Abstract
Urinary tract infections (UTIs) represent one of the most commonly acquired diseases among the general population as well as hospital in-patients, yet remain difficult to effectively and consistently treat. High rates of recurrence, anatomic abnormalities, and functional disturbances of the urinary tract all contribute to the difficulty in management of these infections. However, recent advances reveal important molecular and genetic factors that contribute to bacterial invasion and persistence in the urinary tract, particularly for the most common causative agent, uropathogenic Escherichia coli. Recent studies using animal models of experimental UTIs have recently provided mechanistic insight into the clinical observations that question the effectiveness of antibiotic therapy in treatment. Ultimately, continuing research will be necessary to identify the best targets for effective treatment of this costly and widespread infectious disease.
Keywords: Escherichia coli, epidemiology, urinary tract infection
INTRODUCTION
A variety of bacterial pathogens are responsible for causing urinary tract infections (UTIs), but the most prominent are Escherichia coli, Enterococcus spp., Pseudomonas aeruginosa and mirabilis, Klebsiella pneumoniae, Candida albicans, Enterobacter spp., and coagulase-negative Staphylococci. However, it is the uropathogenic E. coli (UPEC) strains that are the primary causative agents of up to 90% of UTIs. Formally, UPEC is defined traditionally, as other E. coli strains, by the presence of somatic, capsular polysaccharide, and flagellar antigens (O, K, and H respectively). Among UPEC variants, the O antigens 1, 2, 4, 6, 7, 8, 16, 25, and 75 occur more often than others. While the K and H antigens appear to have no significant trends, the K1 antigen, typically associated with ExPEC (extraintestinal pathogenic E. coli) strains causing neonatal meningitis [NMEC], can be found among the more virulent strains of UPEC.[1]
UPEC strains are similar to other pathogenic E. coli strains in that they generally carry larger genomes than K12 or commensal E. coli isolates, most likely due to the survival needs outside the human intestinal tract.[1] To date, the genomes of three UPEC isolates have been fully sequenced: two pyelonephritis isolates, CFT073 (O6:K2:H1) and 536 (O6:K15:H31) and one cystitis isolate, UTI89 (O18:K1:H7). While no unique genetic factors have yet been found specific to UPEC, the sequenced isolates indicate that UPEC encodes more virulence factors than K12/commensal strains.[1] These include α-hemolysin, cytotoxic necrotizing Factor 1 (CNF1), lipopolysaccharide (LPS) modification systems, virulent capsule antigens, iron acquisition systems including the sidereophores aerobactin and enterobactin, proteases, and a variety of pili including Type 1, P, S, and F1C.[1,2]
Clinical challenges to the management of urinary tract infections
Clinical urologists continue to face challenges in the management of UTIs. For instance, the rapid identification and treatment of patients with complicated UTIs remains an important problem. Fortunately, certain anatomic factors contributing to classification of a UTI as complicated can be remedied quickly and effectively to minimize the morbidity associated with the UTI. For example, anatomic urinary tract obstruction (i.e. stone, stricture) can be alleviated with internal or external drainage during the acute infectious period prior to definitive management of the etiology causing the obstruction. However, ultimately the efficacy of future therapy in patients with complicated UTIs will be impaired unless the underlying complicating factor(s) are identified and appropriately managed.[3] Another common etiology of complicated UTIs is functional disturbances of the urinary tract resulting in abnormal micturition that can be neurologic or non-neurologic in origin. A frequently utilized modality to treat the abnormal bladder function in patients with functional disturbances of the urinary tract is clean intermittent catheterization (CIC). This has been shown to be safe and effective since its first introduction by Lapides in 1972.[4] CIC has revolutionized the approach to lower urinary tract disease states. However, an unfortunate consequence of CIC is a high incidence of bacteriuria, with rates ranging from 15–85%.[5,6] The clinical sequela of the bacteriuria is unclear as the incidence of UTI in patients performing CIC varies widely.
