Abstract
Background:
Globularia alypum L. (Globulariaceae) is a shrub growing in the Mediterranean basin and known to be used as a popular medicine for its several pharmacological properties against rheumatism, gout, typhoid, intermittent fever, and diabetes.
Materials and Methods:
The acute and chronic toxicities of a G. alypum L. aqueous leaf extract were studied in animals. Acute toxicity was performed in male and female mice whereas chronic toxicity was realized in male and female rats that orally received the drug at the doses of 300 and 600 mg/kg/24 h for 30 days.
Results:
Acute toxicity showed that the extract, administered by the oral route, does not induce any mortality even for a dose of 10,000 mg/kg. Administered by the intra-peritoneal route to female and male mice, the LD50 of the extract was found to be of 2750 and 2550 mg/kg, respectively. A chronic toxicity study showed that, compared to the control groups that only received the vehicle (water), the drugs affects weight growth (effects more pronounced in female than in male rats), some organs weight after autopsy, hematological and biochemical parameters and histology of some principal organs (lungs: histological grades I to II pulmonary hypertension (PHT), respiratory distress syndrome (ARDS), and lymphoid hyperplasia; esophagus: thinning down of esophageal wall, atrophic muscular coat). The most important finding of the study was the recorded active spermatogenesis induced by the reiterated administrations of the drug that was confirmed by reducing the administered dose and the period of treatment (100 mg/kg/24 h for 15 days).
Conclusion:
It is suggested that the G. alypum L. leaf extract contains active substances with androgenic properties that could be used in human therapy.
Keywords: Active spermatogenesis, acute and chronic toxicity, androgenic properties, Globularia alypum L
INTRODUCTION
Globularia alypum L. (Globulariaceae) is a widely growing shrub in the Mediterranean area known for its uses in popular medicine for several properties: treatment of rheumatism, gout, typhoid, intermittent fever, diabetes[1] and also as effective laxative.[1,2] The plant is also described to possess anti-leukemic,[3] immunosuppressive,[4] anti-ulcer,[5,6] hypoglycemic,[7–9] anti-oxidant,[10,11] myorelaxant, and spasmolytic activities.[12]
Despite all these various demonstrated pharmacological properties, the toxicity of the drug remains practically unknown. In 1998, Skim et al.[13] suggested that the administration of an infusion of G. alyupum leaves has no toxicological effects in rats. From their side, Elbetieha et al.,[14] worked on ethanolic extracts of G. arabica and G. alypum, concluded that these two plants could have some reproductive toxicity in female rats. Hence, this work has been performed to evaluate the toxicity of the drug in animals. It is divided in two parts: acute toxicity study and chronic toxicity study.
MATERIALS AND METHODS
Plant material
Leaves of Gobulaira alypum L. were collected on the hills of Testour (North West Tunisia). After drying in air, the leaves were used to prepare aqueous extract using water. Approximately 50 g of leaf material was ground in a blender with 50 ml of distilled water for 3 min. The homogenate was centrifuged for 30 min at 2000 g and the supernatant filtered and lyophilized.
Quantification of globularin
Globularin, a principal iridoid glucosid present in G. alypum L. leaves,[15] was quantified by a reversed-phase high performance liquid chromatography method with gradient elution and multiwavelength detection[16] using harpagoside (Extrasynthθse, France) as a standard, a commercially compound with very close structural similarity to globularin.[17]
A Waters Series 510 pump was used as the solvent delivery system together with a Waters U6K injector. The system was equipped with a Spectra Physics Focus multiwavelength scanning detector and data manipulation was achieved with an IBM PS/2 computer. The column was a 250 × 4.6 mm2 I.D. Sherisorb 4 μm ODS column (Waters, Milford, MA, USA). Separation was achieved using a mobile phase consisting of acetonitrile: water (30:70) to pH 2.5 with concentrated orthophosphoric acid. The eluent was monitored at 280 nm with complete UV absorbance spectra of each eluting peak available between 200 and 360.
Quantitative analysis was performed on an extract of 10 μg of dried plant material. The concentration of globularin in the aqueous extract of G. alypum L. as determined as from the calibration curve constructed over the range of 0.02–5 μg/ml with each point taken as the mean of three determinations.
Toxicity study
This study is divided in two parts: evaluation of the acute toxicity by the determination of the lethal dose 50 (DL50) of the drug in male and female mice and the determination of the toxicity of the drug after reiterated administrations in male and female rats.
Acute toxicity study in mice
The determination of the acute toxicity of G. alypum L. aqueous extract has been evaluated in the Swiss mouse (Central Animal Breeding House, Tunisia Pharmaceutical Industries Society, Tunisia), of both sexes male and female, weighing 18–20 g and maintained fasting 12 h before the administration of the drug. The drug, dissolved in water, has been administrated by oral and intra-peritoneal routes to ten mice groups according to doses chosen in geometrical progression of 1:3 ratio. A group served as the control group received vehicle (water). The dilutions were administered by oesophagus cannula (0.5 ml/20 g) (oral route) and a syringe equipped with 25 4/10 needles (0.05–1 ml) (intra-peritoneal route). The animals, observed for 14 days following the unique administration of the tested drug, received a standardized food (Animal Alimentation Society—SNA—Borj Cidria, Tunisia) and fresh water ad libitum.
