Abstract
Background:
The methanol extract of Euphorbia hirta L (Euphorbiaceae), which is used in traditional medicines, was tested for in vivo toxicity.
Materials and Methods:
In vivo brine shrimp lethality assay and oral acute toxicity study at single high dose of 5000 mg/kg and observation for 14 days in mice were used to study the toxic effect of E. hirta.
Results:
Brine shrimp lethality assay was used to calculate the median lethal concentration (LC50) of E. hirta (for leaves, stems, flowers and roots) methanolic extracts at concentrations from 100 to 0.07 mg/ml. The LC50 values of 1.589, 1.420, 0.206 and 0.0827 mg/ml were obtained for stems, leaves, flowers and roots, respectively. Potassium dichromate (the positive control) had LC50 value of 0.00758 mg/ml. The acute oral toxicity study of the leaf extract resulted in one third mortality and mild behavioral changes among the treated mice. No significant statistical differences found between body weight, relative (%) and absolute (g) organ weights of treated and untreated groups (P> 0.05). Gross and microscopic examination of the vital organ tissues revealed no differences between control and treated mice. All the tissues appeared normal.
Conclusions:
E. hirta leaves methanol extract has exhibited mild toxic effects in mice.
Keywords: Acute oral toxicity, artemia salina, Euphorbia hirta, methanolic extract
INTRODUCTION
Plants have long been considered as valuable sources of medicines for treating a variety of diseases and ailments.[1] Most, if not all people, have used plants as curatives. New estimates suggest that, in many developing countries, a large number of the civilizations depend heavily on traditional practitioners and medicinal plants to meet their primary health care needs.[2,3] Despite the availability of modern medicine throughout all countries, herbal medicines have often kept popularity for only historical and cultural reasons. However, many people in developed countries have also begun to turn to alternative or complementary therapies, including medicinal plants.[4–6]
Euphorbia hirta L. belongs to the family Euphorbiaceae. It is a small annual herb common in tropical countries. It is frequently seen occupying open waste spaces, banks of watercourses, grasslands, road sides, and pathways.[7,8] E. hirta is a very popular herb amongst practitioners of traditional medicine, widely used as a decoction or infusion to treat various ailments including intestinal parasites, diarrhoea, peptic ulcers, heartburn, vomiting, amoebic dysentery, asthma, bronchitis, hay fever, laryngeal spasms, emphysema, coughs, colds, kidney stones, menstrual problems, sterility and venereal diseases. Moreover, the plant is also used to treat affections of the skin and mucous membranes, including warts, scabies, tinea, thrush, aphthae, fungal afflictions, measles, Guinea-worm and as an antiseptic to treat wounds, sores and conjunctivitis. The plant has a reputation as an analgesic to treat severe headache, toothache, rheumatism, colic pains and also pains during pregnancy. It is used as an antidote and pain relief of scorpion stings and snakebites. The use of the latex to facilitate removal of thorns from the skin is common.[9] The sedative, anxiolytic, analgesic, antipyretic and anti-inflammatory properties of E. hirta have been reported in the literature.[9] Leaf extract of E. hirta increased urine output and electrolytes in rats.[10,11] Furthermore, studies revealed that E. hirta possesses galactogenic, anti-anaphylactic, antimicrobial, antioxidant, anticancer, antifeedant, anti-platelet aggregation, anti-inflammatory, aflatoxin inhibition, antifertility, anthelmintic, antiplasmodial, antiamoebic, antimalarial, larvicidal, repellent and antifeedant activities against Plutella xylostella.[7]
Plant preparations which were medically useful should be non toxic or of low toxicity towards human cells. Therefore, it is necessary to carry out toxicity studies on medicinal plants even though they have been used for decades to determine acceptable from non-acceptable toxicity levels. In this study, for the first time, we evaluated the toxicity of E. hirta separated parts (leaves, stems, flowers and roots) which earlier in this study exhibited antibacterial, antifungal and antioxidant activities against common human pathogenic bacteria, Candida albicans and destructive free radicals. The in vitro Artemia salina L. (Artemiidae) nauplii (brine shrimp larvae) lethality bioassay was used for this purpose, while the Swiss albino mice were used for the in vivo evaluation of the acute oral toxicity. The acute oral toxicity testing was carried out on both sexes of mice according to the guidelines of Organization for Economic Cooperation and Development[12] and with the approval of animal ethics committee, Universiti Sains Malaysia.
MATERIALS AND METHODS
Plant materials
The fresh plant leaves, flowers, stem and root was harvested from various areas in Universiti Sains Malaysia, Penang, Malaysia in June 2009. The taxonomic identity of the plant was confirmed by the botanist of the School of Biological Sciences at Universiti Sains Malaysia. The plant materials were washed under tap water and separated into leaves, flowers, stems and roots. The separated parts were air dried in shade for ten days and then in an oven at 60°C for one to two days, before grinding to a fine powder using an electric blender and stored in clean labelled airtight bottles.
