Abstract
Context:
There is a complex and significant correlation between respiratory disorders and psychiatric conditions. Reliability of self-reported tobacco use has been questioned in recent times.
Aims:
The current study aims at assessment of accuracy of self-reported tobacco use (both smoked and smokeless) among psychiatric out-patients.
Settings and Design:
We recruited 131 consecutive subjects from the out-patient psychiatry department of a tertiary care hospital.
Materials and Methods:
Male patients meeting the study criteria were approached for participation in the study. They were asked about their recent tobacco use history. Those reporting recent use were assessed for severity of dependence using Fagerstrom Test for Nicotine Dependence (FTND)-smoking and FTND-smokeless scales. Quantitative urine cotinine analysis was performed using the Enzyme Linked Immunesorbant Assay (ELISA) method. Based on this method, a (50 ng/ml) cut off score for urinary cotinine level for tobacco use was set.
Statistical Analysis Used:
Concordance between the self-report of tobacco use and urinary cotinine level was assessed using the Cohen's kappa. Additionally, Pearson's correlation coefficient was used to examine the correlation between the FTND-smoking and FTND-smokeless scales and the urinary cotinine levels.
Results:
The values of Cohen's kappa suggest no significant concordance between the self-reported recent tobacco use and urinary cotinine levels for both smoking and smokeless tobacco forms. The discordance was present irrespective of a higher (550 ng/ml) or a lower (50 ng/ml) cut off score for a urinary cotinine level. Pearson's correlation coefficient failed to reveal any significant direct correlation between the FTND scores and urinary cotinine levels.
Conclusions:
It is recommended to use biological markers such as urinary cotinine levels to corroborates the information provided by the patients.
KEY WORDS: Psychiatric illness, tobacco use, urinalysis
INTRODUCTION
There is a complex and significant correlation between respirator disorders and psychiatric conditions. An increased prevalence of respiratory disorders has been reported among patients with psychiatric illnesses.[1–3] It has been observed that psychiatric symptoms might be the first presenting symptoms for small cell lung cancer.[4] However, the physical illnesses including respiratory illness largely remain undetected and untreated. Alarmingly, low rates of 13% (psychiatric inpatients) and 8% (psychiatric outpatients) for physical examination have been reported among psychiatric patients.[5,6] Consequently, most of these conditions are likely to get undetected. Similarly, the rate of psychiatric morbidity is high among patients with lung cancer.[7] Mood and anxiety disorders are also prevalent among both adult and pediatric patients with asthma and severe lung diseases.[8,9]
Smoking rates have been found to be high among the patients with psychiatric illness. Epidemiological studies have found smoking rates among psychiatric patients to be twice that of general population.[10] Thus, a high rate of tobacco use has been implicated as a possible risk factor for respiratory disease in psychiatric patients.[11,12] Most of the literature on tobacco and psychiatric disorders is limited to cigarette smoking, and information on other forms of smoking is limited. Smokeless tobacco use remains understudied and under-researched in the association of respiratory disorders with psychiatric conditions, and it is tempting to speculate that this is due to the potential of oral/smokeless tobacco to adversely impact on respiratory disease in the first place. Nevertheless, tobacco use, smoking as well as smokeless, is the single largest preventable cause of death globally.[13]
The literature on accuracy of self-reported tobacco use among psychiatry patients is limited. Takeuchi et al. (2010) conducted a study on validity of self-report of tobacco use among patients with schizophrenia. In the absence of accurate and precise information on tobacco use, it is likely to get unnoticed and hence unmanaged among patients with psychiatric illness.[14]
The current study aims at assessment of accuracy of self-reported tobacco use (both smoked and smokeless) among male psychiatric out-patients.
