Abstract
Background:
Drug resistance is a major problem in the treatment of tuberculosis (TB). An estimate of drug resistance is extremely important in the epidemiology and control of TB. However, an assessment of the magnitude of drug resistance in TB is not very well described globally and data remains scantier for India. In view of this, we reviewed our data over last five years.
Materials and Methods:
Six hundred and seventy-three Mycobacterium tuberculosis isolates were subjected to drug susceptibility against primary anti-tuberculosis drugs by economic variant proportion method. All isolates resistant to isoniazid and rifampicin were taken as multi-drug resistant (MDR).
Results:
Out of the 673 strains tested, 95 (14.11%) showed monoresistance, 365 (54.23%) strains were found to be resistant to more than one drug. A total of 118 (17.53%) strains were found to be resistant to all the four drugs tested. MDR was seen with 320 (47.54%) isolates. This study observed maximum resistance with rifampicin (74.4%) followed by streptomycin (70.0%), isoniazid (53.2%), and ethambutol (21.7%).
Conclusion:
While this information may not reflect true prevalence of drug resistance in the region, this may help in further planning long term surveillance studies to know the trend of drug resistance in this area.
KEY WORDS: Drug resistance, Mycobacterium tuberculosis, Multi-drug resistance, MDR
INTRODUCTION
Tuberculosis (TB) is the leading cause of death from a curable infections disease. A disease caused by Mycobacterium tuberculosis, TB has affected mankind for over 5000 years, and still continues to be a leading cause of morbidity and mortality.[1] MDR-TB variety has been thrust into the forefront as a serious and life threatening illness in recent years. The advent of HIV/AIDS contributed to this substantially. Globally MDR-TB is a major challenge to programme managers.[1] Epidemiological studies for the assessment of local rates and the detection of MDR-TB are important to optimize drug therapy and prevent the dissemination of resistant strains in the community.[2] Although drug resistance in TB has been reported frequently during the last four decades, the available information from India is localized, inaccurate or incomplete.[3] In order to formulate a national treatment policy reliable and periodic updates on the prevalence of drug resistance for the entire country is needed, which would serve as an indicator of the transmission of drug resistant organisms as well as the efficacy of the National Tuberculosis Programme (NTP).[4] Sir J.J Hospital, Mumbai being one of the biggest tertiary care centers in the Western Maharashtra, India it would be worthwhile to analyze the drug resistance pattern of M tuberculosis isolates from our centre. Keeping this in mind we reviewed last five years’ drug sensitivity data of M. tuberculosis isolates.
MATERIALS AND METHODS
Over a period of five years, a total of 4148 specimens were received for culture for Mycobacteria. Out of this, 2163 constituted pulmonary specimens. M. tuberculosis grew in 903 specimens. Out of 903, 673 M. tuberculosis identified by conventional methods[5] were subjected to drug susceptibility testing against primary anti-tuberculosis drugs by economic variant of proportion method against Isoniazid: 0.2 μg/ml, Ethambutol: 2 μg/ml, Streptomycin: 4 μg/ml, and Rifampicin: 40 μg/ml.[6] All isolates resistant to isoniazid and rifampicin were taken as multi-drug resistant (MDR). This retrospective data analysis was done after obtaining necessary ethical permission from Institutional Ethical Committee.
Bacterial suspension was prepared by adding approximately 4 mg moist weight of a representative sample of the bacterial mass visualized as 2/3 loopful of 3 mm internal diameter 24 Standard Wire Gauge (SWG) wire loop into 0.2 ml of sterile distilled water (D.W.) in 7 ml Bijou bottle containing 2-3 mg glass beads. The suspension was vortexed for 30 sec to produce a uniform suspension. Distilled water, 3.8 ml sterile, was added to given suspension containing approx 1 mg/ml (S1). This suspension was kept on the bench to let the coarse particles settle down. The supernatant was decanted carefully into another clear, sterile MacCartney bottle. The opacity of the bacterial suspension was then adjusted by the addition of distilled water to obtain a concentration of 1 mg/ml of tubercle bacilli by matching with MacFarlands 1 standard. 0.2 ml of S1 was added to 1.8 ml of sterile D.W. to get (S2). To 0.2 ml of S2 1.8 ml of sterile D.W. was further added to get S3. Similarly S4 was made from S3. One standard loopful 3 mm diameter 27 SWG was inoculated onto drug-free as well as drug containing media. The standard strain of M. tuberculosis H37RV was tested with each new batch of medium. The slopes were then incubated at 37°C. Reading of the proportion test was taken on 28th day and again on 42nd day. The growth was recorded as +++ for confluent growth, ++ for more than 100 colonies, and for 1-99 colonies the actual number of colonies were counted. Interpretation of all tests is based on the 42-day readings. For each strain, express the number of organisms resistant to each drug concentration as a percentage of the number of organisms growing on the drug-free slope. The selection of slopes was made for estimating the growth on the drug-free and drug-containing media. Any strain showing ≥1% growth on drug-containing media than that of drug-free media was considered as resistant to that particular drug to that critical concentration. (Any strain with 1% - the critical proportion - of bacilli resistant to any of the four drugs - Streptomycin (S), Isoniazid (H), Rifampicin (R), and Ethambultol (E) was classified as resistant to that drug).