Preparation of the plant extract
Hundred grams of powder of each part was extracted by maceration in methanol (400 ml) for 14 days with frequent agitation. The mixture was filtered through clean muslin cloth followed by double filtration with Whatman No.1 filter paper and the filtrate was concentrated by rotary evaporation under vacuum (vacuum pressure: 500 N/m2) at 40°C until a volume of about 15 ml was reached. Next the concentrate was poured into glass Petri dishes and brought to dryness in an oven at 60°C. The percentage yield of the crude extract was determined for each part and was 11.1, 7.3, 4.7 and 4.1% for leaves, stems, flowers and roots, respectively. The obtained paste-like mass was then stored in parafilm sealed Petri-dishes in a dark cabinet. The extracts were reconstituted by dissolving in methanol to the required concentrations. The reconstituted extracts were maintained at 2-8°C. All plant part extracts were used for the brine shrimp lethality assay. Only leaves extracts were evaluated in vivo.
Brine shrimp lethality test
Toxicity was performed using the A. salina nauplii lethality assay developed by Meyer et al. with some modifications.[13] In this method, the eggs of the brine shrimp, A. salina cysts, were collected from local aquarium shop (George Town, Penang, Malaysia). One gram of A. salina cysts (brine shrimp eggs) was allowed to hatch and mature as nauplii in 1000 ml filtered artificial seawater (3.8% w/v salt in distilled water) for 48h at 25°C under continuous aeration and light regimen. Highly active nauplii were concentrated to a suitable density by placing an artificial light at one end of their incubation beaker and the nauplii rich water closest to the light was removed with a pipette for biological assays. Ten to fifteen nauplii were transferred to each of the two folds serially diluted test (extracts) solutions (100-0.07 mg/ml extract in 4 ml artificial seawater). Potassium dichromate (K2Cr2O7) prepared and serially diluted in artificial sea water to get concentrations between 1000-1.95 μg/ml served as positive control. Artificial sea water was used as negative control. After the 24h incubation at 25°C, a magnifying lens was used to count the number of dead and the mortality percentage was calculated. Larvae were considered dead only if they did not move their appendages for 10 s during observation. Mean percentage mortality was plotted against the logarithm of concentrations. The concentration killing fifty percent of the larvae (LC50) was calculated from the linear equation by taking the antilogarithm. Extract is considered bioactive only if LC50 is less than 1.0 mg/ml.[13]
Acute oral toxicity study
Animal
The experiment was conducted on 12 healthy Swiss albino mice (males and females) weighing 30 to 35 g and aged 8 to 10 weeks, acquired from the Animal House, Universiti Sains Malaysia. Those mice were randomly distributed into two groups, i.e., one treated and the other control groups. Each group consisted of 3 males and three females. The experimental procedures relating to the animals were authorized by Universiti Sains Malaysia Ethical committee (USM/ Animal Ethics Approval/ PPSG/07(A)/044/(2010)(59)) before starting the study and were conducted under the internationally accepted principles for laboratory animal use and care.
Toxicity test
The mice used in the experiment were randomly selected and marked on the tails for individual identification. Six mice of the same sex were kept in standard polypropylene cage. All cages were placed at room temperature with constant humidity. The room was controlled with cycles of 12 h of light and 12 h of darkness. The mice were adapted to the laboratory environment for one week earlier before starting the experiment. Tap water and a standard pellet diet were provided ad libitum (free access to food or water thereby allowing the animal to self-regulate intake according to its biological needs) throughout the experiment, except for the short fasting period where the drinking water was still in free access but no food supply was provided 12h prior to the treatment. The acute oral toxicity of E. hirta methanol crude extracts was evaluated in mice according to the procedures outlined by the Organization for Economic Co-operation and Development.[12] The extracts were suspended in methanol. Following the 12 h fasting period, body weight of the mice was determined and the dose was calculated in reference to the body weight. A single high dose of 5000 mg/kg at 10 ml/kg of crude extracts was administered to both three male and three female mice through the oral route using oral gavage (20 G). Other three male mice and three female mice were given distilled water and were regarded as the control groups. Food was provided to the mice approximately one hour after treatment. The mice were closely observed for any indications of toxicity effect within the first eight hours after the treatment period, and daily further for a period of 14 days. Surviving animals were weighed daily and visual observations for mortality, behavioral pattern changes such as weakness, aggressiveness, food or water refusal, diarrhea, salivation, discharge from eyes and ears, noisy breathing, changes in locomotor activity, clonic convulsion, coma, injury, pain or any signs of illness in each treated group were monitored carefully on daily basis throughout the experiment period.
