Abstract
Rhupus syndrome, the overlap of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), is an extremely uncommon condition. Organ damages found due to SLE are usually mild in rhupus. Lupus pneumonitis in rhupus syndrome has not been reported worldwide. We are reporting a 23-year-old female with bilateral symmetric erosive arthritis, oral ulcer, alopecia, polyserositis, anemia, leucopenia, positive RA-factor, anti nuclear antibody (ANA) and anti ds-DNA. She presented with acute onset dyspnea, high fever, chest pain, tachycardia, tachypnea, hypoxia and respiratory alkalosis. High resolution computed tomography (HRCT)-thorax showed bilateral, basal consolidation with air bronchogram. Repeated sputum and single broncho alveolar lavage (BAL) fluid examination revealed no organism or Hemosiderin-laden macrophage. The diagnosis of rhupus was confirmed by combined manifestations of RA and SLE, and the diagnosis of acute lupus pneumonitis was established by clinico-radiological picture and by excluding other possibilities.
KEY WORDS: Lupus pneumonitis, rheumatoid arthritis, rhupus syndrome, systemic lupus erythematosus
INTRODUCTION
Rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) are common rheumatologic diseases, and considered to be separate diseases. The overlap of RA and SLE was first described by Schur in 1974 as ‘Rhupus’.[1] Rhupus syndrome is a rare clinical condition and there are only a small number of well-documented cases in the literature. The prevalence of rhupus syndrome was found to be far less than expected chance of concurrence (0.09% versus calculated 1.2%).[2] Rhupus syndrome has clinical manifestations of both RA and SLE but the organ damages found due to SLE are usually mild.
We are reporting a young lady with bilateral acute lupus pneumonitis in rhupus syndrome, a rare condition with only few cases reported worldwide. Lupus pneumonitis in rhupus syndrome has not been reported so far in literatures.
CASE REPORT
A 23-year-old female, diagnosed as RA five years back and on treatment with analgesics, hydroxychloroquine and methotrexate, had developed high grade intermittent fever for seven days, joint pain with swelling involving bilateral elbow and wrist joints, dyspnea and cough with scanty mucoid expectoration for four days. She was transferred to our respiratory care unit as she was not responding to parenteral antibiotics (intravenous cefepime 2 gm twice daily and levofloxacin 500 mg daily for 10 days). On general survey, following were the findings: oral temperature 104°F, moderate pallor, blood pressure 110/72 mmHg, pulse 110/min, regular, respiratory rate 32/min with accessory muscles working, alopecia, oral ulcer, swan neck deformities of both hands with ulnar deviation of wrist joints. There was no skin nodule, skin rash, lymphadenopathy, hepato-splenomegaly, puffy fingers, sclerodactyly, or she did not give history of Raynaud's phenomenon, dysphagia, hemoptysis or any bleeding episodes, muscle weakness, seizures or abnormal behavior. Chest examination revealed impaired percussion notes in both infrascapular areas with decreased vesicular breath sound and bilateral basal crepitations.
Investigations showed normochomic normocytic anemia with hemoglobin of 9.1 gm/dl, total white blood cell (WBC) count of 1,800/mm3 with neutrophil 83%, lymphocyte 17%; platelet count of 225,000/mm3, erythrocyte sedimentation rate at 110 mm in the1st hour and fasting blood sugar at 78 mg/dl. Urine examination, renal and liver function tests were normal. X-ray of both hands showed juxta articular osteopenia, loss of carpometacarpal and metacarpophalangeal joint spaces [Figure 1]. Her X-ray chest postero-anterior view showed bilateral lower zone consolidation with bilateral pleural effusion [Figure 2]. High resolution computed tomography (HRCT) thorax showed bilateral, multiple areas of consolidation in lower lobes with bilateral small pleural effusion [Figure 3]. Electrocardiography showed sinus tachycardia and echocardiography showed mild pericardial effusion with slightly thickened pericardium and mild pulmonary arterial hypertension. Ultrasonography of whole abdomen was normal. Arterial blood gas analysis (ABG) showed hypoxia (PaO2- 54 mm Hg with 4 litre of oxygen/min) with respiratory alkalosis (PH- 7.48, PaCO2- 32 mm Hg). Pleural fluid examination showed cell count 540 cells/mm3 with lymphocyte 65%, neutrophil 30%, eosinophil 05%; sugar 89 mg/dl; protein 3.39 mg/dl; lactate dehydrogenase 2,783 units/liter and adenosine deaminase 51.3 units/liter; Ziehl-Neelsen stain negative; Gram stain and culture negative and Mycobacterium DNA polymerase chain reaction was negative. Consecutive 3 days sputum examinations revealed no acid fast bacilli (AFB) or organisms and one time bronchiolo-alveolar lavage (BAL) examination from the affected areas was also negative for organism, AFB and Hemosiderin-laden macrophage. Special investigation showed RA factor was positive (1:480); antinuclear antibody (ANA) was 3+ (1:320, homogenous) and anti-dsDNA with the immunofluorescence technique (IFT) using Crithidia luciliae as a substrate was positive.