MATERIALS AND METHODS
All the male subjects attending the psychiatry out-patient department of the tertiary care multispecialty teaching hospital constituted the sample frame for the current study. The subjects were recruited from the follow up OPD in order to ensure adequate participation of the subjects with regards to self-report. All male patients aged 18 or more coming for follow-up visit were approached for participation in the study. Those willing to participate and giving informed consent were included in the study. Subjects with significant cognitive impairment (MMSE score <24) or having a significant medical co-morbidity interfering with participation in the study were excluded from the study. A total of 175 consecutive male patients were approached for participation in the study. Five subjects were excluded as they were having significant cognitive deficit or significant medical co-morbidity. Finally, 131 consecutive male subjects (75% of all patients approached) were included in the study. Since this is the first reported study of its kind, it was left to the study group to arrive at the sample size. Each potential subject recruited in the study was to serve its own control using the urinalysis report. So it was decided to collect the study sample over a 2-month period. This was done with an aim to conduct a pilot study on this non-researched issue.
The study subjects were asked about their recent tobacco use during the past week. Active tobacco use was defined as daily use of tobacco products. Information was gathered for both smoking and smokeless forms of tobacco. Those reporting recent tobacco use were asked “How many cigarettes/biri/gutkha/etc do you use per day?”; “Which is your most preferred tobacco product?” Additionally, they were assessed for severity of dependence using the Fagerstrom Test for Nicotine Dependence (FTND) (smoking as well as smokeless). FTND-smoking is a widely used six-item questionnaire used to screen for severity of dependence on smoked tobacco.[15] FTND-smokeless (FTND-ST) is a nine-item instrument used to evaluate the level of nicotine dependence for smokeless tobacco.[16]
Subsequently 50 ml of urine samples were collected from each subject under close supervision and were submitted for laboratory analysis. Quantitative urinary cotinine was done by using Enzyme Linked Immunesorbant Assay (ELISA) kits of Calbiotech Inc., USA, which uses solid phase competitive ELISA. The assay was carried out as directed by the manufacturers. The detection limit of the cotinine assay was 2 ng/ml.
Data was analyzed using SPSS ver. 17. The concordance between the self-report of tobacco use and urinary cotinine level was assessed using the Cohen's kappa. Additionally, Pearson's correlation coefficient was used to find the correlation between the FTND-smoking and FTND-smokeless scales and the urinary cotinine levels.
There is no unanimity of appropriate cut off value of urine cotinine levels to screen for recent tobacco use. The recommended cut off values range from 50 to 550 ng/ml in different studies.[17,18] The Calbiotech ELISA method recommends a cut off value of >50 ng/ml.
The ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975 as revised in 1983 were followed. Conditions of anonymity and confidentiality were ensured throughout the conduct of the study.
RESULTS
A total of 131 male subjects attending the psychiatry out-patient department were recruited in the study. The mean age of study subjects was 31.05 (SD±11.66) years. Eighty-six (65%) of the subjects were from urban background and 70% were married. Self-reported recent use of cigarettes and biri was reported by 8 (6.1%) and 16 (12.1%) of the subjects, respectively. Self-reported use of smokeless tobacco products was by 13 (9.8%) for gutkha, 8 (6.1%) for tobacco powder, and 2 (1.5%) for khaini, respectively. The findings have been summarized in Figure 1. The mean FTND scores were 2.59 (SD±1.37) and 3.35 (SD±1.72) for smoking and smokeless forms.
Figure 1.
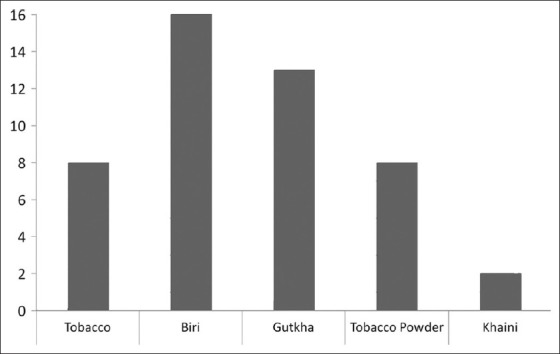
Self-reported use of different tobacco products by study subjects (smoking and smokeless)
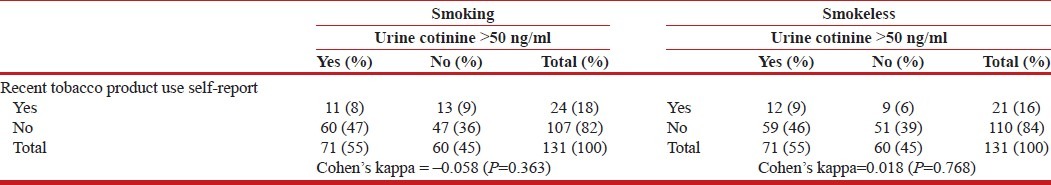
In order to assess the accuracy of self-reported recent tobacco use, an ELISA method for quantitative urinary cotinine level was used as biomarker. The values of Cohen's kappa suggest no significant concordance between the self-reported recent tobacco use and urinary cotinine levels for both smoking and smokeless tobacco forms [Table 1]. The discordance was present at the recommended cut off value of >50 ng/ml as recommended for the Calbiotech ELISA method.