Histopathology of the vital organs
All the vital organs (heart, kidneys, liver, lung and spleen) isolated from each individual mouse were fixed in 10% (v/v) formalin, routinely processed and embedded in paraffin wax. Paraffin sections of 5.0 μm thickness were cut using a rotary microtome, fixed onto glass slides and stained with haematoxylin and eosin for histological examination. The slides were examined by a histopathologist under (OLYMPUS BX50) compound light microscope provided with CCD camera and image capture software. The magnified images of the tissue structures were captured for further histopathological analysis.[14]
Organs and body weight statistical analysis
On the 15th day, all the surviving mice were sacrificed. Vital organs such as heart, kidneys, liver, lung and spleen were autopsied and examined macroscopically for any lesions or abnormalities. Body weight and weight of the organs from the control and the test groups were measured and recorded. The relative organ weight of each animal was then calculated as follows. Relative organ weight: (absolute organ weight × 100%)/ body weight of mice on the day of sacrifice.[15] All of the individual organs were observed macroscopically and their appearance was compared between both treated and control groups. Statistical analysis to assess the significant difference between both groups was conducted by running an independent sample t-test using SPSS (version 16.0) spreadsheet application. The level of significance used in this analysis was 5%.
RESULTS
Brine shrimp lethality
The crude methanol extract of flowers and roots of E. hirta showed positive result indicating that they are biologically active or toxic. Leaves and stems had less toxic effects on the brine shrimp nauplii. The lethality was found to be directly proportional to the concentration of the extract. 100% mortality was observed in the first six tubes with the highest concentrations (100, 50, 25, 12.5, 6.25, 3.125 mg/ml) of all the plant parts extracts. The concentrations that could kill 50% of the A. salina nauplii (LC50) obtained from E. hirta leaves, stems, flowers and roots extracts and that of the positive control, potassium dichromate, are presented in Table 1. LC50 values of 1.589, 1.420, 0.206 and 0.0827 mg/ml were obtained from stems, leaves, flowers and roots, respectively. Potassium dichromate had LC50 value of 0.00758 mg/ml. Nearly zero mortality was noticed in all the negative control tubes. Figure 1 shows a stereo microscopic image of a dead A. salina nauplii treated with the crude methanolic extract of E. hirta flowers for 24 h. No movement was seen in the dead nauplii appendages during the observation per.
Table 1.
LC50 of methanolic extract of Euphorbia hirta parts (values are expressed as an average of triplicates)

Figure 1.
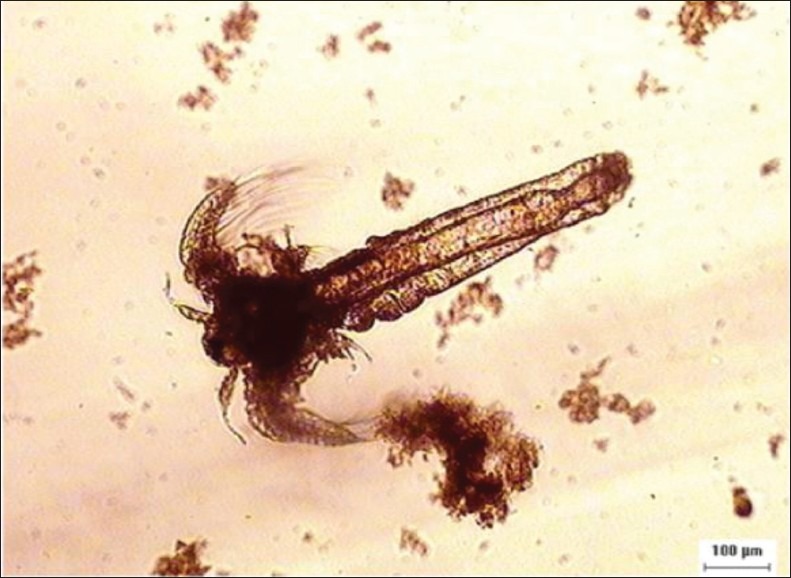
Stereo microscope image of Artemia salina nauplus after 24 hrs treatment with 100 mg/ml Euphorbia hirta flower extract
Acute oral toxicity study
The lethality and toxicity effect of the methanol extract of E. hirta leaves on the appearance and behavioral pattern of mice are shown in Tables 2 and 3, respectively. There were two deaths among the male mice; one was detected during the first 8 h and the second after 5 days of feeding the mice with the extract. During the 8h observation period, the extract treated animals were inactive, refused food and water and had increase in the breathing and heart beat. Thereafter, most animals became active and behaved normally. Generally, the male mice showed a more sensitive response to oral administration of E. hirta leaf extract. No significant changes in general appearance or behavioral pattern were noted till the end of 14 days. Normal weight gain was detected in the treated and the control groups. No statistically significant differences existed in the absolute (g) and relative weights (%) of almost all the isolated organs between the treated and the control mice (P > 0.05), Table 4. Only the female spleen relative weight index had probability value of less than 0.05. However, macroscopic examination of the vital organs didn’t reveal any abnormality [Figure 2]. Histopathologic analysis of the tissues showed that all the vital organs from the control and test groups were normal [Figure 3]. No inflammation, hemorrhage, fluid accumulation or any unfavourable changes were seen in the studied tissue sections. Few small foci of cellular swelling were noted in one of the dead males’ liver. However the damage was considered mild and non-specific, and could have been due to a number of aetiological factors such as toxic or immunologic insult.
