Abstract
The pandemic of swine flu (H1N1) influenza spread to involve the whole world rapidly. Many patients manifested a mild clinical illness but some developed pneumonia and respiratory failure. High mortality was observed in patients with severe disease. Among survivors, studies are limited. Ground-glass opacities on a high-resolution computerized tomography scan and reduced diffusion capacity were noted after 3 months in a study. But long-term complications in patients with swine flu pneumonia have not been studied well. We are presenting an unusual case of swine flu pneumonia who developed interstitial lung disease after recovery.
KEY WORDS: Complications, diffuse parenchymal lung disease, Influenza A virus H1N1 subtype, pulmonary fibrosis
INTRODUCTION
Swine flu pneumonia cases of varying severity were reported from many parts of the world during the last epidemic. Since the first report of pneumonia caused by the H1N1 virus in Mexico, several severe cases have been found throughout the world.[1,2] The histopathologic changes observed in this type of pneumonia were consolidation, focal squamous metaplasia, diffuse alveolar damage, pulmonary edema, and acute pulmonary hemorrhage.[3] Ground-glass opacities (GGOs) in high-resolution CT scan (HRCT) imaging and reduced diffusion capacity for carbon monoxide (DLCO) have also been reported at 3 months in a short-term follow-up study.[4] Long-term follow-up studies on radiologic-pathologic correlation of fibrosis in swine flu cases are not available, especially the data from our country. We are reporting a case of swine flu pneumonia who developed interstitial lung disease (ILD) during recovery.
CASE REPORT
A 29-year-old female presented, in November 2009, with fever, nonproductive cough of 3- to 4-day and dyspnea of 2-day duration. On examination of the chest, basal crepitations were present on both sides. The chest skiagram showed bilateral consolidation predominantly involving lower zones. Investigations revealed hemoglobin 10.9 g/ dl, total leucocyte count (TLC) 4300/μl, and differential leucocyte count – neutrophil 80.7%, lymphocyte 17.5%, and platelet count 1.6 ×105/μl. The biochemical profile showed blood sugar 90 mg/dl, blood urea 35 mg/dl, serum creatinine 0.8 mg/dl, total protein 6.9 g/dl, bilirubin 0.6 mg/ dl, and SGOT/SGPT 384 and 186 U/l, respectively. The throat swab sample for the H1N1 PCR test was positive. In PCR testing, the sample was processed and RNA extraction was done with a kit (Qiagen kit, Hildane, Germany). Then RT-PCR was done with a real-time amplification machine under thermocycling conditions with reverse transcription at 50°C for 30 min. Activation took place at 95°C for 10 min and then PCR amplification was carried out at 95°C for 15 s and at 55°C for 30 s (45 cycles). She was diagnosed as a case of H1N1 community acquired pneumonia. The patient was put on empirical antibiotics, oseltamivir (75 mg twice daily for 15 days) and oxygen supplementation.
During the next 2 days, the condition of the patient worsened as she had increased dyspnea. She was also unable to maintain oxygen saturation. Arterial blood gas (ABG) analysis on high flow oxygen (FiO2: 60%) showed PO2 44.6 mmHg, PCO2 42.2 mmHg, pH 7.53, SaO2 84.5%, and bicarbonate 34.3 meq/l. She was intubated and put on mechanical ventilatory support. Initially, the patient was put on a pressure control mode with positive end-expiratory pressure (PEEP) of 6 cm of H2O. After few days of ventilatory support, she developed pneumothorax on the right side of the chest which was involving almost 40 % of hemithorax along with surgical emphysema on the same side for which an intercostal drain tube (ICD) was placed. After development of pneumothorax, the PEEP was reduced to 5 cm of H2O. Tracheal culture was sterile on the 12th day. Afterward, it became positive for Enterobacter cloacae on the 26th day and Pseudomonas aeruginosa on the 42nd day. TLC also showed an increasing trend and was 19,500/μl on the 18th day after admission. Tracheostomy was done on the 20th day of admission.
Gradually, she started improving. With recovery, ventilatory support was gradually weaned off on the 50th day. During weaning from the ventilator, the patient was put on the synchronized intermittent mandatory ventilation (SIMV) and pressure support mode. The ICD was also removed and tracheostomy was closed. She was discharged on the 64th day of admission. The HRCT scan at the time of discharge showed ground-glass haziness and areas of consolidation in upper lobes and bilateral lower lobes suggestive of alveolar inflammation [Figure 1a and b]. At the time of discharge, hematologic and biochemical investigations were normal. ABG at the time of discharge was done on room air (FiO2: 21%) which showed PO2 82.0 mmHg, PCO241.6 mmHg, pH 7.42, SaO2 95.2%, and bicarbonate 27.2 meq/l.
Figure 1.