Toxicity study by reiterated administrations in rats
Administration of the product
This study has been undertaken in male and female Wistar rats, divided in six groups of 10 rats each according to the following protocol:
Groups C–M: P = 195.11 ± 2.28 g (n = 10): the male control group receiving only the vehicle (water)
Group G1: P = 197.50 ± 2.13 g (n = 10): the male group receiving 300 mg/kg/24 h of G. alypum L. aqueous extract
Group G2: P = 198.00 ± 2.37 g (n = 10): the male group receiving 300 mg/kg/24 h of G. alypum L. aqueous extract
Group C–F: P = 170.75 ± 2.14 g (n = 10): the female control group receiving only the vehicle (water)
Group G3: P = 171.00 ± 3.14 g (n= 10): the female group receiving 300 mg/kg/24 h of G. alypum L. aqueous extract
Group G4: P = 172.50 ± 3.18 g (n = 10): the female group receiving 300 mg/kg/24 h of G. alypum L. aqueous extract
G. alypum L. aqueous water extract dissolved in water was administered to animals according to the above-mentioned doses with oesophagus cannula (1 ml/100 g) for 30 days (from day D1 to day D30). During the treatment period, the animals, maintained by groups of 10 in Macrolon cages (26.6 × 42.5 × 15 cm3) under the following conditions: temperature = 20 ± 1°C, relative humidity = 55%, lighting = 200 h Lux/m2, received a standardized food (Animal Alimentation Society—SNA—Borj Cedria, Tunisia) and fresh water ad libitum.
Investigations carried out during the treatment period
During the treatment period, the animals were clinically followed up, daily weighed, determination of food and water consumption, measure of blood pressure and cardiac pulse rate at root tail level with a B.P. Recorder apparatus.
Animals sacrifice
Twenty-four hours after the last treatment (D31), all the animals were killed by anesthesia with pentobarbital (40 and 50 mg/kg i.p., respectively, in female and male rats) and bleeding. Immediately after the killing, the principal organs were removed, weighed for each animal of a given group and fixed in 10% neutral buffered formalin for histological examination. Likewise, blood samples, realized by puncture at retro-orbital sinus and abdominal aorta, were collected for biochemical and hematological investigations.
Investigations carried out after animal sacrifice
Hematological investigations
Erythrocytes and leukocytes were determined with a Leuco-ery-counter B.C.200 Brand. Leukocytes formula was established by microscopic examination of smears after M.G.G. coloration. Hematocrit and hemoglobin were respectively determined by means of the gravity and cyanmethemoglobin method.
Biochemical investigations
Concentrations of blood glucose, cholesterol and triglycerides were determined by enzymatic methods (Sera Pak Kits, Ames, Italia). Lipids and proteins were, respectively, measured by the sulfovanillic technique and the refractometric method. Glutamic-oxalo-acetic and glutamic-pyruvic transaminases (GOT and GPT) and alkaline phosphatases (ALP) were evaluated by colorimetric methods (Biomérieux Kits).
Histological examination of organs
The organs fixed in 10% neutral buffered formalin were processed for paraffin embedding, sliced into 4–5 μm pieces with a microtome and systematically stained with the usual hematoxyllin and eosin coloration. Moreover and in certain cases, it has been used the following histo-chemical colorations: PAS (Periodic Acid Schiff), Gomori's method, Masson Trichrome,, and Perls staining.
Statistical analysis
All results in the text, tables,, and figures are expressed as mean ± SEM. Statistical analysis of the results were performed by using Student's t-test.
RESULTS
Quantification of globularin
Harpagoside and globularin were,, respectively,, eluted at 8.65 ± 0.02 min (n = 12) and 6.53 ± 0.01 min (n = 20). The calibration curve was linear with a correlation coefficient of 0.9987. Intra assay precision was determined as 2.1%. The concentration of globularin in the aqueous extract was of 23.30 ± 0.70% (n = 10).
Toxicity study
Acute toxicity
No mortality was observed in experimental animals when the drug was orally administered. The LD50 could not therefore be quantified by oral route, the oral LD0 was found to be greater than 10,000 mg/kg. However, it has been possible to quantify the LD50 of G. alypum L. aqueous extract when the drug was administered by the intra-peritoneal route. The results with 95% confidence limits[18] were, respectively, for male and female mice of 2550 (2318–2805) and 2750 (2358–3206) mg/kg.
Toxicity by reiterated administrations
Clinical examination
No mortality was observed in all experimental animal groups. Daily clinical examination did show any significant change in gross general behavior (spontaneous motor activity, reactivity, gait, respiration). Likewise, it has not been noted any concerning food and water consumption between G. alypum L treated animals (300 and 600 mg/kg/24 h) and control groups (vehicle:water). However, it has been noted on day D5 the appearance of liquid stools in the four treated groups of animals. This discrepancy, totally absent in the control groups and more pronounced in female treated rats, lasted until D25.
Weight growth curves in rats
The weight growth curves in male and female rats are, respectively, presented in Figures 1a and b where it is possible to note that reiterated administrations of the tested extract induced significant differences compared to the control groups that only received the vehicle (distilled water). These differences, more pronounced in female than in male rats, showed a dose-dependent decrease in growth weight. In females receiving the daily dose of 300 mg/kg, the decrease in weight growth was starting at D2, so that from D11 to D24, it induced a decrease in growth weight versus initial weight measured at D0. The decrease of weight compared to the one measured at D0 was more pronounced when the drug was daily administered at the dose of 600 mg/kg to the females: it started on D1 and was maintained until the end of the treatment [Figure 1b]. The impact of the drug was different in male rats: at the daily dose of 300 mg/kg, the drug induced a significant decrease of weight growth compared to the control group that started at D7 (P < 0.05). This decrease lasted to D19 and, from this date no difference in weight growth was observed between the control group and the group treated with at daily 300 mg/kg (P > 0.05). When male rats were treated at the daily dose of 600 mg/kg, a significant decrease of weight growth in treated animals was observed from D1 to D7 compared to the control group (P < 0.05). Likewise, it has been noted in this treated group a significant decrease in weight growth versus initial weight measured at D0 from D8 to D22 (P < 0.05) but, from D23 to the end of the treatment period, no significant difference between treated and control groups was recorded (P > 0.05) [Figure 1a].