Table 2.
Potential toxic effects of methanol leaf extract of Euphorbia hirta in mice

Table 3.
General appearance and behavioral observations for control and treated groups
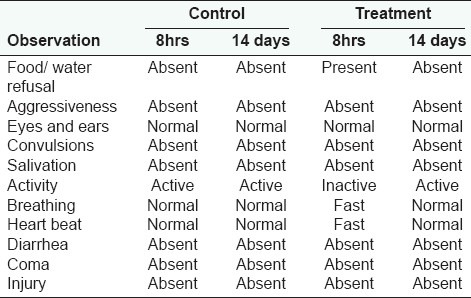
Table 4.
Effect of methanol leaf extract of Euphorbia hirta on the relative (%) and absolute (g) weights of organs (n = 3)
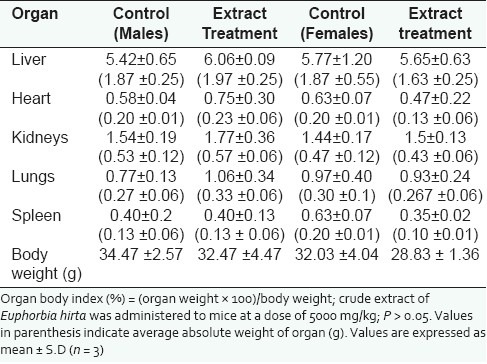
Figure 2.
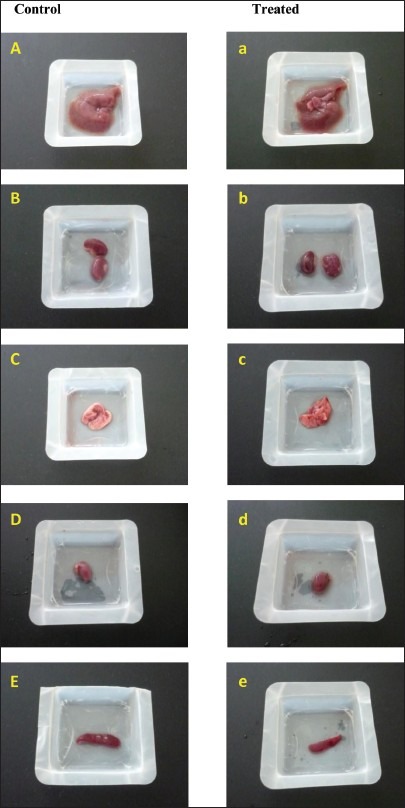
Gross observation of systemic organs (Liver (A, a), kidney (B, b), Lung (C, c), Heart (D, d) and spleen (E, e) from control and extract treated mice
Figure 3.