Table 1.
Concordance between self.report of recent tobacco product use and urine cotinine levels (N=131)
There was a statistically significant negative correlation between the FTND-smoking form and urine cotinine levels (correlation coefficient=–0.59, P=0.02). However, no such correlation was observed between FTND-ST scores and urine cotinine levels (correlation coefficient=–0.08, P=0.75).
DISCUSSION
We recruited the subjects from follow-up psychiatry out-patient so that they could provide information about their tobacco use. The findings of the current study raise concerns about the reliance on the self-report for tobacco use among psychiatric out-patients.
There is a complex and significant correlation between respiratory disorders and psychiatric conditions. An increased prevalence of respiratory disorders has been reported among patients with psychiatric illnesses.[1–3] It has been observed that psychiatric symptoms might be the first presenting symptoms for small cell lung cancer.[4] A higher prevalence of paraneoplastic syndrome in this cancer type and higher metastasis rates to brain might be possible explanations for this association. A high rate of tobacco use has been implicated as a possible contributor to this association.[10,11] Cigarette smoking has been postulated to a common underlying factors for both respiratory illness and panic attacks among patients with these co-morbidities.[19]
However, the physical illness including respiratory illness largely remains undetected and untreated. Alarmingly low rates of 13% (psychiatric inpatients) and 8% (psychiatric outpatients) of physical examination have been reported among psychiatric patients.[5,6] Consequently, most of these conditions are likely to get undetected. An out-patient study from India reported the rate of undetected respiratory illness to be 15%, second only to hypertension.[20]
Similarly, the rates of psychiatric morbidity is high among patients with lung cancer.[7] Mood and anxiety disorders are also prevalent among both adult and pediatric patients with asthma and severe lung diseases.[8,9] Anxiety and depressive symptoms are predictive of poorer asthma management, associated functional impairment, and inferior treatment outcomes among asthma patients.[21,22]
The accuracy of self-report of tobacco use was found to be low in the current study when it was cross checked with urinary cotinine levels for both smoking and smokeless forms. Even among those reporting recent use of tobacco products, the FTND scores were not find to be directly correlated with the urinary cotinine levels. In fact, there was a negative correlation between the FTND-smoking scale scores and urine cotinine levels. Both the smoking and smokeless versions of FTND have been shown to be valid and reliable instruments for assessing tobacco dependence.[15,16] These findings suggest that psychiatric out-patients tend to under report recent use of tobacco products. Additionally, the severity of tobacco dependence (as estimates by FTND) does not correlate with the amount of tobacco consumed by these individuals.
