulating blood flow within the kidneys, and balancing platelet function,[19] whereas COX-2 triggers by response to a variety of pro-inflammatory stimulation.[20] COX-2 regulates prostaglandin production by regulating arachidonic acid pathway in inflammatory cells for healing and repairing.[21] Therefore, inhibition of COX, and inhibiting the release of prostaglandins, is an important way to suppress inflammatory response.
In the present investigation, V. negundo reduced inflammation by inhibiting COX-2 receptor activity. Hong et al. reported that a number of medicinal plants showed anti-inflammatory activity via inhibition of the COX-2 receptor namely Aristolochia debilis, Cinnamomum cassia, Cinnamomum loureirii, Curcuma zedoaria, Eugenia caryophyllata, Pterocarpus santalius, Rehmania glutinosa, and Tribulus terrestris[22] but the activity on COX-1 receptor of these plants are remain unclear. V. negundo inhibits the COX-2 receptor without significant interfering to the COX-1 receptor. Selective COX-2 inhibitors can also inhibit peripheral pain responses when given intrathecally,[23] whereas a selective COX-1 inhibitor has no effect.[24] COX-1 inhibition leads to varying degrees of gastric ulcerations, perforations, or obstructions. Therefore, ideal anti-inflammatory drugs should inhibit COX-2 without interfering COX-1. The major drawback for analgesic/anti-inflammatory traditional drugs that provides optimum therapeutic efficacy without the gastro-toxicity which arises mainly COX-1 inhibition along with COX-2 receptors.
The study showed that oil of V. negundo prevented carrageen an-induced inflammation via COX-2 inhibition. Further studies are required to elucidate the molecular mechanism of the action V. negundo.
CONCLUSION
This finding indicates that V. negundo leaf oil is a potent anti-inflammatory agent and its acts via the inhibition of COX-2 receptor without interfering COX-1 inhibition.
ACKNOWLEDGEMENTS
The authors gratefully acknowledged the financial support by Defense Research and Development Organization (DRDO), Ministry of Defense, Govt. of India.
Footnotes
Source of Support: Financial support by Defense Research and Development Organization (DRDO), Ministry of Defense, Govt. of India
Conflict of Interest: None declared.
REFERENCES
- 1.Jabeen A, Khan M, Ahmad M, Zafar M, Ahmad F. Indigenous uses of economically important flora of Margallah Hills National Park Islamabad Pakistan. Afr J Biotech. 1999;8:763–84. [Google Scholar]
- 2.Banerji A, Chadha MS, Malshet VG. Isolation of 5-hydroxy-36-73’4’-pentamethoxyflavone from Vitex negundo. Phytochemistry. 1969;8:511–2. [Google Scholar]
- 3.Sehgal CK, Taneja SC, Dhar KL, Atal CK. ‘2’-p-hydroxybenzoyl mussaenosidic acid a new iridoid glucoside from Vitex negundo. Phytochemistry. 1982;21:363–6. [Google Scholar]
- 4.Sehgal CK, Taneja SC, Dhar KL, Atal CK. ‘6’-p-hydroxybenzoyl mussaenosidic acid an iridoid glucoside from Vitex negundo. Phytochemistry. 1983;22:1036–8. [Google Scholar]
- 5.Achari B, Chowdhuri US, Dutta PK, Pakrashi SC. Two isomeric flavones from Vitex negundo. Phytochemistry. 1984;23:703–4. [Google Scholar]
- 6.Singh V, Dayal R, Bartley J. Volatile constituents of Vitex negundo leaves. Planta Med. 1999;65:580–5. doi: 10.1055/s-2006-960832. [DOI] [PubMed] [Google Scholar]
- 7.Tiwari OP, Tripathi YB. Antioxidant properties of different fractions of Vitex negundo Linn. Food Chem. 2007;100:1170–6. [Google Scholar]
- 8.Devi PR, Kumari SK, Kokilavani C. Effect of Vitex negundo leaf extract on the free radicals scavengers in complete Freund's adjuvant induced arthritic rats. Indian J Clin Biochem. 2007;22:143–7. doi: 10.1007/BF02912899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azhar-Ul-Haq, Malik A, Anis I, Khan SB, Ahmed E, Ahmed Z, et al. Enzyme inhibiting lignans from Vitex negundo. Chem Pharm Bull. 2004;52:1269–72. doi: 10.1248/cpb.52.1269. [DOI] [PubMed] [Google Scholar]