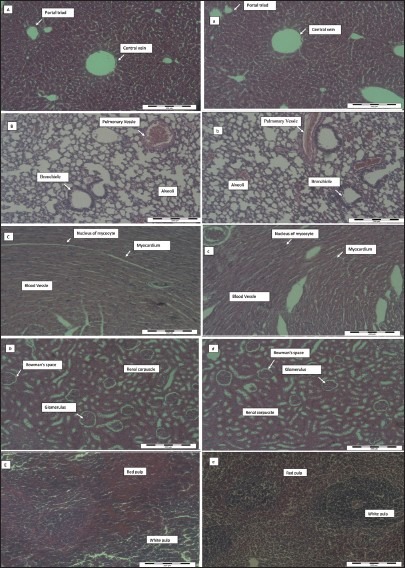
Histopathological analysis of the vital organs from control and Euphorbia hirta leaf extract treated mice at 5000 mg/kg body weight. Capital letters (A-E) indicate control liver, lung, heart, kidney and spleen, respectively, while small letters indicate the treated ones. There is no significant pathological in tissues from both groups
DISCUSSION
In the present study, we elucidated acute toxicity effects of E. hirta on behavior, organs body weight index and histopathology of liver, spleen, lung, heart and kidney of mice and A. salina. The brine shrimp (A. salina) cytotoxicity assay has been reported to be an efficient, inexpensive and relatively rapid way to detect toxic compounds, requiring only low amounts of sample, i.e., < 20 mg.[13,16] Use of higher animals to determine mortality is laborious and severely restricts the number of substances that can be tested.[17] The brine shrimp cytotoxicity assay has broadly been considered as a convenient method for preliminary assessment of toxicity and it has been used for the detection of fungal toxins,[18] food additives,[19] plant extract toxicity, heavy metals, cyanobacteria toxins,[20] pesticides,[21] and cytotoxicity testing of dental materials.[22,23]
The results on brine shrimps assay indicated that the E. hirta leaves and stems had LC50 values of more than 1.0 mg/ml; therefore, according to Meyer et al.[13] E. hirta leaves and stems might be nontoxic to human. This result is of great importance since, earlier in this study the leaves were shown to be the most active against the screened bacteria and Candida albicans and had the strongest antioxidant activities when measured by DPPH and ferric reducing power assays and also had the highest phenolic and flavonoids contents. In contrast, flowers and roots extracts had LC50 values less than 1000 μg/ml. Therefore, flowers and roots might be toxic to human. Roots extract was the most toxic with LC50 value (0.0827 mg/ml) but less toxic than that of the positive control potassium dichromate (0.00758 mg/ml). A. salina bioassay has previously been shown to be a good indicator of antitumor activity. Therefore, extracts toxic towards A. salina should also be tested for toxicity towards human tumor cell lines.[24]
So far no studies have been reported on the toxicity evaluation of E. hirta separated parts; most of the studies were on the whole plant. Adedapo et al.[25] reported that E. balsamifera, E. heterophylla, E. hirta, E. hyssopifolia, and E. lateriflora have toxic potentials. Khandoker et al.[26] showed that the E. hirta extract had moderate cytotoxic effect (LC50 37.07 μg.ml-1) against A. salina (brine shrimp nauplii) in respect to ampicillin trihydrate (LC50 16.87 μg.ml-1). Ethanolic extract of E. hirta leaves was also found to be a potent molluscicidal against L. acuminate snail with a lethal concentration of 20 mg/L at 24 h and 17 mg/l at 48 h.[27] In the light of above results, toxicity results from animals will also be necessary as a way to definitively judge the safety of this plant. Until that point, caution must be taken when consuming the plant.
Acute toxicity is usually defined as “the adverse change(s) occurring immediately or a short time following a single or short period of exposure to a substance or substances or as adverse effects occurring within a short time of administration of a single dose of a substance or multiple doses given within 24 h”. And the adverse effect is any effect that causes functional impairment and/or biochemical lesions which may affect the performance of the whole animal or that decrease the organ's ability to respond to additional challenges.[21] Consequently, a chemical that enters the organism via the oral route during a limited time and produces any adverse effect with little latency is orally and acutely toxic. It has been claimed that when properly performed and closely observed, an acute toxicity test can give more information about the biologic properties of a chemical compound than any other single test.[28]
In this study of acute oral toxicity, 12 Swiss albino mice from both sexes were used to observe the toxicity effects of methanol crude extract of E. hirta leaves. Both sexes were used for regulatory purposes. Studies have shown that mice give better prediction for human acute lethal dose compared to rats and therefore mice were used in this study.[14] The crude extract was administered orally; thus, the mice were fasted before taking the dose to avoid food and other chemicals in the digestive tracts from affecting the reaction(s) of the extract constituents. Generally, the oral route administration is the most convenient and commonly used route when studying acute toxicity. It costs less and is painless to the animal.[14] Therefore, no need for using anesthesia during the extract administration. On the other hand, the same route used by the traditional healers in treating their patients was used in the test animals. This would make any results in the mice easily translatable to what would be expected in the human. All the procedures were performed based on the appropriate OECD guideline.[12]
E. hirta has a long history of folkloristic use in the treatment of various ailments in almost every part of the world. The decoction of the flowering and fruiting plants is used in the treatment of respiratory tract infections and asthma, as well as against, chronic bronchitis, cough, pulmonary disorders and in removing worms in the bowel. Leaves infusion is prescribed to nursing mothers to increase lactation.[29] Because it is generally known that E. hirta is used to treat various ailments in humans and also there were no previous reports for any toxicity effects of the plant, it is assumed to be non toxic. Therefore in this limit dose oral acute toxicity test, a very high dosage level of 5000 mg/kg of crude extract was administered to the tested mice.[12]
In this study, one third mortalities were detected in the treated mice. Males were more sensitive to the extract. Less than 50% deaths were reported in this study, suggesting that the LD50 is higher than 5000 mg/kg. According to the following chemical labelling and classification of acute systemic toxicity based on oral LD50 values as recommended by the Organisation for Economic Co-operation and Development:[12] very toxic, < 5 mg/kg body weight; toxic, > 5 < 50 mg/kg; harmful, > 50 < 500 mg/kg; and no label, > 500 < 2000 mg/kg. LD50 of more than 5000 mg/kg of E. hirta methanol leaves extract is an indication that the extract is safe. However, lethal effect being found in this test indicated that the methanol crude extract of E. hirta had mild toxicity when given orally at 5g/kg body weight. Still the results of the current study agree with the wide use of this plant by traditional healers as traditional medicine since we used a very high dose of the crude extract that is equivalent to 300 g crude extract/60kg body weight or in a simpler way a plateful of the extract and this dose is much higher than what one is suggested to take to treat certain ailments. On the other hand, previous studies indicated that the aqueous extract of the antispasmodic principle of the plant was toxic to the mice, but the alcoholic extract was non toxic.[29] This indicates that there may be some toxic substance that can only be extracted in water and be responsible for the plant toxicity. Thus, when compared to previous studies, the methanolic extract is found more active and less toxic than water extract.