The issue of reliability of self-report about tobacco use among psychiatric patients has not been studied adequately. Takeuchi et al. (2010) reported the first study of reliability of self-report of smoking among patients with schizophrenia.[14] The correlation between the self-reported smoking and breath CO levels was lost with an increase in duration of psychiatric illness. We could not come across any other study on this issue. We made use of urinary cotinine levels as a biomarker for recent tobacco use. Urine cotinine level has been recognized as a useful biomarker of recent tobacco use. Use of urinary cotinine levels (>50 ng/ml) to validate the self-report of tobacco use has been recommended in medical settings.[23]
Use of tobacco products by psychiatric patients is associated with a poor treatment response and worsening of the long-term course.[24] Additionally, it exposes these individuals to the harmful effects of tobacco. Tobacco use continues to be the single largest preventable cause of death globally. Tobacco use is a likely contributor to a relatively higher mortality rate seen among patients with psychiatric disorders.[25] Smoking has also shown to increase the requirement of neuroleptics.[26]
Co-morbid use of tobacco products by psychiatric patients is likely to get unnoticed if not assessed properly. Mentally ill receive tobacco treatment on only 12% of their visits to a psychiatrist and 38% of their visits to a primary care physician.[27]
A focus on the presenting axis I psychiatric illness could overshadow the tobacco use problem among these individuals. Use of biomarkers such as urinary cotinine level can help improve the recognition rate of recent tobacco use by these patients. This information would be of help while planning an appropriate management for them.
The current study has certain strengths. We used an objective biomarker in urine cotinine levels to corroborate the self-report. Additionally, we carried out a quantitative analysis of the urine cotinine levels. We analyzed the data using the two extreme cut off values of urinary cotinine levels. This was done keeping in mind use of different threshold for this value across studies.[17,18] However, the concordance rates were poor with both these cut-off values.
However, it is a pilot study with a relatively small sample size. Use of the study subjects as self-controls partly takes care of the issue of sample size. A conclusive sample size could not be estimated due to absence of prior work. The current study sample comprised of a heterogeneous group of different psychiatric disorders as we recruited a consecutive sample presenting to the out-patient psychiatry department. The issue needs to be studied among specific psychiatric illness groups. Also the findings need to be replicated in larger samples from different centers and settings. Additionally, we recruited only male subjects in the current study from a follow up out-patient setting. It would be interesting to compare the findings from female psychiatric patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kendrick T. Cardiovascular and respiratory risk factors and symptoms among general practice patients with long-term mental illness. Br J Psychiatry. 1996;169:733–9. doi: 10.1192/bjp.169.6.733. [DOI] [PubMed] [Google Scholar]
- 2.Bennett N, Dodd T, Flatey J. Health survey for England, London: Her magesty's stationery office. 1994 [Google Scholar]
- 3.Dickey B, Normand SL, Weiss RD, Drake RE, Azeni H. Medical morbidity, mental illness, and substance use disorders. Psychiatr Serv. 2002;53:861–7. doi: 10.1176/appi.ps.53.7.861. [DOI] [PubMed] [Google Scholar]
- 4.Benros ME, Laursen TM, Dalton SO, Mortensen PB. Psychiatric disorder as a first manifestation of cancer: A 10-year population-based study. Int J Cancer. 2009;124:2917–22. doi: 10.1002/ijc.24274. [DOI] [PubMed] [Google Scholar]
- 5.Bunce DF, Jones R, Badger IW. Medical illness in psychiatric patients: Barriers to diagnosis and treatment. South Med J. 1982;75:887–91. doi: 10.1097/00007611-198208000-00010. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre S, Romano J. Is there a stethoscope in the house (and is it used)? Arch Gen Psychiatry. 1977;34:1147. doi: 10.1001/archpsyc.1977.01770220029002. [DOI] [PubMed] [Google Scholar]