Figure 1.
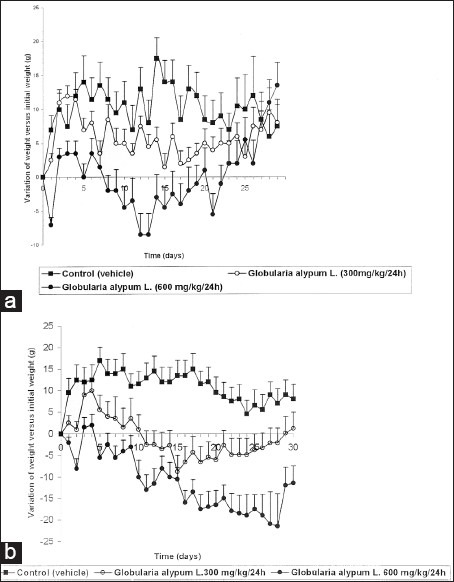
Effect of G. alypum on weight growth in the male rat (a) and in the female rat (b)
Effect of G. alypum L. on the blood pressure and cardiac pulse rate in the rat
Administered at the daily dose of 600 mg/kg, G. alypum L. did not induce any significant effect on the blood pressure and heart pulse rate in male rats [Figures 2a and 3a] whereas, at the daily dose of 300 mg/kg, G. alypum L. induced an increase of blood pressure at the end of the treatment. Compared to the control group, this effect is not significant (P > 0.5). The same applies for the effect of the drug on the heart pulse rate in male rats [Figures 2a and 3a]. At the daily dose of 300 and 600 mg/kg, it has been recorded in female rats, a biphasic phenomenon: increase (from D1 to D25) followed by decrease (from D25 to D30) of the blood pressure and heart pulse rate in female rats [Figures 2b and 3b]. The more pronounced effect was observed when the drug was administered at the daily dose of 300 mg/kg. Compared to the control group, this effect is not significant (P > 0.5).
Figure 2.
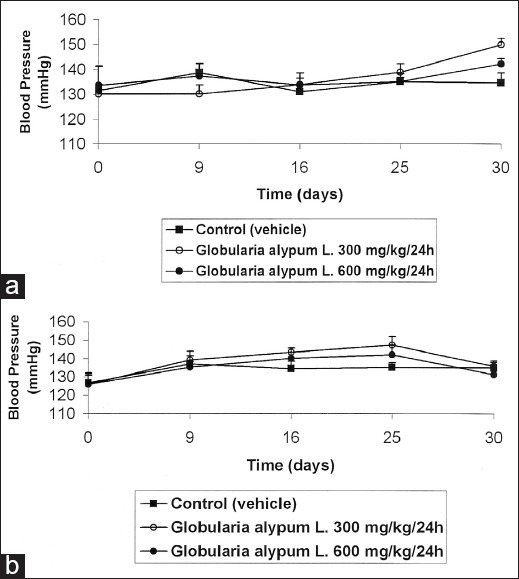
Effect of G. alypum on blood pressure in the male rat (a) and in the female rat (b)
Figure 3.
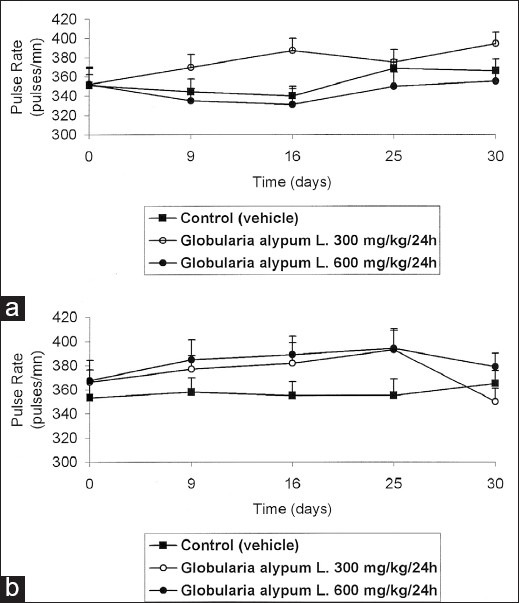
Effect of G. alypum on cardiac pulse in the male rat (a) and in the female rat (b)
Organs weight after autopsy
Determination of organs weight after autopsy showed that in males treated at the daily dose of 600 mg/kg in comparison to the control group a significant decrease of lungs weight (P < 0.5) and an increase of stomach weight (P < 0.01) and small intestine and duodenum (P < 0.05) were reported [Table 1].
Table 1.
Effect of Globularia alypum L. on organs weight
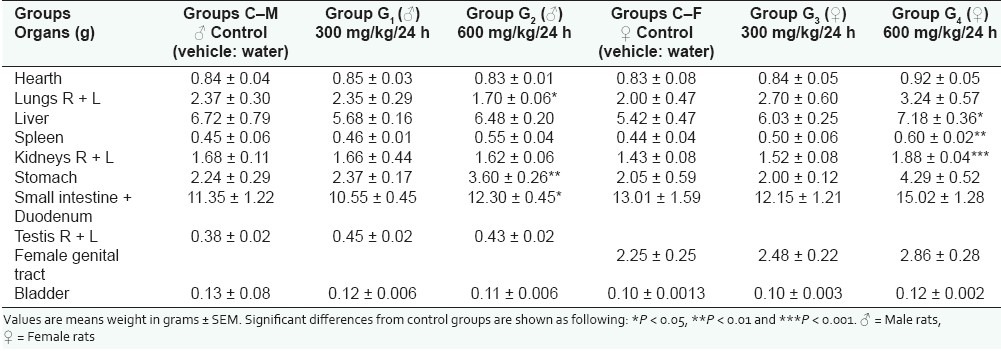
In female rats, the recorded modifications in comparison to the control group were observed in the group of animals treated at the daily dose of 600 mg/kg in which the studied drug induced a significant increase of liver weight (P < 0.05), of spleen weight (P < 0.01), of kidney weight (P < 0.001), and stomach weight (P < 0.05) [Table 1].
