Abstract
Nocardia species is rarely encountered in cystic fibrosis (CF) patients. Its isolation usually implies colonization. Of all other Nocardia species, Nocardia transvalensis is very unusual and is clinically distinguishable because of its resistance to aminoglycosides, a standard antinocardial therapy. We report a case of N. transvalensis pulmonary infection in a CF patient.
KEY WORDS: Cystic fibrosis, Nocardia transvalensis, Nocardiosis
INTRODUCTION
Most cystic fibrosis (CF) patients have chronic pulmonary infections and episodic exacerbations requiring intravenous antibiotic therapy. Early in the disease, the bacteria most commonly responsible are Staphylococcus aureus, Haemophilus influenzae and Klebsiella. Later, Pseudomonas aeruginosa becomes the predominant organism. Nocardia species are rarely encountered in this population. There are only a few case reports showing isolation of Nocardia from CF patients, but it did not appear to be pathogenic in this population.[1,2] We report a case of a pediatric patient with CF harboring Nocardia transvalensis that did appear to be pathogenic in this patient.
CASE REPORT
A 19-year-old Caucasian male patient with CF presented with an acute exacerbation of his respiratory symptoms. He was diagnosed with CF shortly after birth, secondary to meconium ileus. His past medical history was significant for pansinusitis (requiring multiple surgeries), pancreatic exocrine insufficiency as well as numerous upper and lower respiratory tract infections. Sputum specimens in his prior admissions had yielded mucoid Pseudomonas aeruginosa, Klebsiella pneumoniae, Aspergillus and Candida species. He reported good compliance with his medications, which included inhaled corticosteroids, inhaled dornase alfa, inhaled bronchodilators, inhaled tobramycin, pancreatic enzymes, along with cotrimoxazole and azithromycin prophylaxis.
Physical exam revealed a poorly nourished young male with a body mass index of 19 kg/m2, clubbing and bilateral coarse breath sounds on chest auscultation. Chest X-ray showed bilateral bronchiectasis consistent with CF, but no acute cardiopulmonary process. He was empirically initiated on anti-pseudomonal therapy, which included intravenous ceftazidime and tobramycin. Despite 2 weeks of intervention, his respiratory symptoms deteriorated. He reported increased malaise, cough and right-sided pleuritic chest pain. He required supplemental oxygen in view of dyspnea and drop in oxygen saturation into the 80s. Examination was remarkable for decreased breath sounds in the right lower lung fields. Chest roentgenogram and computed tomography (CT) scan demonstrated right lower lobe pneumonia. Meanwhile, sputum culture grew gram positive branching filaments and Pseudomonas aeruginosa. A modified Ziehl-Neelsen acid fast stain showed an intermittent staining pattern [Figure 1]. The pathogen was tentatively identified as Nocardia species. The isolate was subsequently sent to the Mycobacterial/Nocardia Research Laboratory of the University of Texas in Tyler, Texas, for polymerase chain reaction (PCR) and antibiotic susceptibility. The organism was finally identified as N. transvalensis, which was sensitive to cotrimoxazole and linezolid and resistant to amikacin.
Figure 1.
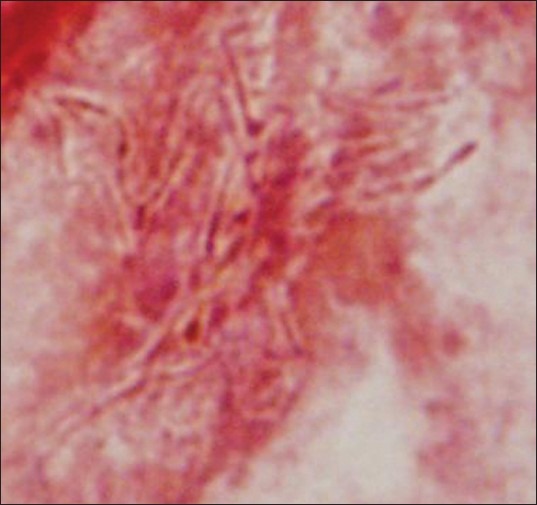
A modified Ziehl-Neelsen acid fast stain showed an intermittent staining pattern
Sputum isolate of Nocardia could be either colonization or infection. Given the poor response to the usual antipseudomonal therapy, he was presumptively commenced on a trial of intravenous cotrimoxazole (20 mg/kg/day) and linezolid (40 mg/kg/day). Over the next 2 weeks of intravenous therapy, dramatic clinical improvement was evident, with resolution of chest pain and cough. His pulmonary function tests also showed improvement and returned back to baseline. He was discharged home on 4 weeks of oral cotrimoxazole and 1 week of oral linezolid. Two follow-up sputum cultures did not yield Nocardia species, and the infiltrates resolved on the chest X-ray after 6 weeks of completion of treatment.
DISCUSSION
Most Nocardia pulmonary infections are primary, but it can spread to the lungs from other sites. Pulmonary nocardiosis may manifest as an acute or chronic infection, and the most frequent predisposing factors are chronic obstructive pulmonary disease, bronchiectasis, pulmonary fibrosis, emphysema, asthma, neoplastic disease, organ transplant, human immunodeficiency virus infection and long-term corticosteroid therapy.[3] A study in Japan concludes that the most common predisposing factors for nocardial infection were therapy by immunosuppressive agents (22.4%), cancer (6.6%), diabetes (3.6%), tuberculosis (3.3%) and acquired immunodeficiency syndrome (2%).[4] Our patient had received long-term inhalational corticosteroids and, although the dosage was very low, this may have contributed to culture positivity of Nocardia.