Figure 1.
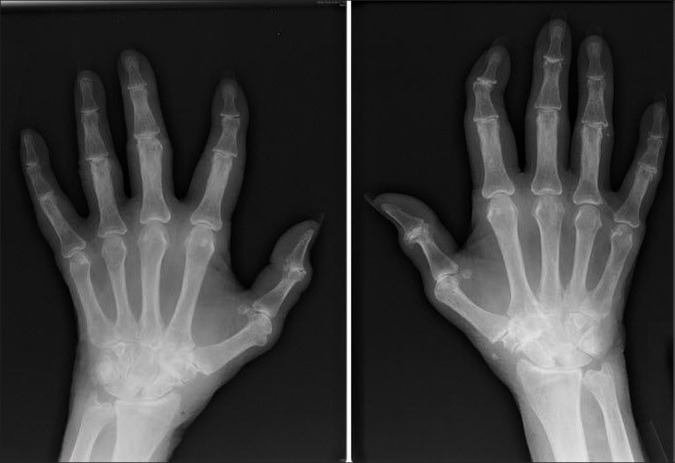
X-ray of both hands showing juxta articular osteopenia, loss of carpo-metacarpal and meta-carpophalangeal joint spaces
Figure 2.
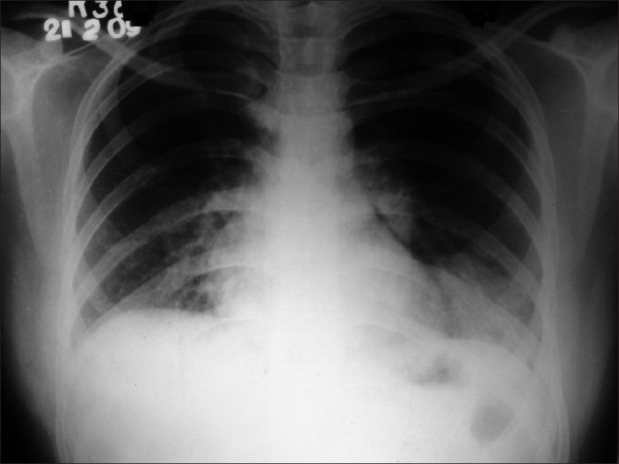
Chest X-ray postero-anterior view shows bilateral lower zone consolidation with bilateral pleural effusion
Figure 3.
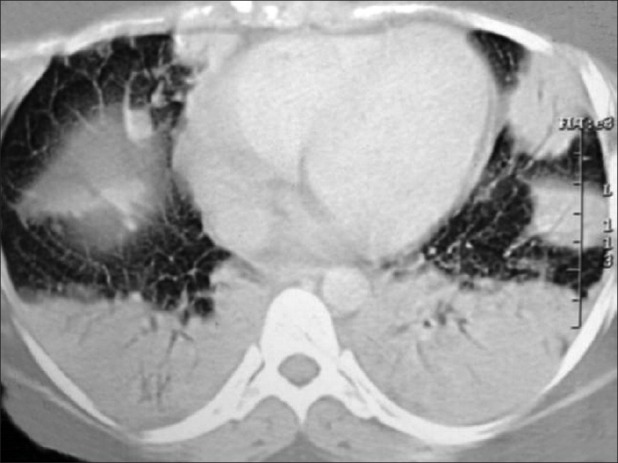
HRCT scan thorax showing bilateral lower lobe consolidation
We had diagnosed the case as bilateral lupus pneumonitis and started treatment with methyl prednisolone intravenous 1,000mg/day for three days, followed by oral prednisolone 40 mg/day for two weeks then tapered over two months.