Results of hematological and biochemical investigations
Hematological investigations showed a polycythemia in the female group treated by the daily dose of 600 mg/kg (P < 0.5) [Table 2]. It has also been observed in this group of animals, compared to the control group, an increase of the rate of neutrophil granulocytes (P < 0.5). Likewise, in both female groups treated by the daily doses of 300 and 600 mg/kg, it has been recorded a significant lymphopenia (P < 0.05) [Table 2]. In the male groups, the only recorded modifications compared to the control group are represented by the diminution of the monocytes rate observed in both groups treated by the daily dose of 300 and 600 mg/kg (P < 0.01 and P < 0.05) [Table 2]. On the other hand, the results of biochemical analysis revealed in the male group treated by the drug at the daily dose of 600 mg/kg a significant hyperglycemia (P < 0.05) as well as a dose-dependent hyperlipemia when the animals are, respectively, treated by the daily doses of 300 and 600 mg/kg (P < 0.05 and P < 0.01) [Table 3]. Table 3 shows that the studied drug induced in female rats treated by the daily doses of 300 and 600 mg/kg a significant hypercholesterolemia compared to control group (P < 0.05) and a significant dose-dependent hyperparaprotidemia affecting more the males (P < 0.1) than the females (P < 0.5). Finally, enzymes dosage revealed a significant dose-dependent increase of ALP enzymes rates in both male and female animals (P < 0.5) [Table 2].
Table 2.
Effect of Globularia alypum L. on biochemical and hematological parameters
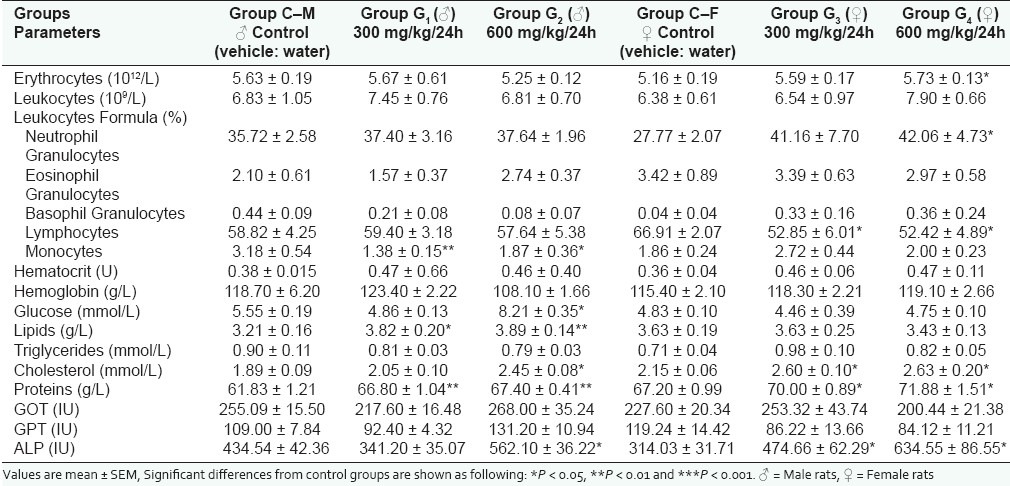
Table 3.
Results of histological examinations
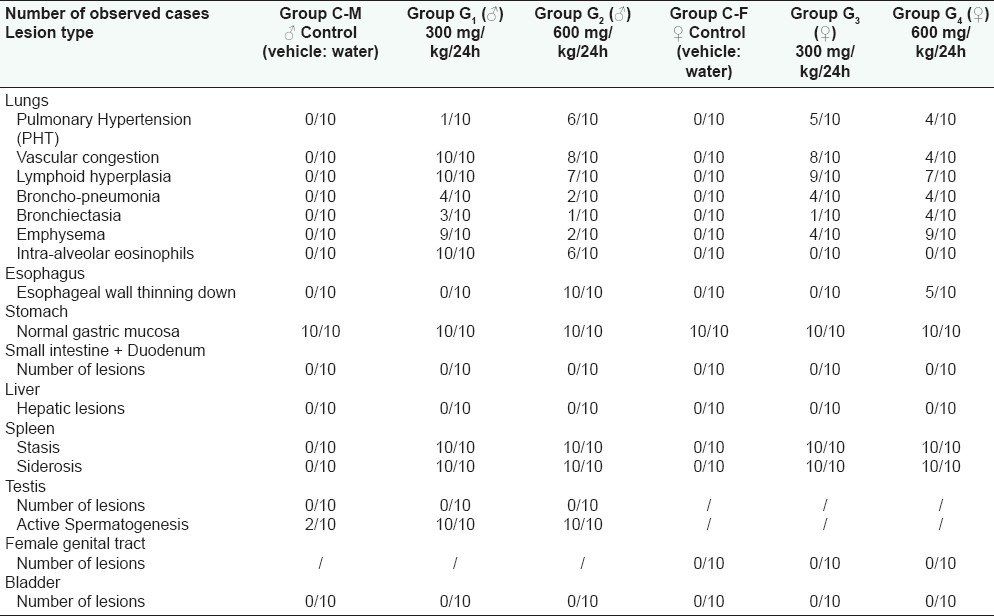
Results of histological examination
Histopathological examination of the organs removed at the end of the treatment revealed, in comparison with the control groups, the existence of important histological modifications induced by the chronic administration of the drug.