The incidence of symptomatic UTI developing in patients with normal lower urinary tract function and untreated bacteriuria can also vary dependent upon patient population. Specifically, in pregnant women, the rate of development of symptomatic UTI with untreated bacteriuria can be as high as 30%, which can then precipitate preterm labor.[7] Therefore, nearly every pregnant woman diagnosed with bacteriuria on screening urine cultures will be offered treatment to avoid the complications associated with preterm labor. Similarly, untreated bacteriuria detected on screening urine cultures in school-age children with normal urinary tracts has the potential to develop into a symptomatic UTI in approximately 10%.[8] Lastly, in one study, untreated bacteriuria detected on weekly screening urine cultures in 14 patients over a six-month period who performed CIC with a normal upper urinary tract resulted in five symptomatic UTIs developed during the 323 week follow-up period.[9] The authors of this study concluded that “attempts to eradicate bacteriuria should be deferred until proven beneficial”. Clearly, the treatment of bacteriuria is highly dependent upon patient demographics and better descriptors are needed to identify those patients that have a need for continuous antibiotic therapy to prevent sequelae.
Intracellular lifestyle of UPEC
UTIs are the result of a complex series of interactions between the uropathogen and the host that can result either in asymptomatic disease (commensalism) or symptomatic disease 3.[3,10] of the bladder, kidney, or both. Important factors that influence the outcome of UTIs include: virulence factors of the uropathogen, functional and anatomical status of the urinary tract, inoculum size, genetic factors, and the competency of the host immune system.[11,12]
The bladder itself is a hostile environment for bacteria in general. Except for P. mirabilis which produces a urease,[2] there are no nutrients available in the urine. Furthermore, bacteria that come into contact with the epithelial cells of the bladder can trigger innate immune responses initiated by Toll-like receptor 4 (TLR4), among others, which is responsive to the LPS produced by many bacteria, and UPEC specifically. This immune cascade results in the production of various proinflammatory cytokines and chemoattractants, including IL-8, important in neutrophil recruitment. The host can also respond with other innate immune phagocytes such as macrophages, or by producing antimicrobial peptides, and, the most drastic response, total exfoliation of the superficial layer of bladder epithelial cells (recently reviewed in:12, 13).[13,14]
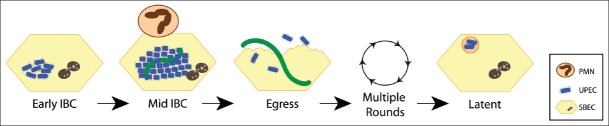
Once thought to be strictly an extracellular pathogen, recent studies from the murine cystitis model[15–20] have established a new intracellular paradigm of UPEC infection within superficial bladder epithelial cells. Intracellular growth within superficial epithelial cells of the bladder provides many advantages to UPEC including: access to nutrients enabling robust intracellular growth,[15,19,21] evasion of professional phagocytic cells,[22–24] avoidance of expulsion,[16,17,25] and protection from the antibacterial properties of urea, ammonium, and osmolarity of urine.[26] Time-lapse fluorescence video microscopy of infected murine bladders revealed that UPEC proceed through a complex developmental pathway forming biofilm-like communities termed intracellular bacterial communities (IBCs).[21] This study was instrumental in that it unified previously described pathogenic events from single time points into a comprehensive model of UTI pathogenesis that elucidates four separate developmental stages of the IBC pathway. After invasion, early IBCs appear as loose collections of non-motile rods that rapidly divide within the cytoplasm of the bladder epithelial cells.[21] Middle IBCs occur approximately 6-8 h following infection as colonies of tightly packed, coccoid bacteria exhibiting biofilm-like traits with slower growth rates.[21] Concurrent with maturation into the coccoid morphology, a subpopulation of the intracellular bacteria becomes filamentous. During late stage IBCs, coccoid bacteria differentiate into motile rods that disperse from the IBC in a flagellar-mediated process called fluxing.[21] Finally, egressing out of the infected epithelial cells enables UPEC to re-enter the IBC cascade through multiple rounds, albeit with slower kinetics to eventually establish a latent infection.[21] Additionally, the subpopulation of elongated, filamentous bacteria inhibit killing by polymorphonuclear neutrophils PMNs,[24] as well as promoting adhesion to the underlying layer of cells in the bladder after exfoliation, allowing the bacteria to enter the latent phase of the infection cascade [Figure 1].[13]
Figure 1.