On follow-up, X-ray chest postero-anterior view after one month showed there was complete clearance of consolidations [Figure 4]. ABG findings were normal, on pulse-oximetery SpO2 was 96% in room air, six minute walking distance was 340 meters and on spirometry FEV1/FVC ratio was 84%, FEV1 and FVC were 82% and 86% of predicted values, respectively. No relapse was observed during tapering period or after stopping corticosteroids.
Figure 4.
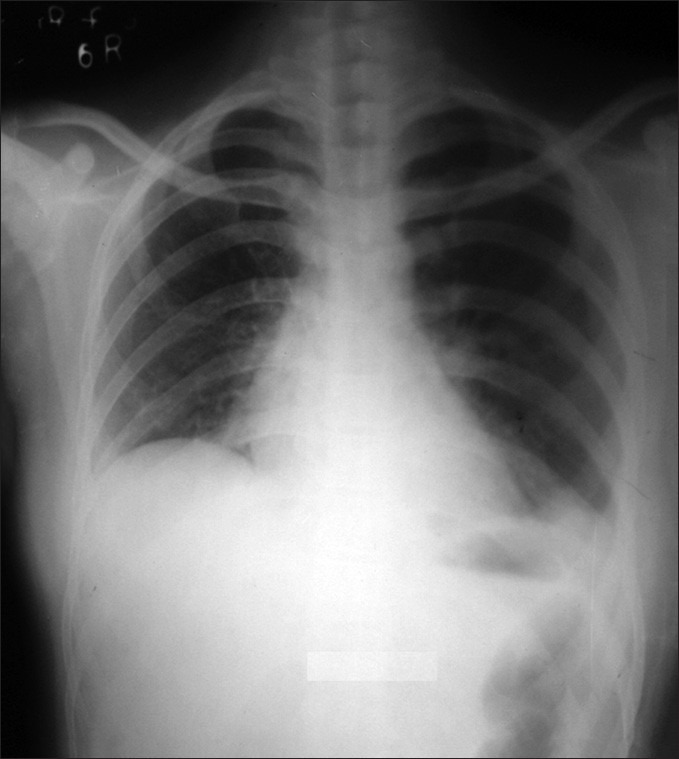
Follow-up X-ray chest postero-anterior view after one month showed complete clearance of consolidations
DISCUSSION
The diagnostic criteria for rhupus was suggested as inflammatory symmetrical erosive polyarthritis, positive RA factor, clinical features suggestive of SLE and positive anti-dsDNA or anti-smith auto-antibodies.[3] A retrospective study in China observed that the mean age of rhupus was 36.8 years; SLE developed about 7.7 years after initial presentation and SLE associated severe organ damages other than hematopoietic abnormalities were less frequent.[4] Features of RA are dominated by erosive polyarthritis in all cases and rheumatoid nodules in around 40% of the cases.[2] SLE is usually manifested by cutaneous (butterfly skin rash, photosensitivity and alopecia), hematological (leukopenia and thrombocytopenia), serosal (pleural and pericardial effusion) and mucosal involvement.[2] We diagnosed the case as rhupus by clinical manifestations of RA and SLE with positive RA factor, ANA and ds-DNA. We excluded the possibilities of other differential diagnosis of rhupus like RA patients with extrarticular manifestations, SLE patients with arthritis and mixed connective tissue disease.