Lungs
Histological examination of this organ showed the existence of a pronounced thinning of pulmonary arteriolar wall meaning the presence of histological grades I to II pulmonary hypertension (PHT) [Figure 4a]. This lesion has been observed in about 50% of the treated females at the daily doses of 300 and 600 mg/kg whereas, in the males it only has been recorded when the animals were treated at the daily dose of 600 mg/kg affecting by this way 60% of them [Table 3]. Moreover, it has been observed in treated males that the intra-alveolar spaces were invaded by eosinophil granulocytes associated to a septa capillary congestion that would correspond to a beginning of a respiratory distress syndrome (ARDS) [Figure 4a]. It has to be noted that this observation was totally absent in the female rats. It has also to be pointed out that the observed vascular congestion was nearly constant in the all the groups of animals treated with G. alypum L. but with a lesser grade in the female group receiving a daily dose of 600 mg/kg. Furthermore, it has been observed an important lymphoid hyperplasia in all treated groups with a frequency varying between 70% and 100% [Table 3]. Finally, it has also been recorded a distrophic dilatation of pulmonary alveoli constituting emphysematous bullae affecting selectively males and females, respectively, receiving the drug at the daily dose of 300 and 600 mg/kg.
Figure 4.
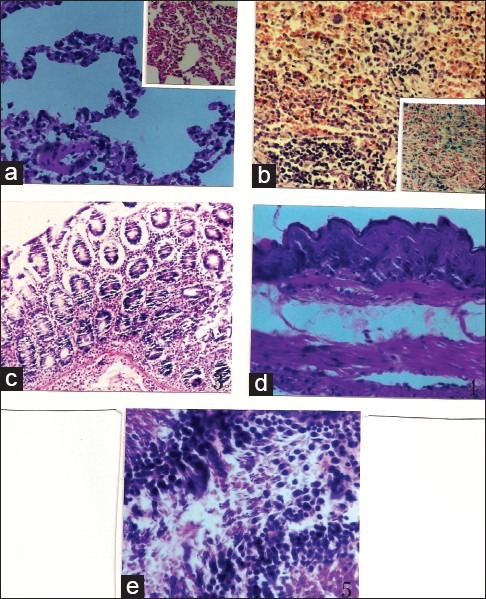
Results of histological examination (a) Histological aspect of lungs (H and E, × 230): emphysematous bullae and pronounced thinning of arteriolar wall (PHT) Inset: Septa capillary congestion and eosinophil granulocytes invasion, (b) Spleen (H and E, × 230): stasis and siderosis Inset: (H and E, × 230) Perls: blue coloration of hemosiderin, (c) Gastric mucosa (H and E, × 230): normal histological aspect, (d) Esophagus (H and E, × 230): thinning down of esophageal wall, (e) Testis (H and E, × 576): active spermatogenesis: seminiferous tubules invaded by spermatozoa
Digestive tract
Esophagus
When the drug was administered at the daily dose of 300 mg/kg, it did not affect the esophageal wall that appeared of normal aspect. In the opposite, when the drug is administered at the daily dose of 600 mg/kg, it has been noted a thinning down of the esophageal wall [Figure 4d]. If the mucous membrane remained intact under the effect of the drug, it was not the same for the muscular coat that became more atrophic. This lesion affected, respectively, 50% and 100% of treated female and male rats [Table 3].
Stomach and intestines
Histological aspect of gastric and intestinal mucosa was found strictly normal in all studied groups. It has notably not been observed any loss of substance nor inflammatory infiltrate or lymphoid hyperplasia [Figure 4c].
Liver
In all groups of animals, the histological architecture of the liver was found normal. Hepatocytes examination did not particularly reveal any necrosis or steatosis. Portal areas were without any particularity and Kupffer's cells were not modified. It has not also been observed any fibrosis or siderosis.
Spleen
In most observed cases, it has been observed that the normal histological structure of the spleen was respected. The white pulp was of variable size whereas the red pulp was congestive with stasis and siderosis [Figure 4b].
Kidneys
In general, the histological structure of the glomerulus was respected in all the observed groups and it has neither been observed membrane thinning nor hyperplasia of mesangial cells or deposits of immunoglobulins. The same applies for tubules that normal histological structure was well preserved.
Genital organs
Histological examination of female organs did not show any particularity. On the opposite, histological examination of the testis in the male rat demonstrated that, compared to the control that received the vehicle (water), G. alypum L. extract administrated at the daily doses of 300 and 600 mg/kg induced an active spermatogenesis. Indeed, observation of Table 3 and Figure 4e shows that 100% of treated animals display an active spermatogenesis with seminiferous tubules invaded par by spermatozoa. Following this observation, it has been decided to conduct another study by reducing the administered dose and the treatment period. In this respect, G. alypum L. aqueous extract has been administered at the daily dose of 100 mg/kg for 15 days to a group of 10 male rats weighing 198.13 ± 2.03 g. During the treatment period, a control group of 10 male rats weighing 199.43 ± 2.12 g received the vehicle (water). At day D16, all the animals were killed and the testis of experimental animals were removed for histological examination. Anatomopathological examination represented in Table 4 and Figure 5 showed that a very active spermatogenesis has been recorded in animals (100%) receiving the studied drug whereas, in the control group receiving the vehicle (water), only 20% and 50%, respectively, presented a very active and an active spermatogenesis. Moreover, a case of azoospermia has been recorded the control group [Figure 5a and b] whereas, in treated animals, examination of seminiferous tubules revealed that they were invaded by spermatozoa where all cell-lineages were represented in germinal epithelium. The same applies for epididymis that was revealed containing a significant amount of spermatozoa [Figure 5d and e].