The diagnosis of Nocardiosis is mainly by isolation of the organism and identification of species from sputum or bronchoalveolar lavage fluid. Petersen et al. first reported a case of an 8-year-old child with CF harboring N. farcinica in bronchoalveolar lavage fluid.[5] Radiographic findings are variable and include infiltrates, consolidation, lung masses, single or multiple nodules, pleural effusions and subpleural plaques.
In our case, the patient's sputum isolated Pseudomonas aeruginosa and partially acid fast bacteria on admission, and he was on antipseudomonal treatment. After 2 weeks of therapy, our patient had worsening of clinical symptoms like cough, chest pain and dyspnea. Chest X-ray showed new infiltrate in the right lower lobe and sputum isolated N. transvalenis. Sputum isolation of Nocardia from CF patients does not necessarily imply disease, but it may represent colonization; hence, the need for treatment should be assessed on an individual basis. Rosett and Hodges et al, have recommended criteria to categorize colonization versus disease.[6] According to the criteria, our patient was judged to have been diseased rather than colonized.
Studies have assessed that cotrimoxazole prophylaxis is not a protective factor for preventing breakthrough Nocardiosis in immunocompromised patients.[7,8] Similarly, our patient, even though on cotrimoxazole prophylaxis, was diagnosed as having Nocardiosis. A unique feature of N. transvalenis is it being resistant to amikacin and other aminoglycosides, while all other Nocardia species, like N. farcinica, N. nova and N. asteroides are typically susceptible. N. transvalenis is highly susceptible to cotrimoxazole, third-generation cephalosporin, imipenem and linezolid. Hence, we escalated the cotrimoxazole from prophylactic dose to higher dose (20 mg/kg/day) and added linezolid. After 2 weeks of intravenous therapy, significant improvement in clinical symptoms was noticed and he was switched to oral therapy for 4 weeks. Repeat sputum specimen after 6 weeks of treatment did not yield N. transvalensis. Using a specific RT–N. farcinica–PCR, Bittar et al. demonstrated that Nocardia sp, despite treatment, were detected and cultured during the long follow-up in their patient.[9] There are no prospective studies that suggest effective therapy and duration for Nocardial infection. Most case reports and infectious disease specialists suggest to treat with, initially, two susceptible drugs intravenously and to continue with a prolonged period of oral antibiotics.
Although there are no pathognomonic signs or symptoms of Nocardiosis in a patient with CF, a high suspicion is necessary to make a timely diagnosis and treatment, which is associated with improved clinical outcomes. Presumptive diagnosis can be made if partially acid fast filamentous branching rods are seen either in sputum or bronchoalveolar lavage fluid.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lumb R, Greville H, Martin J, Sangster N, Colmes M. Nocardia asteroides isolated from three patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis. 2002;21:230–3. doi: 10.1007/s10096-001-0687-8. [DOI] [PubMed] [Google Scholar]
- 2.Barrio MI, Martínez MC, Prados C, Girón RM, Maiz L, Martínez MT Grupo de Fibrosis Quística de Neumomadrid. Isolation of Nocardia species in patients with cystic fibrosis. Arch Bronconeumol. 2008;44:109–12. doi: 10.1016/s1579-2129(08)60021-x. [DOI] [PubMed] [Google Scholar]
- 3.Lederman ER, Crum NF. A case series and focused review of nocardiosis: Clinical and microbiological aspects. Medicine (Baltimore) 2004;83:300–13. doi: 10.1097/01.md.0000141100.30871.39. [DOI] [PubMed] [Google Scholar]
- 4.Kageyama A, Yazawa K, Ishikawa J, Hotta K, Nishimura K, Mikami Y. Nocardial infections in Japan 1992 to 2001, including the first report of infection by Nocardia transvalensis. Eur J Epidemiol. 2004;19:383–9. doi: 10.1023/b:ejep.0000024706.02325.c0. [DOI] [PubMed] [Google Scholar]
- 5.Petersen BE, Jenkins SG, Yuan S, Lamm C, Szporn AH. Nocardia farcinica isolated from bronchoalveolar lavage fluid of a child with cystic fibrosis. Pediatr Infect Dis J. 2007;26:858–9. doi: 10.1097/INF.0b013e31805cdbff. [DOI] [PubMed] [Google Scholar]
- 6.Rosett W, Hodges GR. Recent experiences with nocardial infections. Am J Med Sci. 1978;276:279–85. doi: 10.1097/00000441-197811000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Peleg AY, Husain S, Qureshi ZA, Silveira FP, Sarumi M, Shutt KA, et al. Risk Factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: A matched case-control study. Clin Infect Dis. 2007;44:1307–14. doi: 10.1086/514340. [DOI] [PubMed] [Google Scholar]
- 8.van Burik JA, Hackman RC, Nadeem SQ, Hiemenz JW, White MH, Flowers ME, et al. Nocardiosis after bone marrow transplantation: A retrospective study. Clin Infect Dis. 1997;24:1154–60. doi: 10.1086/513654. [DOI] [PubMed] [Google Scholar]
- 9.Bittar F, Stremler N, Audié JP, Dubus JC, Sarles J, Raoult D, et al. Nocardia farcinica lung infection in a patient with cystic fibrosis: A case report. J Med Case Reports. 2010;4:84. doi: 10.1186/1752-1947-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]