Treated mice showed hypoactivity during the first 24 h of the extract administration and this may be due to the sedative activity of the plant extract. Lanhers et al.[10,30] reported that extracts of E. hirta have been shown to have analgesic, anti-pyretic, anti-inflammatory and sedative effects in laboratory animals. The fast heart beat and breathing detected in the treated mice may be due to the vasodepressive activity of the plant. Hazelton and Hellerman[31] studied the effects of euphorbia on blood pressure and respiration in dogs and found that euphorbia administered intravenously resulted in a marked but fleeting vasodepression with little effect on respiration. On the other hand, treated mice refused water and food during the first day of extract administration due to the restlessness and sedative activity of the plant. Observation of food intake is important in the study of safety of a product with therapeutic purpose, since proper consumption of nutrients is crucial to the physiological status of the animal and to the accomplishment of the proper response to the substance tested instead of a false response due to improper nutritional conditions. Furthermore, the tested animals did not show any statistically significant changes in the body weight increment. Body weight alterations are indices of adverse effects of drugs and chemicals and it will be significant if the body weight loss occurred exceeds 10% from the initial body weight.[32] There was no change in body weight between control and treated groups. The body weight gain was similar in both treated and control groups. The differences were not statistically significant indicating that it did not have any adverse side effects on the body weight.
The absolute (g) and relative weights (%) of all the isolated organs (liver, kidneys, lungs, spleen and heart) between the treated and the control groups remained normal, indicating that the plant extract was non toxic in these vital organs. The organ weight is an important indicator of physiological and pathological state in human and animals. The relative organ weight is fundamental to diagnose whether the organ was exposed to the injury or not.[32] The heart, liver, kidney, spleen and lungs are the primary organs affected by metabolic reaction caused by toxicants.[33] The absolute organ weight has also been observed to be a relative sensitive indicator of nephrotoxicity for known nephrotoxicants. An increase in kidney weight, either absolute or relative, indicates nephrotoxicity.[34] The methanol leaf extract of E. hirta did not induce any toxic effect on the kidneys and the other organs going by this indicator, since the absolute and relative weights of the organs were not significantly different from control values.
There was agreement between the LC50 results obtained from brine shrimp lethality assay and the LD50 from the oral acute toxicity test in mice. LC50 was found to be 1.42 mg/ml and according to Meyer et al.,.[13] the plant may not be toxic since only LC50 values of less than 1.0 mg/ml are considered biologically active. On the other hand, LD50 was found to be more than 5000 mg/ml since the plant caused lethality of only one third of the tested animals. Our results are supported with previous studies which demonstrated that there is a good correlation (r = 0.85; P < 0.05) between the LC50 of the brine shrimp lethality assay and the LD50 of the acute oral toxicity assay in mice. Based on the correlation result; the brine shrimp LC50 < 10 μg/ml possesses LD50 between 100 and 1000 mg/kg; LC50 < 20 μg/ml possesses LD50 between 1000 and 2500 mg/kg, and LC50 > 25 μg/ml possesses LD50 between 2500 and 8000 mg/kg.[15] We can assume that the LD50 of oral acute toxicity for E. hirta leaves methanol extract also will be more than 2500 mg/kg, because the LC50 of brine shrimp lethality assay is 1420 μg/ml.
The low LC50 values obtained from the roots and flowers of E. hirta plant against A. salina may support the claim that many members of the spurge family, to which these plants belong, are poisonous to human and animals. This poisonous property is attributed to toxic compounds present in these plant parts. Thus, caution must be considered when using this plant and further work is needed to isolate and characterise the toxic compounds. On the other hand, the A. salina bioassay has previously been shown to be a good indicator of antitumor activity. It is suggested that the plant extract has a high variety of bioactive substances and may contain compounds that possess cytotoxicity effects. Therefore, extracts toxic towards A. salina are also suggested to be tested for toxicity towards human tumor cell lines.