Table 4.
Spermatogenesis study after administration by the oral route of Globularia alypum L. aqueous extract 100 mg/kg/24 h for 15 consecutive days to the male rat
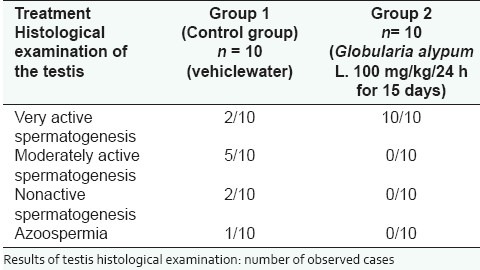
Figure 5.
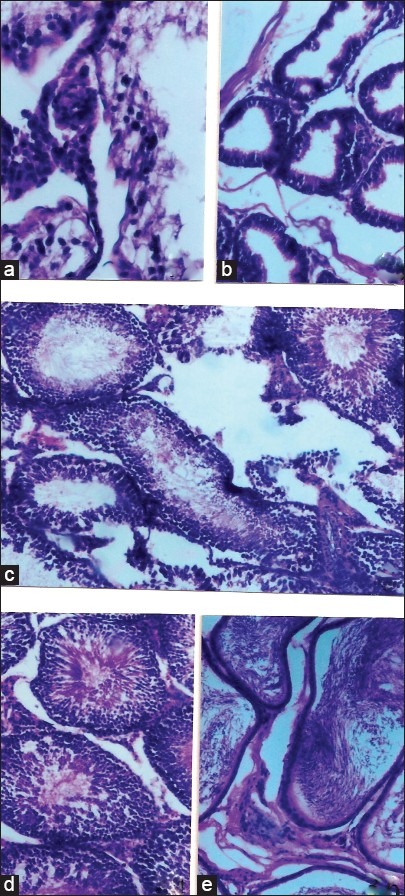
Spermatogenesis study. Results of histological examination. Control group (vehicle: water) (a) Testis (H and E, × 230): Quasi total absence of spermatozoa and germinal cells in seminiferous tubules, (b) Epididymis devoid of spermatozoa, (c) Non active spermatogenesis: approximately 50% of seminiferous tubules are devoid of spermatozoa, Group treated (G. alypum L. 100 mg/kg/24h for 15 days), (d) Testis (H and E, × 560): seminiferous tubules are invaded by spermatozoa and germinal epithelium contains all cell lineages, (e) Epididymis (H and E, × 560): rich in spermatozoa
DISCUSSION
The acute toxicity study showed that when G. alypum L. aqueous leaf extract is administered orally to female and male mice even for a dose of 10,000 mg/kg, it does not produce any death up to 14 following the unique administration of the product. This result may suggest that a very low toxicity of the drug when given orally.
Through an intra-peritoneal route, G. alypum L. aqueous leaf extract induced death in female and male mice which allowed quantification of LD50 as 2750 and 2550 mg/kg, respectively. This data may also confirm the low toxicity of the drug.
Since the drug was found to be very low toxic when given by orally route, it has decided to study its toxicity in male and female rats by using relatively high administered doses (300 and 600 mg/kg/24 h) for 30 days. No mortality was observed in treated animals from D1 to D30. However, daily clinical examination showed the appearance of liquid stools from D5 to D25 that were more pronounced in female than in male rats. This observation confirms the purgative and laxative properties related to the drug and reported by Balansard and Delphaut.[2]
This increase in defecation can also explain the impact of the drug on weight growth observed in treated animals and, in particular in females. In male rats receiving the daily dose of 300 mg/kg, the weight loss started at D7, lasted to D19 but, from this date, the weight growth curves of treated animals and control became super imposable and statistical analysis did not show any significant differences between the two groups (P > 0.05). At the same dose, the weight loss in females started from the beginning of the treatment (D2) and was significant compared to the control group and to the initial weight (P < 0.05). The impact of the drug on weight growth was more pronounced in females at the daily dose of 600 mg/kg as it induced a significant decrease of weight growth versus the initial weight from the beginning to the end of the treatment. The decrease of weight growth versus the initial weight in males treated at the daily dose of 600 mg/kg lasted to D22 but, from D23 to D30, no significant difference was observed between control and treated animals.
The effect of G. alypum L. aqueous leaf extract on the blood pressure and cardiac pulse rate was not dose-dependent; the most pronounced effect occurred when the drug was administered at the daily dose of 300 mg/kg. In male rats, the blood pressure increased from 130.11 ± 3.87 (D0) to 150.10 ± 2.46 mmHg (D30). This effect was followed by an increase of cardiac pulse rate from 352.00 ± 16.79 (D0) to 395.00 ± 11.67 pulse/min (D30). A biphasic phenomenon has been recorded in female rats: increase of blood pressure from 125.92 ± 5.95 (D0) to 147.14 ± 4.74 mmHg (D25) followed by a decrease of blood pressure to 135.83 mmHg from D25 to D30. The same applies for the cardiac pulse rate that enhanced from 366.41 ± 10.13 to 392.85 ± 17.03 pulse/min (from D0 to D25) and decrease to 350.10 ± 10.43 pulse/min min (from D25 to D30).