RESULTS
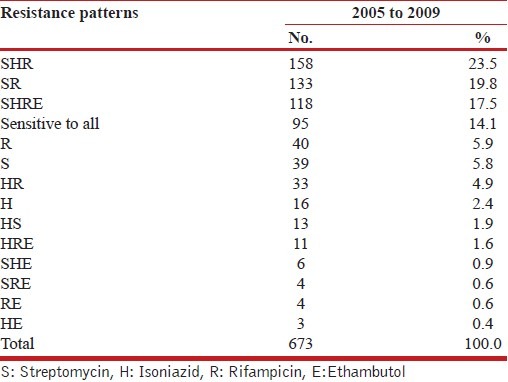
Out of the 673 M. tuberculosis isolates tested for drug sensitivity against first line drugs (S, H, R, E), 95 (14.1%) strains were found to be sensitive to all the four drugs. Ninety-five isolates (14.11%) showed resistance to a single drug. This type of resistance was seen with rifampicin, streptomycin and isoniazid. A total of 365 (54.23%) strains were found to be resistant to more than one drug. The commonest resistance pattern seen amongst the isolates was RHS (23.5%) followed by RS (19.8%). Remaining 118 (17.53%) isolates were found to be resistant to all the four drugs tested [Table 1].
Table 1.
Drug Resistance profiles (2005-2009)
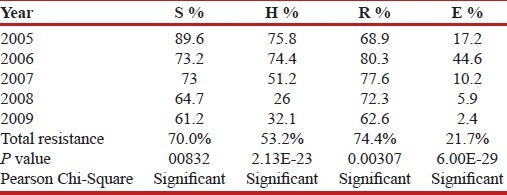
Further analysis was done to find out resistance to the individual drug. Total resistance was calculated as the sum total of resistance to that drug individually or in combination. This study observed maximum resistance with rifampicin (74.4%), followed by streptomycin (70.0%), isoniazid (53.2%), and ethambutol (21.7%) [Table 2].
Table 2.
Distribution of resistance to the drug (individually and in combination)
Isolates resistant to isoniazid and rifampicin were taken as MDR. Out of 673 strains tested, 320 (47.54%) strains were found to be MDR. Figure 1 shows the trend of MDR in these five years. In the initial years (2005-65.5%, 2006-68.3%, 2007-46.5%) the percentage of MDR strains was quite high. However, in the year 2008 and 2009 the study noted 22.7% and 25.2% of MDR, respectively.
Figure 1.