- 7.Fallowfield L, Ratcliffe D, Jenkins V, Saul J. Psychiatric morbidity and its recognition by doctors in patients with cancer. Br J Cancer. 2001;84:1011–5. doi: 10.1054/bjoc.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HF, Kunik ME, Molinari VA, Hillman SL, Lalani S, Orengo CA, et al. Functional impairment in COPD patients: The impact of anxiety and depression. Psychosomatics. 2000;41:465–71. doi: 10.1176/appi.psy.41.6.465. [DOI] [PubMed] [Google Scholar]
- 9.Limbos MM, Joyce DP, Chan CK, Kesten S. Psychological functioning and quality of life in lung transplant candidates and recipients. Chest. 2000;118:408–16. doi: 10.1378/chest.118.2.408. [DOI] [PubMed] [Google Scholar]
- 10.Atlanta: US Department of Health and Human Services; 2000. Reducing Tobacco Use: A Report of the Surgeon General. Available at www.cdc.gov/tobacco/sgr/sgr2000/index.htm . [Google Scholar]
- 11.Jeste DV, Gladsjo JA, Lindamer LA, Lacro JP. Medical comorbidity in schizophrenia. Schizophr Bull. 1996;22:413–30. doi: 10.1093/schbul/22.3.413. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Mora N, Medina O, Francisconi B, Meza NW, Rossi N, Colmenares F, et al. Risk factors for respiratory disease in chronic psychiatric in patients. Euro J Psych. 2007 [Google Scholar]
- 13.Murray C, Lopez A. Evidence-based health policy – Lessons from the global burden of disease study. Science. 1996;274:740–3. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi T, Nakao M, Shinozaki Y, Yano E. Validity of self-reported smoking in schizophrenia patients. Psychiatry Clin Neurosci. 2010;64:274–8. doi: 10.1111/j.1440-1819.2010.02082.x. [DOI] [PubMed] [Google Scholar]
- 15.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 16.Ebbert JO, Patten CA, Schroede DA. The Fagerström test for Nicotine Dependence-Smokeless Tobacco (FTND-ST) Addict Behav. 2006;31:1716–21. doi: 10.1016/j.addbeh.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorber SC, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11:12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 18.Zielińska-Danch W, Wardas W, Sobczak A, Szołtysek-Bołdys I. Estimation of urinary cotinine cut-off points distinguishing non-smokers, passive and active smokers. Biomarkers. 2007;12:484–96. doi: 10.1080/13547500701421341. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin RD, Pine DS. Respiratory disease and panic attacks among adults in the United States. Chest. 2002;122:645–50. doi: 10.1378/chest.122.2.645. [DOI] [PubMed] [Google Scholar]
- 20.Singh GP, Chavan BS, Kaur P, Bhatia S. Physical illnesses among psychiatric outpatients in a tertiary care health institution: A prospective study. Indian J Psychiatry. 2006;48:52–5. doi: 10.4103/0019-5545.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nouwen A, Freeston MH, Labbé R, Boulet LP. Psychological factors associated with emergency room visits among asthmatic patients. Behav Modif. 1999;23:217–33. doi: 10.1177/0145445599232002. [DOI] [PubMed] [Google Scholar]
- 22.Laszlo G, Nicholson EM, Denison J, Goddard PR. Adverse effect of previous bronchial asthma on disability in chronic airflow obstruction. Lancet. 2000;356:737–8. doi: 10.1016/S0140-6736(00)02636-2. [DOI] [PubMed] [Google Scholar]
- 23.Rebagliato M. Validation of self reported smoking. J Epidemiol Community Health. 2002;56:163–4. doi: 10.1136/jech.56.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodd S, Brnabic AJ, Berk L, Fitzgerald PB, de Castella AR, Filia S, et al. A prospective study of the impact of smoking on outcomes in bipolar and schizoaffective disorder. Compr Psychiatry. 2010;51:504–9. doi: 10.1016/j.comppsych.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen PB, Juel K. Mortality and causes of death in first admitted schizophrenic patients. Br J Psychiatry. 1993;163:183–9. doi: 10.1192/bjp.163.2.183. [DOI] [PubMed] [Google Scholar]
- 26.Salokangas RK, Saarijärvi S, Taiminen T, Lehto H, Niemi H, Ahola V, et al. Effect of smoking on neuroleptics in schizophrenia. Schizophr Res. 1997;23:55–60. doi: 10.1016/S0920-9964(96)00083-7. [DOI] [PubMed] [Google Scholar]
- 27.Hitsman B, Moss TG, Montoya ID, George TP. Treatment of tobacco dependence in mental health and addictive disorders. Can J Psychiatry. 2009;54:368–78. doi: 10.1177/070674370905400604. [DOI] [PMC free article] [PubMed] [Google Scholar]