- 10.Lodhi A, Choudhary I, Malik A, Ahmad S. ‘a-Chymotrypsin inhibition studies on the lignans from Vitex negundo Linn. J Enzyme Inhib Med Chem. 2008;23:400–5. doi: 10.1080/14756360701584653. [DOI] [PubMed] [Google Scholar]
- 11.Umamaheswari M, Asok Kumar K, Somasundaram A, Sivashanmugam T, Subhadradevi V, Ravi TK. Xanthine oxidase inhibitory activity of some Indian medical plants. J Ethnopharmacol. 2007;109:547–51. doi: 10.1016/j.jep.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Azhar UH, Malik A, Khan MT, Khan SB, Anwar-Ul-Haq, Ahmad A, et al. Tyrosinase inhibitory lignans from the methanol extract of the roots of Vitex negundo Linn.and their structure–activity relationship. Phytomedicine. 2006;13:255–60. doi: 10.1016/j.phymed.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Tandon VR, Gupta RK. Anti-inflammatory activity and mechanism of action of Vitex negundo Linn. Int J Pharmacol. 2006;2:303–8. [Google Scholar]
- 14.Gupta RK, Tandon VR. An experimental evaluation of anticonvulsant activity of Vitex negundo. Indian J Physiol Pharmacol. 2005;49:163–72. [PubMed] [Google Scholar]
- 15.Leblanc Y, Roy P, Boyce S, Brideau C, Chan CC, Charleson S, et al. SAR in the alkoxy lactone series: The discovery of DFP, a potent and orally active COX-2 inhibitor. Bioorg Med Chem Lett. 1999;9:2207–12. doi: 10.1016/s0960-894x(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 16.Tan-no K, Nakajima T, Shoji T, Nakagawasai O, Niijima F, Ishikawa M, et al. Anti-inflammatory effect of property through inhibition of nitric oxide production on carrageenan-induced mouse paw edema. Biol Pharm Bull. 2006;29:96–9. doi: 10.1248/bpb.29.96. [DOI] [PubMed] [Google Scholar]
- 17.Oku H, Ishiguro K. Cyclooxygenase-2 inhibitory 14-naphthoquinones from Impatiens balsamina L. Biol Pharm Bull. 2002;25:658–60. doi: 10.1248/bpb.25.658. [DOI] [PubMed] [Google Scholar]
- 18.Jocelyne G, Kevin B, Robert G, Joseph M, Denis R. Carrageenan-induced paw edema in rat elicits a predominant prostaglandin E2 (PGE2) response in the central nervous system associated with the induction of microsomal PGE2 Synthase-1. J Biol Chem. 2004;279:24866–75. doi: 10.1074/jbc.M403106200. [DOI] [PubMed] [Google Scholar]
- 19.Xie WL, Chipman JG, Robertson DL, Erikson RL, Simmons DL. Expression of a mitogen-responsive gene encoding prostaglandin syntheses is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991;88:2692–6. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman M, McMahon AT. Do cyclooxygenase-2 inhibitors provide benefits similar to those of traditional non-steroidal anti-inflammatory drugs with less gastrointestinal toxicity? Ann Intern Med. 2000;132:134–43. doi: 10.7326/0003-4819-132-2-200001180-00008. [DOI] [PubMed] [Google Scholar]
- 21.Flower RJ. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974;26:33–67. [PubMed] [Google Scholar]
- 22.Hong CH, Hur SK, Oh OJ, Kim SS, Nam KA, Lee SK. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J Ethnopharmacol. 2002;83:153–9. doi: 10.1016/s0378-8741(02)00205-2. [DOI] [PubMed] [Google Scholar]
- 23.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–5. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 24.Yaksh TL, Dirig DM, Conway CM, Svensson C, Luo ZD, Isakson PC. The acute antihyperalgesic action of nonsteroidal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 (COX-2) but not COX-1. J Neurol Sci. 2001;21:5847–53. doi: 10.1523/JNEUROSCI.21-16-05847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