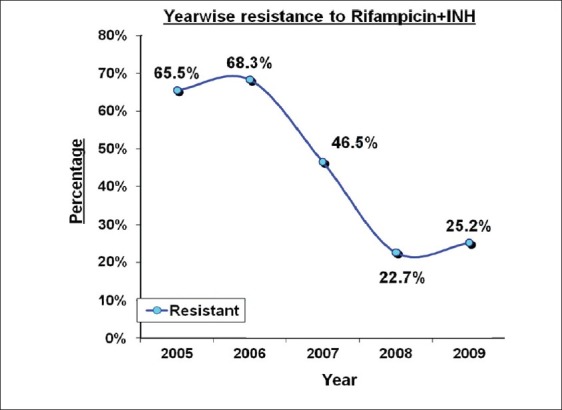
MDR (2005-2009)
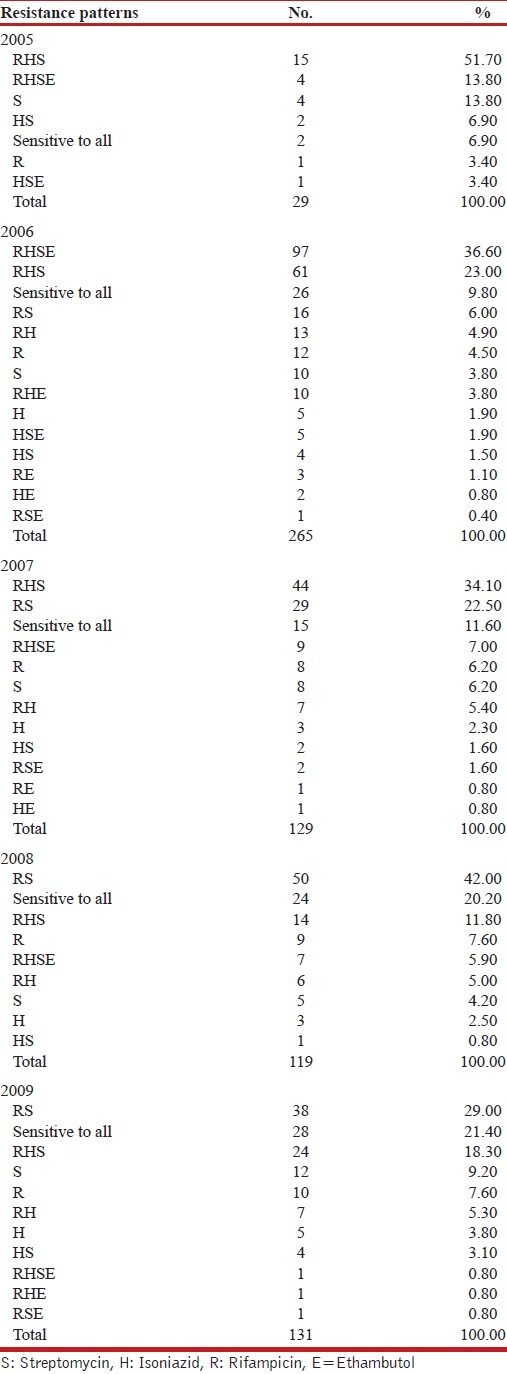
An attempt was made to analyze the resistance patterns observed in these five years, predominant resistance patterns noted were SHR (2005), SHRE (2006), SHR (2007), RS (2008 and 2009). The number of strains sensitive to all four drugs tested showed an increasing trend i.e. 6.9% to 21.4% [Table 3].
Table 3.
Drug Resistance profiles: 2005-2009
DISCUSSION
Anti-TB drugs are a two-edged sword while they destroy pathogenic Mycobacterium tuberculosis, they also select for drug resistant bacteria against which those bugs are then ineffective.[7] A review by WHO of a series of 63 surveys of drug resistant TB carried out between 1985-1994 led to the conclusion that the problem of drug resistance was global.[8] The pattern of drug resistance varies from place to place and at different periods of time. It is important to know the drug resistance pattern in that area to formulate an effective drug regime. With this in mind, we have reviewed our drug sensitivity data of Mycobacterium tuberculosis isolates. The study sample constituted strains of M. tuberculosis isolated from pulmonary tuberculosis cases over a period of five years (2005-2009). It included both inpatients and out patients.
Out of 673 M. tuberculosis isolates tested for drug sensitivity against first line drugs (S, H, R, E), 578 (85.9%) strains were found to be resistant to either one or more drugs [Table 1]. The prevalence of primary/initial drug resistance observed in different studies from India was found to be about 18.8% (7.9% - 27.1%).[9–15] Similarly, the prevalence of acquired drug resistance ranged from 25 to 100% in Indian studies.[16–20] These studies were conducted in different institutes and tertiary care centers. Even though these studies do not really reflect the overall status of drug resistance problem in India, but the message from these studies is clear that there is high drug resistance seen with Mycobacterium tuberculosis. Our study also noted high level of drug resistance (85.9%). However, in the present study, no such clear distinction between primary and acquired resistance was possible.