Major organ involvements are rare, though there are scattered reports of lupus encephalitis in Rhupus.[5] Lupus pneumonitis is an unusual and life threatening complication of SLE; usually occurs during flare-up or rarely as a presentation. Lupus pneumonitis presents with acute onset of fever, cough, tachypnea and hypoxia. The usual radiological signs of lupus pneumonitis are consolidations in one or more areas usually basal and bilateral and often associated with pleural effusion and pulmonary arterial hypertension.[6] Cavitation is extremely rare and suggests other possibilities too. The mortality of lupus pneumonitis is around 50%, rest of them responds dramatically to steroids and some require cyclophosphamide.[7] The diagnosis of Lupus pneumonitis is essentially by exclusion of other causes of lung infiltration such as infective pneumonia (bacterial, mycobacterial, fungal and viral), organizing pneumonia (OP), alveolar hemorrhage, pulmonary embolism, etc. We excluded infective pneumonia by repeated sputum and single BAL fluid examination; alveolar hemorrhage by the absence of hemoptysis and Hemosiderin-laden macrophage in BAL fluid and the syndrome of acute reversible hypoxemia by the presence of abnormal chest X-ray and HRCT-scan of thorax.
Organizing pneumonia, a rare complication of RA that may simulate Lupus pneumonitis, is usually not life threatening, subacute in onset, the mean age of onset around 50-60 years and begins with a mild flu-like illness with fever, cough, malaise, mild dyspnoea, anorexia and weight loss.[8] The radiographical pattern in OP secondary to connective tissue disease (CTD) is reticularnodular, and thus, indistinguishable from idiopathic pulmonary fibrosis while that of idiopathic OP is usually patchy alveolar infiltration.[9] CT findings are commonly accompanied by ground glass opacity, nodules (indicating bronchiolitis), and the findings of non-specific/usual interstitial pneumonia (in CTD-associated OP). Relapses are common upon stopping or reduction of corticosteroids to <20mg/day, thus often leading to prolonged treatment.[8] Moreover, prognosis of CTD-associated OP appears less favorable than idiopathic OP.[9] We excluded the possibility of CTD associated OP by absence of typical clinic-radiological picture and good response to treatment without relapse. The diagnosis of acute lupus pneumonitis was established by the typical clinical manifestations, HRCT findings and dramatic response to corticosteroids.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Schur PH. Systemic lupus erythematosus. In: Beeson PB, editor. Cecil-loeb textbook of medicine. 13th edn. Philadelphia: WB Saunders; 1971. p. 821. [Google Scholar]
- 2.Panush RS, Edwards NL, Longley S, Webster E. ‘Rhupus’ Syndrome. Arch Intern Med. 1988;148:1633–6. [PubMed] [Google Scholar]
- 3.Satoh M, Ajmani AK, Akizuki M. What is the definition for coexistent rheumatoid arthritis and systemic lupus erythematosus? Lupus. 1994;3:137–8. doi: 10.1177/096120339400300215. [DOI] [PubMed] [Google Scholar]
- 4.MU R, YE H, Chen S, LI ZG. A retrospective clinical study of Rhupus syndrome. Zhonghua nei ke za zhi. 2006;45:540–3. [PubMed] [Google Scholar]
- 5.Wang JG, Tang HH, Tan CY, Liu Y, Lin H, Chen YT. Diffuse lupus encephalopathy in a case of rhupus syndrome. Rheumatol Int. 2009;30:961–3. doi: 10.1007/s00296-009-1007-3. [DOI] [PubMed] [Google Scholar]
- 6.Matthay RA, Schwarz MI, Petty TL, Stanford RE, Gupta RC, Sahn SA, et al. Pulmonary manifestations of systemic lupus erythematosus: Review of twelve cases of acute lupus pneumonitis. Medicine. 1975;54:397–409. doi: 10.1097/00005792-197509000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Wiedemann HP, Matthay RA. Pulmonary manifestations of systemic lupus erythematosus. J Thorac Imaging. 1992;7:1–18. doi: 10.1097/00005382-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Cordier JF. Cryptogenic organising pneumonia. Eur Respir J. 2006;28:422–46. doi: 10.1183/09031936.06.00013505. [DOI] [PubMed] [Google Scholar]
- 9.Lamblin C, Bergoin C, Saelens T, Wallaert B. Interstitial lung diseases in collagen vascular diseases. Eur Respir J. 2001;18:69S–80s. [PubMed] [Google Scholar]