CONCLUSION
Our present study is valuable since it could indicate about the toxic parts of the plant that may help to employ the plant as an antimicrobial or antioxidant agent. E. hirta leaves methanol extract was found to be fairly nontoxic when brine shrimp lethality assay was performed. While acute oral toxicity study showed mild toxic effects in mice. These results may primarily suggest E. hirta leaves to be consumed as an antimicrobial or antioxidant agent in known dosages, especially in poor rural communities, where conventional drugs are expensive and unaffordable. However, detailed experimental analysis on the sub acute and chronic toxicity is necessary for further support of this extract safety, and clinical trials are needed to be performed before any new phytotherapeutic agent from this plant can be generally recommended for use.
ACKNOWLEDGEMENTS
The authors acknowledge the Islamic Development Bank for the financial support to carry out this research and special thanks to Associate Prof. Dr. Gurjeet Kaur from INFFORM, USM for the histopathology examination of the tissue sections. Yuet Ping Kwan was supported by MyPhD fellowship from Ministry of Higher Education, Government of Malaysia, Malaysia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.da Costa JM, Campos AR, Brito SA, Pereira CB, Souza EO, Rodrigues FG. Biological screening of Araripe basin medicinal plants using Artemia salina Leach and pathogenic bacteria. Pharmacogn Mag. 2010;6:331–4. doi: 10.4103/0973-1296.71792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajazuddin, Saraf S. Evaluation of physicochemical and phytochemical properties of Safoof-E-Sana, a Unani polyherbal formulation. Pharmacognosy Res. 2010;2:318–22. doi: 10.4103/0974-8490.72332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalaivanan K, Pugalendi KV. Antihyperglycemic effect of the alcoholic seed extract of Swietenia macrophylla on streptozotocin-diabetic rats. Pharmacognosy Res. 2011;3:67–71. doi: 10.4103/0974-8490.79119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasheed N, Gupta VC. Standardization of a compound Unani herbal formulation “Qurs-e-Luk” with modern techniques. Pharmacognosy Res. 2010;2:237–41. doi: 10.4103/0974-8490.69115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhope SG, Nagore DH, Kuber VV, Gupta PK, Patil MJ. Design and development of a stable polyherbal formulation based on the results of compatibility studies. Pharmacognosy Res. 2011;3:122–9. doi: 10.4103/0974-8490.81960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnaraju AV, Rao VN, Sundararaju D, Vanisree M, Tsay HS, Subbaraju GV. Biological screening of medicinal plants collected from Eastern Ghats of India using Artemia salina (Brine Shrimp Test) Int J Appl Sci Eng. 2006;2:115–25. [Google Scholar]
- 7.Rajeh BM, Zuraini Z, Sasidharan S, Latha LY, Amutha S. Assessment of Euphorbia hirta L. leaf, flower, stem and root extracts for their antibacterial and antifungal activity and brine shrimp lethality. Molecules. 2010;15:6008–18. doi: 10.3390/molecules15096008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anonymous. Euphorbiahirta L. [Last Accessed on 2010 May 31]. Available at: http://florabase.calm.wa.gov.au/browse/profile/4629. 2008 .
- 9.Anonymous. Euphorbiahirta L. [Last Accessed on 2010 Apr 1]. Available at: www.pfaf.org/database/plants.php? Euphorbia hirta 2010 .