Fourteen different drug resistance patterns were observed after testing 673 strains of M. tuberculosis [Table 1]. The commonest pattern observed was SHR. 578 (85.9%) strains were found to be resistant to more than one drug. In previously treated cases, the proportion of strains resistant to three or four drugs was significantly greater than among new cases. This relationship was found globally as well regionally and suggested amplification of resistance.[4] Resistance against two or three drugs are difficult to treat and often result in treatment failure.[21]
Further analysis was done to find out the total resistance to the individual drug (i.e., resistance to drug alone or in combination with other drug). Analysis of almost 90,000 strains from countries between 1994 and 2002 confirmed that, globally, more strains were resistant to isoniazid than to any other drug (range 0-42%). In general, isoniazid and streptomycin resistance was more prevalent than rifampicin or ethambutol resistance.[4] However, the present study noted maximum resistance with rifampicin (74.4%) followed by streptomycin (70.0%), isoniazide (53.2%), and ethambutol (21.7%) [Table 2]. Deodhar et al.,[22] also from Mumbai reported 58.55% drug resistance to rifampicin 46.95% to streptomycin, 30.41% to isoniazid, and 3.67% to ethambutol. There is also high incidence of rifampicin resistance reported from Mumbai by Chougule et al.[23] Trivedi and Desai form Gujarat (India) reported acquired drug resistance to isoniazid, rifampicin, and streptomycin as 55.8%, 37.3%, and 26.9%, respectively. Jesudason et al.,[24] (Vellore, Tamil Nadu) reviewed their data of past 20 years (1980-2000) and found that there had been a spurt in 2000 in resistance to isoniazid, streptomycin, and rifampicin. Rifampicin is potent bactericidal and sterilizing drug and hence the most important drug in DOTS program. Resistance to rifampicin may lead to the failure of DOTS program.[25] It is also said that isoniazid and streptomycin are the main gateways to acquisition of additional resistance.[4] In a study by Liu et al.,[26] single drug resistance to isoniazid and streptomycin was most common. Amiri et al.,[27] from Tehhran-Iran reported maximum resistance to streptomycin (85%) and the lowest resistance to etambutol (56%). Even the present study noted the lowest resistance (21.7%) to ethambutol.
Among the drug resistant isolates, there was a decrease in prevalence of resistance to each drug tested when trends were analyzed over the five years period. This decrease was found to be statistically significant [Table 2]. We tried to analyze further the drug resistance pattern is changing year wise [Table 3]. As shown in the Table 3 in the year 2005 and 2007 the commonest pattern seen was SHR. In the year 2006, majority of the strains were resistant to all the four drugs tested. While in the year 2008 and 2009 the trend remains to be same, i.e., majority of the strains were found to be resistant to rifampicin and streptomycin. This analysis of patterns also showed that the number of strains sensitive to isoniazid was increasing so also the strains sensitive to all four drugs.
MDR has been a topic of growing interest and posing threat to control of TB. Current estimates report, the prevalence of primary and acquired MDR in India as 3.4% and 25%, respectively.[9] Figure 1 shows the trend of MDR (year wise) in the present study. The percentage of MDR strains was quite high in the initial reasons (2005-65.5%, 2006-68.3%, 2007-46.5%). One of the main reasons could be that in the initial years the DST was carried out only in selected and complicated cases which were not responding to the treatment. Afterwards the DST was introduced almost on regular basis. In the years 2008 and 2009 the percentage of MDR strains was 22.7% and 25.2%, respectively.
In 1994, WHO - IUATLD carried out a surveillance which concluded that the problem of MDR-TB is global; the median prevalence of primary and acquired MDR was 4% (0-14.4%) and 13% (0-54.4%), respectively. A second WHO-IUATLD global project on drug surveillance carried out in 1996-1999 in 58 countries found that the median primary and acquired MDR was 1% (0-14%) and 9% (0-48%), respectively.[9] The total prevalence of initial multi-drug resistance in India varied between 0-5%.[9,12–15] The prevalence of acquired MDR ranged from 6-100%.[9,18–20] The crisis of MDR-TB in Mumbai has been well documented.[28] Multi drug resistance occurring primarily as a consequence of poor treatment services could lead to emergence of XDR-TB if MDR-TB is not managed properly. There were an estimated 0.5 million cases of MDR-TB in 2007. The countries that ranked first to fifth in terms of total numbers of MDR-TB cases in 2007 were India (131 000), China (112 000), the Russian Federation (43 000), South Africa (16 000) and Bangladesh (15 000). By November 2009, 57 countries had reported at least one case of XDR-TB.[1]
CONCLUSION
Routine surveillance of drug resistance profiles found in specific populations of patients provides information that is useful for adapting strategies for effective retreatment within NTPs. One important limitation of this study is that previous treatment histories, demographies and other patient data were not available for analysis, restricting our ability to derive concrete conclusions. Finally it is beyond the scope of this study to demonstrate any association between the observed increase or decrease in resistance to specific drug and any specific cause. Whether the increase in resistance resulted from failure of previous empiric treatment regimen, or from some other cause, remains to be determined. However, larger drug sensitivity data with uniform testing methodologies with vigorous quality assurance needs to be generated to formulate the new drug policies.