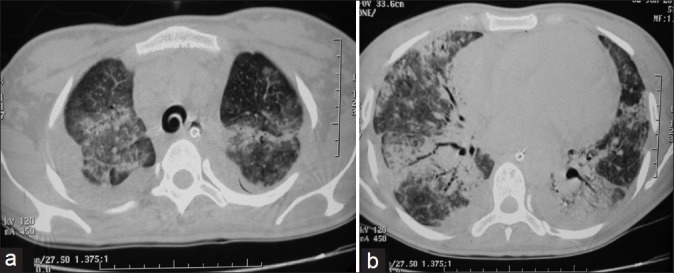
High-resolution CT scan at the time of discharge showing ground-glass haziness with areas of consolidation in (a) upper lobes and (b) bilateral lower lobes suggestive of alveolar inflammation.
After 1 year of her illness, she presented to us with complains of dyspnea on exertion and nonproductive cough. On examination of the chest, bilateral late inspiratory crepitations were present. During a 6-min walk test (6MWT), she was able to walk a distance of 342 m, and pulse oximetry showed a fall in arterial oxygen saturation from 97% to 78%. A restrictive pattern was found on lung function tests (FEV1= 0.84 l, 27.1% of predicted; FVC = 1.01 l, 28% of predicted, with the FEV1/FVC ratio being 83.2%). The patient was unable to perform the diffusion test because of a low vital capacity (1.01 l). A chest X-ray showed bilateral lower zone reticulonodular shadows [Figure 2a]. The HRCT scan showed reticulonodular shadows and ground-glass haziness. The dominant picture was reticulonodular shadowing. There were areas of honeycombing and traction bronchiectasis. Findings remained unchanged even in a prone position [Figures 2b, 3a and b]. Her bronchoscopy was done which showed a normal tracheobronchial tree. Bronchoalveolar lavage (BAL) and transbronchial lung biopsy (TBLB) were done. The microscopic examination of BAL showed total cells to be 400/μl and the differential count was 60% lymphocytes and 40% neutrophils. BAL was negative for acid-fast bacilli (AFB). The histopathologic examination of the TBLB sample with the hematoxylin and eosin stain showed chronic nonspecific inflammatory cells and fibroblasts [Figure 4a]. TBLB was negative for malignancy and tuberculosis. When special Van Gieson's stain was used, the pathological examination revealed multiple areas of fibrocollagenous foci suggestive of interstitial fibrosis [Figure 4b].
Figure 2.
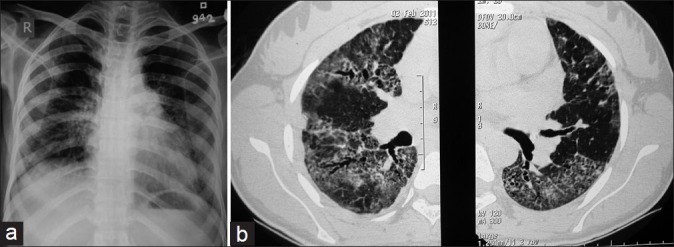
(a) Chest X-ray on follow-up showing reticulonodular shadows predominantly involving a bilateral lower zone with an apical-basal gradient. (b) High-resolution CT scan on follow-up showing ground-glass haziness with reticulonodular shadows involving bilateral lungs, traction bronchiectasis, and peripheral honeycombing.
Figure 3.
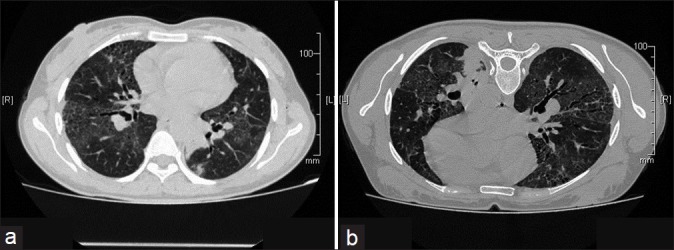
HRCT on follow up after 2 years in supine (a) and prone (b) position showing ground glass opacities, interstitial fibrosis and traction bronchectasis in bilateral lung fields. The opacities do not change in prone position.
Figure 4.
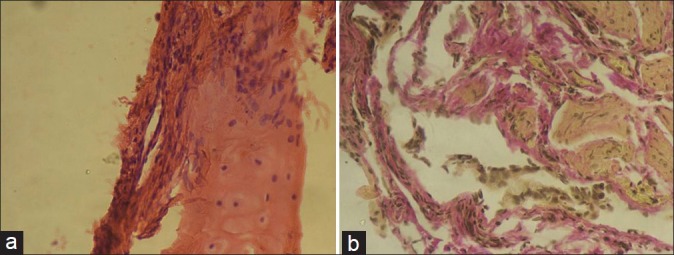
(a) Transbronchial lung biopsy (hematoxylin and eosin stain) showing fibroblasts adjacent to the bronchial cartilage (arrow). (b) Transbronchial lung biopsy (Van Gieson's stain) showing multiple areas of collagenous tissue (stained in pink color) and fibroblasts (elongated cells with brown-colored nuclei).
DISCUSSION
Uncomplicated swine flu influenza generally causes mild self-limiting illness, but a small percentage of patients develop severe pneumonia and respiratory failure. Various factors have been identified for the occurrence of this severe illness, like pregnancy, health status of the patient prior to illness, comorbid conditions, and secondary bacterial infections. In our case, though there was no underlying medical illness and comorbid conditions, secondary bacterial infections might had played a role in worsening the disease.