- 10.Lanhers MC, Fleurentin J, Dorfman P, Mortier F, Pelt JM. Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta. Planta Med. 1991;57:225–31. doi: 10.1055/s-2006-960079. [DOI] [PubMed] [Google Scholar]
- 11.Johnson PB, Abdurahman EM, Tiam EA, Abdu-Aguye I, Hussaini IM. Euphorbia hirta leaf extracts increase urine output and electrolytes in rats. J Ethnopharmacol. 1999;65:63–9. doi: 10.1016/s0378-8741(98)00143-3. [DOI] [PubMed] [Google Scholar]
- 12.OECD (Organization for Economic Co-operation and Development) Guideline for testing of chemicals: 2001, Acute oral Toxicity-Fixed Dose Procedure, No. 420 [Google Scholar]
- 13.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–4. [PubMed] [Google Scholar]
- 14.Syahmi AR, Vijayarathna S, Sasidharan S, Latha LY, Kwan YP, Lau YL, et al. Acute oral toxicity and brine shrimp lethality of Elaeis guineensis Jacq., (oil palm leaf) methanol extract. Molecules. 2010;15:8111–21. doi: 10.3390/molecules15118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahgal G, Ramanathan S, Sasidhara S, Mordi M, Ismail S, Mansor SM. Brine shrimp lethality and acute oral toxicity studies on Swietenia mahagoni (Linn.) Jacq. Seed methanolic extract. Phcog Res. 2010;2:215–20. doi: 10.4103/0974-8490.69107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solis PN, Wright CW, Anderson MM, Gupta MP, Phillipson JD. A microwell cytotoxicity assay using Artemia salina. Planta Med. 1993;59:250–2. doi: 10.1055/s-2006-959661. [DOI] [PubMed] [Google Scholar]
- 17.Prior MG. Evaluation of brine shrimp (Artemia salina) larvae as a bioassay for mycotoxins in animal feedstuffs. J Comp Med. 1979;43:352–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Harwig J, Scott P. Brine shrimp (Artemia salina L.) larvae as a screening system for fungal toxins. Appl Microbiol. 1971;21:1011–6. doi: 10.1128/am.21.6.1011-1016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerster HW, Schaeffer DJ. Brine shrimp (Artemia salina) nauplii as a teratogen test system. Ecotoxicol. Environ Safe. 1983;7:342–9. doi: 10.1016/0147-6513(83)90079-9. [DOI] [PubMed] [Google Scholar]
- 20.Jaki B, Orjala J, Bürji HR, Sticher O. Biological screening of cyanobacteria for antimicrobial and molluscicidal activity, brine shrimp lethality, and cytotoxicity. Pharm Biol. 1999;37:138–43. [Google Scholar]
- 21.Barahona MV, Sanchez-Fortu’n S. Toxicity of carbamates to the brine shrimp Artemia salina and the effect of atropine, BW284c51, iso-OMPA and 2-PAM on carbaryl toxicity. Environ Pollut. 1999;104:469–76. [Google Scholar]
- 22.Pelka M, Danzl C, Distler W, Petschelt A. A new screening test: toxicity testing of dental materials. J Dent. 2000;28:341–5. doi: 10.1016/s0300-5712(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 23.Milhem MM, Al-Hiyasat AS, Darmani H. Toxicity testing of restorative dental materials using brine shrimp larvae (Artemia salina) J Appl Oral Sci. 2008;16:297–301. doi: 10.1590/S1678-77572008000400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cock IE. Assessment of the toxicity of selected Australian native plant extracts using the Artemia franciscana nauplii bioassay. Internet J Toxicol. 2008;5:1–12. [Google Scholar]
- 25.Adedapo AA, Abatan MO, Olorunsogo OO. Toxic effects of some plants in the genus Euphorbia on haematological and biochemical parameters of rats. Vet Arhiv. 2004;74:53–62. [Google Scholar]
- 26.Khandoker ZH, Habib RM, Nikkon F, Rahman M, Karim RM. Antibacterial and Antineoplastic Effect of Root of Euphorbia hirta L. Drug Inven Today. 2009;1:10–2. [Google Scholar]
- 27.Sharma S, Singh T, Vijayvergia R. Molluscicidal activity of some medicinal plants. J Herbal Med Toxicol. 2009;3:155–7. [Google Scholar]
- 28.Walum E. Acute oral toxicity. Enviro Health Perspect. 1998;106:497–503. doi: 10.1289/ehp.98106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idu M, Obaruyi GO, Erhabor JO. Ethnobotanical uses of plants among the binis in the treatment of ophthalmic and ENT (Ear, Nose and Throat) ailments. Ethnobot Leaflets. 2008;13:480–96. [Google Scholar]
- 30.Lanhers MC, Fleurentin J, Cabalion P, Rolland A, Dorfman P, Misslin R, et al. Behaviord Effects of Euphorbia hirta L.: Sedative and Anxiolytic Properties. J Ethnopharmacol. 1990;29:189–98. doi: 10.1016/0378-8741(90)90055-x. [DOI] [PubMed] [Google Scholar]
- 31.Hazeleton LW, Hellerman RC. Studies on pharmacology of Euphobia pilulifera. J Am Pharm Assoc. 1948;37:491–7. [PubMed] [Google Scholar]
- 32.Vaghasiya YK, Shukla VJ, Chanda SV. Acute oral toxicity study of Pluchea arguta boiss extract in mice. J Pharmacol Toxicol. 2010;6:113–23. [Google Scholar]
- 33.Dybing E, Doe J, Groten J, Kleiner J, Brien JO, Renwick AG, et al. Hazard characterization of chemicals in food and diet: dose response, mechanism and extrapolation issues. Food Chem Toxicol. 2002;42:237–82. doi: 10.1016/s0278-6915(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 34.Swain SR, Sinha BN, Murthy PN. Subchronic toxicity studies of the hydroalcoholic extract of Rungia pectinata leaves. Pharmacologyonline. 2008;2:461–6. [Google Scholar]