ACKNOWLEDGEMENTS
The authors would like to thank the Dept. of Chest and TB, Sir J. J. Hospital and G. T. Hospital, Mumbai for providing the clinical material and the details of the patient. Authors would also like to acknowledge help of Dr. Abhiram Kasbe, Associate Professor, P.S.M., T.N. Medical College, Mumbai for statistical analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Central TB Division Directorate General of Health Services. Ministry of Health and Family Welfare Nirman Bhawan. New Delhi: [cited in 2010]. TB. Burden of the disease in India. TB India 2010: RNTCP Status report 2010. Available from: http://www.tbcindia.org . [Google Scholar]
- 2.Surucuoglu S, Ozkutuk N, Celik P, Gazi H, Dinc G, Kurutepe S, et al. Drug resistant pulmonary tuberculosis in western Turkey: Prevalence, clinical characteristics and treatment outcome. Ann Saudi Med. 2005;25:313–8. doi: 10.5144/0256-4947.2005.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paramasivan CN. An overview of drug resistant tuberculosis in India. Indian J Tuberc. 1998;45:73–81. [Google Scholar]
- 4.Paramasivan CN, Venkataraman P. Drug resistance in tuberculosis in India. Indian J Med Res. 2004;120:377–86. [PubMed] [Google Scholar]
- 5.Baron EJ, Peterson LR, Finegold SM. Bailey and Scott's Diagnostic Microbiology. 9th ed. St Louis: The CV Mosby Company; 1994. [Google Scholar]
- 6.Venkatraman P, Paramasivan CN. Bacteriological methods in laboratory diagnosis of tuberculosis. Chennai: TRC(ICMR); 1999. pp. 33–7. [Google Scholar]
- 7.Johnson R, Streicher EM, Louw GE, Warren RM, van Helden PD, Victor TC. Drug resistance in Mycobacterium tuberculosis. Curr Issues Mol Biol. 2006;8:97–111. [PubMed] [Google Scholar]
- 8.Cohn DL, Bustreo F, Raviglione MC. Drug resistant tuberculosis: Review of the worldwide situation and the WHO/IUATLD Global Surveillance Project: International Union against Tuberculosis and Lung Disease. Clin Infect Dis. 1997;24(Suppl 1):S121–30. doi: 10.1093/clinids/24.supplement_1.s121. [DOI] [PubMed] [Google Scholar]
- 9.Prasad R. MDR TB: Current status. Indian J Tub. 2005;52:121–31. [Google Scholar]
- 10.Espinal MA, Laszlo A, Simonsen L, Boulahbal F, Kim SJ, Reniero A, et al. Global trends in resistance to anti-tuberculosis drugs: World Health Organization-International Union against tuberculosis and lung disease working group on anti-tuberculosis drug resistance surveillance. N Engl J Med. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. [DOI] [PubMed] [Google Scholar]
- 11.Prevalence of drug resistance in patients with pulmonary tuberculosis presenting for the first time with symptoms at chest clinics in India, I: Findings in urban clinics among patients giving no history of previous chemotherapy. Indian J Med Res. 1968;56:1617–30. [PubMed] [Google Scholar]
- 12.Jena J, Panda BN, Nema SK, Ohri VC, Pahwa RS. Drug resistance pattern of Mycobacterium tuberculosis in a chest disease hospital of armed forces. Lung India. 1995;13:56–9. [Google Scholar]
- 13.Paramasivan CN, Venkataraman P, Chandrashekhran V, Bhatt S, Narayanan PR. Surveillance of drug resistant in tuberculosis in two districts of south India. Int J Tuberc Lung Dis. 2002;6:479–84. doi: 10.5588/09640569512977. [DOI] [PubMed] [Google Scholar]