Anatomopathological examination of the principal organs showed that the daily-administrated doses (300 and 600 mg/kg) of the drug for 30 days did not have any significant impact on the histological structure of stomach, intestines, liver, glomerulus, tubules, bladder, and female genital tract. Moreover, with the exception of the red pulp that was found congestive with stasis and siderosis, in general, the normal histological structure of the spleen was preserved. The recorded toxic signs concern first of all the esophagus and occurred at high daily administered dose (600 mg/kg). Histological examination at this level revealed a thinning down of the esophageal wall and an atrophy of the muscular coat. This lesion was selectively more pronounced in male than in female rats. The second toxic effect of the drug was observed in the lung where histopathological examination demonstrated a pronounced thinning of pulmonary arteriolar wall responsible of PHT affecting selectively female than male rats. On the opposite, the observed intra-alveolar spaces eosinophil granulocytes invasion corresponding probably to a beginning of a ARDS selectively affected male than female rats. The drug also induced at this level a vascular congestion, an important lymphoid hyperplasia, and a dystrophic dilatation of pulmonary alveoli with nearly the same importance in all male and female treated animals.
However, the most important result of this study is the recorded active spermatogenesis induction following the reiterated administration of G. alypum L. aqueous leaf extract. Recorded active spermatogenesis was shown when the animals are treated at the daily doses of 300 and 600 mg/kg for 30 days but, also when the administrated dose and the period of treatment are reduced (100 mg/kg for 15 days). This active spermatogenesis induction may suggest that the plant extract could contain androgenic active substances responsible of the recorded active spermatogenesis. This argumentation is supported by the results of biochemical and hematological analysis. Taking into consideration the stimulant effect of androgens on erythropoiesis,[19] it is possible to suggest that the polycythemia, observed in the female group treated at the daily dose of 600 mg/kg, may result from a stimulation of erythropoietin, consecutive itself to the action of some active substances contained in the drug. Our argumentation is supported by the organs weight after autopsy results that showed in the same group of animals, compared to the control group, a significant increase of kidney weight (P < 0.001). Taking into consideration the fact that the erythropoietin source is practically exclusively renal in rat[20] and that androgens are described as possessing a trophy effect on kidneys,[21] it is therefore possible to conclude to an androgen-like action of G. alypum L. Two more results argue in favor of G. alypum L. androgenic properties. The first one is concerned by the recorded hyperprotidemia and hyperglycemia. Hyperprotidemia was observed in all treated groups with a more pronounced effect in males than in females whereas, recorded hyperglycemia was only significant in males treated with the daily dose of 600 mg/kg (P < 0.05). Since, on the one hand, androgens are known to possess anabolic effects by increasing protein synthesis from amino acids[22–24] and to cause glucose metabolism troubles with insulin resistance[25,26] on the other hand, it is therefore possible to attribute that the recorded active spermatogenesis could result from an androgenic effect induced by the drug. The second one is represented by the recorded dose-dependent hyperlipemia observed in males and the hypercholesterolemia that occurred in males and females. Although the mechanism of action of androgens on lipids metabolism is not totally elucidated, it is reported that androgens may cause dyslipidemia,[26] can activate adipocytes lipase[27] and increase the release of free fatty acids.[28] Moreover, it has been shown that some androgens cause changes in cholesterol levels by increasing LDL cholesterol and decreasing HDL cholesterol.[29] The recorded hypercholesterolemia observed in females receiving the daily doses of 300 and 600 mg/kg could be explained by the fact that the drug, inducing a significant secretion of androgens at the expense of estrogens, would annihilate the protective effect exerted by these hormones.
CONCLUSION
The study of acute toxicity performed on mice demonstrated that the toxicity G. alypum L. aqueous extract by oral route is very low (LD50 very high). A chronic toxicity study in rats at the doses of 300 mg/kg/24 hand 600 mg/kg/24 h during 30 days showed actual impact of the drug on growth weight, principal organs, biochemical, and hematological parameters. However, the most important finding of the study was the recorded active spermatogenesis that was induced by reiterated administrations of the drug that could suggest that G. alypum L. leaf extract contains active substances with androgenic properties which could be used in human therapy.
ACKNOWLEDGEMENTS
The authors are thankful to Anatomy and Pathologic Cytology Laboratory (Farhat Hached Hospital, 4000 Sousse, Tunisia) for histological examinations and A.M. Laboratory (35, Rue de Palestine, 1000 Tunis, Tunisia) for biochemical and hematological analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sezik E, Tabat M, Yesilada E, Honda G, Goto K, Ikeshiro Y. Traditional medicine in Turkey I. Folk medicine in Northeast Anatolis. J Ethnopharmacol. 1991;35:191–6. doi: 10.1016/0378-8741(91)90072-l. [DOI] [PubMed] [Google Scholar]