Superimposed bacterial infections are common in patients on ventilator. Pneumatocele and subsequent pneumothorax due to secondary bacterial infection have also been reported in a patient with H1N1 pneumonia.[5] Our case also had pneumothorax. This could be either due to barotrauma related to mechanical ventilation or due to secondary bacterial infection leading to the development of pneumatocele and its subsequent rupture.
In swine flu pneumonia cases, GGOs in chest imaging and reduction in DLCO have been found in a study at 3-month follow-up.[4] In our patient, ground-glass haziness, reticulonodular shadows, honeycombing, and traction bronchiectasis were observed in the HRCT scan done 1 year after the onset of the illness. These findings along with histopathology were compatible with the development of pulmonary fibrosis.
Long-term sequelae including pulmonary fibrosis may occur after either ARDS or ventilator-associated pneumonia.[6,7] There are very few studies available that describe the long-term sequelae of swine flu pneumonia.[8,9] A recent study showed that 10% of the patients who developed ARDS following H1N1 infection had pulmonary fibrosis on chest imaging during the follow-up.[8] The diagnosis in this study was based only on HRCT scan findings. In our patient, the HRCT findings of fibrosis were also substantiated by the histopathology of bronchial biopsy samples. Similar to the findings of Mineo et al., the pattern of the distribution of fibrosis in our patient was peripheral.[8] Pulmonary fibrosis has also been reported in patients with diseases like severe acute respiratory syndrome (SARS). In patients who recovered from SARS, persistent GGOss, reticular opacities, and pathologic findings of fibrosis have been reported during their follow-up visits.[10,11]
A recent study from China has reported high levels of transforming growth factor-beta 1 (TGF-β1) in patients of severe swine flu pneumonia even after recovery.[12] This may be responsible for the development of pulmonary fibrosis in swine flu pneumonia survivors. TGF-β1 has a major role in the development of pulmonary fibrosis.[13,14] It induces pulmonary fibrosis by various mechanisms like increased deposition of extracellular matrix proteins, stimulation of fibroblast chemotactic migration, and fibroblast-to-myofibroblast transition. In conclusion, swine flu pneumonia patients can have serious long-term consequences such as pulmonary fibrosis. Clinicians should be vigilant for such complications and the patients need to have an adequate follow-up even after clinical recovery from the swine flu pneumonia.
Footnotes
Source of Support: No support was taken from funding agencies at any stage of evaluation of case or preparation of the manuscript
Conflict of Interest: None declared.
REFERENCES
- 1.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Pandemic (H1N1) 2009. Update 91. [Last accessed on 2010 Mar 12]. Available from: http://www.who.int/csr/don/2010_03_12/en/index.html .
- 3.Gill JR, Sheng ZM, Ely SF, Guinee DG, Beasley MB, Suh J, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134:235–43. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai L, Gu L, Cao B, Zhai XL, Lu M, Lu Y, et al. Clinical features of pneumonia caused by influenza A (H1N1) virus in Beijing, China. Chest. 2011;139:1156–64. doi: 10.1378/chest.10-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaime MB, Luis AG, Luis MD, Ixchel CM, Patricio SD. Parenchymal injury, fibrosis, and pneumatocele in an H1N1-positive patient. Chest. 2010;138:38A. [Google Scholar]
- 6.Dubaybo BA, Carlson RW. Post-infectious ARDS: mechanisms of lung injury and repair. Crit Care Clin. 1988;4:229–43. [PubMed] [Google Scholar]
- 7.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 8.Mineo G, Ciccarese F, Modolon C, Landini MP, Valentino M, Zompatori M. Post-ARDS pulmonary fibrosis in patients with H1N1 pneumonia: role of follow-up CT. Radiol Med. 2011;117:185–200. doi: 10.1007/s11547-011-0740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toufen C, Jr, Costa EL, Hirota AS, Li HY, Amato MB, Carvalho CR. Follow-up after acute respiratory distress syndrome caused by influenza a (H1N1) virus infection. Clinics (Sao Paulo) 2011;66:933–7. doi: 10.1590/S1807-59322011000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–8. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ketai L, Paul NS, Wong KT. Radiology of severe acute respiratory syndrome (SARS): The emerging pathologic-radiologic correlates of an emerging disease. J Thorac Imaging. 2006;21:276–83. doi: 10.1097/01.rti.0000213581.14225.f1. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y, Deng BC, Zhou Y, Wang Y, Cui W, Wang W, et al. Immunological features in patients with pneumonitis due to Influenza A H1N1 infection. J Investig Allergol Clin Immunol. 2011;21:44–50. [PubMed] [Google Scholar]
- 13.Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, et al. Smad3 is key to TGF-beta-mediated epithelial to mesenchymal transition, fibrosis, tumour suppression and metastasis. Cytokine Growth Factor Rev. 2006;17:19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharyya P, Acharya D, Roychowdhury S. Role of matrix metalloproteinases in the pathophysiology of idiopathic pulmonary fibrosis. Lung India. 2007;24:61–5. [Google Scholar]