- 14.Sphia V, Balasangameshwara VH, Jagannatha PS, Kumar P. Initial drug resistant among tuberculosis patients under DOTS programme in Bangalore city. Indian J Tuberc. 2004;52:17–21. [Google Scholar]
- 15.Mahadeo B, Kumar P, Agrawal SP, Chauhan LS, Sriknataramu N. Surveillance of drug resistant to anti-tuberculosis drugs in districts of Hoogli in West Bengal and Mayurbhanj in Orissa. Indian J Tuberc. 2005;52:5–10. [Google Scholar]
- 16.Prevalence of drug resistance in patients with pulmonary tuberculosis presenting for the first time with symptoms at chest clinics in India II. Findings in urban clinics among all patients, with or without history of previous chemotherapy. Indian J Med Res. 1969;57:823–35. [PubMed] [Google Scholar]
- 17.Trivedi SS, Desai SC. Primary anti tuberculosis drug resistance and acquired Rifampicin resistance in Gujarat, India. Tubercle. 1988;69:37–42. doi: 10.1016/0041-3879(88)90038-4. [DOI] [PubMed] [Google Scholar]
- 18.Jain NK, Chopra KK, Prasad G. Initial and acquired isoniazid and rifampicin resistance to M. tuberculosis and its implications for treatment. Indian J Tuberc. 1992;39:121–4. [Google Scholar]
- 19.Chowgule RV, Deodhar L. Pattern of secondary acquired drug resistance to anti tuberculosis drug in Mumbai, India - 1991-1995. Indian J Chest Dis Allied Sci. 1998;40:23–31. [PubMed] [Google Scholar]
- 20.Deivanayagam CN, Rajasekaran S, Venkatesan R, Mahilmaran, Ahmed PR, Anniadurai S. Prevalence of acquired MDR-TB and HIV co-infection. Indian J Chest Dis Allied Sci. 2002;44:237–42. [PubMed] [Google Scholar]
- 21.Worls Health Organization. Guidelines for the management of drug resistant tuberculosis. WHO. 1997. [cited in 1997]. Available from: http://whqlibdoc.who.int/hq/1997/WHO_TB_96:210_(Rev1).pdf .
- 22.Deodhar L, Priya M, Sweta C. Drug resistance in tuberculosis. Bombay Hosp J. 1999. [cited in 1999]. Available from: http://www.bhj.org/journal/1999_4103_july99/original_253.htm .
- 23.Chowgule RV, Deodhar L. Pattern of secondary acquired drug resistance to anti tuberculosis drug in Mumbai, India–1991-1995. Indian J Chest Dis Allied Sci. 1998;40:23–31. [PubMed] [Google Scholar]
- 24.Jesudason MV, Mukundan U, Saaya R, Vanitha K, Lalitha MK. Resistance of Mycobacterium tuberculosis to the first line anti tubercular drugs: A twenty year review. Indian J Med Microbiol. 2003;21:127–8. [PubMed] [Google Scholar]
- 25.Huq AKMS, Khan ABMBR, Sabur SAMA, Azad A, Miah NU. Study of case finding for pulmonary tuberculosis in outpatient departments of general hospitals Bangladesh. Chest Heart Bull. 1995:25–30. [Google Scholar]
- 26.Liu Y, Jiang G, Zhao L, Fu Y, Li Y, Bi Z, et al. Drug resistance of Mycobacterium tuberculosis in a nationwide epidemiological survey in China in the year of 2000. Zhonghua Je He Hu Xi Za Zhi. 2002;25:224–7. [PubMed] [Google Scholar]
- 27.Amiri MV, Mirsaeidi SA, Mohajer K, Mansoori SD, Tabarsi P, Masjedi R. Pattern of drug resistance in pulmonary TB patients. Tanaffos. 2003;2:47–51. [Google Scholar]
- 28.Udwadia ZF. India's multidrug-resistant tuberculosis crisis. Ann N Y Acad Sci. 2001;953:98–105. doi: 10.1111/j.1749-6632.2001.tb11365.x. [DOI] [PubMed] [Google Scholar]