- 2.Balansard J, Delphant J. Globularia alypum L: A forgotten purgative. Rev Phytothér. 1948;12:213–34. [Google Scholar]
- 3.Caldes G, Prescot A, King JR. A potential antileukemic substance present in Globularia alypum L. Planta Med. 1975;27:72–6. doi: 10.1055/s-0028-1097763. [DOI] [PubMed] [Google Scholar]
- 4.Fehri B, Tebbett IR, Freiburger B, Karlix J. he immunosuppressive effects of Globularia alypum L. extract. Phytother Res. 1996;10:539–40. [Google Scholar]
- 5.Fehri B, Aiache JM. Effects of Globularia alypum L. on the gastrointestinal tract. J Nat Prod. 2010;3:141–6. [Google Scholar]
- 6.Ben Merabet K, Abed A. Some aspects of Algerian traditional pharmacopeia. Pharm Maghreb. 1982;2:18–28. Spécial. [Google Scholar]
- 7.Skim F, Kaaya A, Jaouhari JT, Lazrek BH, Jana M, El Amri H. Hypoglycaemic activity of Globularia alypum leaves in rats. Fitoterapia. 1999;70:382–98. [Google Scholar]
- 8.Jouad H, Maghrani M, Eddouks M. Hypoglycaemic effect of Rubus fructicosis L. and Globularia alypum L. in normal and speptozotocin-induced diabetic rat. J Ethnopharmacol. 2002;81:351–6. doi: 10.1016/s0378-8741(02)00118-6. [DOI] [PubMed] [Google Scholar]
- 9.Zennaki S, Krouf S, Taleb-Senouci D, Bouchenak M. Globularia alypum L. lyophilized methanolic extract decreases hyperglycemia and improves antioxidant status in various tissues of streptozotocin-induced diabetic rats. J Complement Integr Med. 2009;6:34. [Google Scholar]
- 10.Khlifi S, El Hachimi Y, Khalil A, Es-Safi N. In vitro antioxidant effect of Globularia alypum L. hydromethanolic extract. Indian J Pharmacol. 2005;37:227–31. [Google Scholar]
- 11.Es-Safi NE, Kollman A, Khlifi S, Ducrot PH. Antioxidative effect of compounds isolated from Globularia alypum L. structure-activity relationship. Lebenstittel-Wissenschaft Technol. 2007;40:1246–52. [Google Scholar]
- 12.Chokri A, Doukali K, El Abida K, Ben Cheikh R. Myorelaxant and spasmalytic effects of Globularia alypum L. extract on rabbit jejunum. Int J Pharmacol. 2010;6:608–15. [Google Scholar]
- 13.Skim F, Lazrek BH, El Amri H, Kaaya A, Jana M. Toxicological studies of Globularia alypum and Zygophyllium gaetulum in rats. Phytother Res. 1998;12:592–4. [Google Scholar]
- 14.Elbetieha A, Oran SA, Alkofahi A, Damani H, Raies AM. Fetotoxic potentials of Globularia arabica and Globularia alypum (Globulariaceae) in rats. J Ethnopharmacol. 2000;72:215–9. doi: 10.1016/s0378-8741(00)00246-4. [DOI] [PubMed] [Google Scholar]
- 15.Bernard P, Lallemand MM, Balansard G. Aromatic acids and the flavonoid compounds of the leaves of the globe daisy (Globularia alypum) Plant Med Phytother. 1974;8:174–9. [Google Scholar]
- 16.Chandhury RK, Sticher O. New iridoid glucosides and a lignan diglucoside from Globularia alypum L. Hel Chem Acta. 1981;64:3–15. [Google Scholar]
- 17.Fehri B, Lamaison JL, Petit-Jean-Freytet C, Karlix J, Tebbett IR. High performance liquid chromatography analysis of globularin in Globularia alypum L. Boll Chim Farm. 1997;136:250–2. [Google Scholar]
- 18.Litchfield JT, Wilcoxon FA. A simplified method of evaluating dose-effect. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 19.Freid W, Jonasson O, Lang G, Schwartz F. The hematologic effect of androgen in uremic patients. Study of packed cell volume and erythropoietin responses. Ann Intern Med. 1973;79:823–7. doi: 10.7326/0003-4819-79-6-823. [DOI] [PubMed] [Google Scholar]
- 20.Bondurant MC, Koury MJ. Anemia induces accumulation of erythopoietin in the kidney and the liver. Mol Cell Biol. 1986;6:2731–3. doi: 10.1128/mcb.6.7.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouvenot G, Lodovici F, Delboy C. Androgens, corticosteroids and erythropoiesis. Cah Med. 1973;14:1017–23. [PubMed] [Google Scholar]
- 22.Schroeder E, Vallejo A, Zheng L, Stewart Y, Flores C, Nakao S, et al. Six-weeks improvements in muscle mass and strength during androgen therapy in older men. J Gerontol A Biol Sci Med Sci. 2005;60:1586–92. doi: 10.1093/gerona/60.12.1586. [DOI] [PubMed] [Google Scholar]
- 23.Grunfeld C, Kotler D, Dobs A, Glesby M, Bhasin S. Oxandrolone in the treatment of HIV-associated weight loss in men: A randomized, double-blind, placebo-controlled study. J Acquir Immune Defic Syndr. 2006;41:304–14. doi: 10.1097/01.qai.0000197546.56131.40. [DOI] [PubMed] [Google Scholar]
- 24.Giorgi A, Weatherby RP, Murphy PW. Muscular strength, body composition and health responses to the use of testosterone enanthate: A double blind study. J Sci Med Sport. 1999;2:341–55. doi: 10.1016/s1440-2440(99)80007-3. [DOI] [PubMed] [Google Scholar]
- 25.Lee CH, Kuo SW, Hung YJ, Hsieh CH, He CT, Yang TC, et al. The effect of testosterone supplement on insulin sensitivity, glucose effectiveness, and acute insulin response after glucose load in male type 2 diabetics. Endocr Res. 2005;31:139–48. doi: 10.1080/07435800500320653. [DOI] [PubMed] [Google Scholar]
- 26.Prouteau S. Physiopathology of anabolic androgenic steroids abuse. Ann Med Psychol. 2008;166:838–42. [Google Scholar]
- 27.Tan KC, Shiu SW, Pang RW, Kung AW. Alterations in hepatic lipase and lipoproteins subfractions with transdermal testosterone replacement therapy. Clin Endocrinol. 1999;51:765–9. doi: 10.1046/j.1365-2265.1999.00882.x. [DOI] [PubMed] [Google Scholar]
- 28.Rebuffe-Scrive M, Marin P, Bjorntorp P. Effect of testosterone on abdominal adipose tissue in men. Int J Obes. 1991;15:791–5. [PubMed] [Google Scholar]
- 29.Tokar S. Liver damage and increased heart attack risk caused by anabolic steroid use. University of California - San Francisco. 2006. Feb, [Retrieved on 2011 Nov 24]. Available from: http://www.medicalnewstoday.com/releases/38069.php .