Intracellular lifestyle of UPEC. The cartoon depicts the stages through which UPEC (blue and green) proceeds during infection of superficial bladder epithelial cells (SBECs). The intracellular residence protects from polymorphonuclear leukocyte (PMN) attack. See text for details
Studies have demonstrated that the IBC pathway is neither limited to a single UPEC isolate nor to a single mouse strain.[27] For instance, IBC formation was found to occur in five genetic backgrounds of inbred mice strains.[27] Additionally, the majority of UPEC strains isolated from women with different UTI syndromes [asymptomatic bacteriuria, recurrent cystitis, pyelonephritis, and acute cystitis] were competent for IBC formation in the murine cystitis model.[27] Although each of the UPEC strains from the UTI syndromes were proficient at IBC formation differences were observed in the size and number of IBCs as well as the kinetics of infection.[27] Intriguingly, IBCs derived from acute cystitis isolates were both significantly smaller and less numerous when compared to the other UTI syndromes. These results suggest that IBC formation is a common attribute of UPEC strains and that the propensity of a given strain to form IBCs may be associated with persistence in the urinary tract.[27]
Strikingly, evidence of the IBC pathogenic cycle was found in the urines of women with acute cystitis.[28] Rosen et al., observed that IBCs shed into the urine of women with cystitis were indistinguishable from exfoliated IBCs found in mouse urine upon subsequent histologic examination.[28] Furthermore, filamentous uropathogens 20 μm in length were found in the urines from several other Gram-negative species including UPEC, Proteus mirabilis, Klebsiella pneumoniae, and Enterobacter aerogenes.[28] However, neither evidence of exfoliated IBCs nor filaments were found in the urines of women with an asymptomatic infection and women infected by Gram-positive bacteria.[28] This evidence is consistent with recent reports that intracellular bacteria were not observed in biopsies of patients with neurogenic bladder who experience numerous episodes of asymptomatic bacteriuria.[29] Indeed, when biopsies were performed on women with recurrent UTIs it was determined that 88% of biopsies contained bacteria upon the urothelium by scanning electron microscopy SEM analysis and bacteria could be recovered from 50% of the biopsies of women with sterile urine cultures.[30]
Clinical implications of IBC cascade
The evidence that an intracellular bacterial reservoir is established in both mice[15–20] and humans[28] has several important clinical considerations for the management of UTIs. Prophylactic antibiotic therapy has been a mainstay for treatment of recurrent urinary tract infections. With the rise in antibiotic-resistant pathogens and the discussions of whether all patients require antibiotic therapies, it is important to identify new algorithms for treatment built upon evidence-based investigations. To fully evaluate the effectiveness of antibiotic treatment against UTIs, Mulvey and colleagues recently evaluated the efficacy of eight different classifications of antibiotics on the eradication of intracellular UPEC in the mouse model for human UTI. These investigators determined that three-day oral treatment with any of the antibiotics, including those capable of intracellular accumulation, had no effect on the latent bacterial burden in the bladder.[31,32] Importantly, the quiescent intracellular reservoirs avoided elimination despite the presence of antibiotic concentrations in the urine that greatly exceeded the minimal inhibitory concentration.[31] When the bacteria were liberated from the bladder epithelium, the antibiotic sensitivity was enhanced, suggesting that the intracellular compartment, as well as the IBC structure, provide significant protection from antibiotic therapies. These observations further complicate evaluation of treatments given that sterile urine does not correlate with sterile bladder tissue.[32] These results could provide an explanation to the epidemiological findings that upwards of 68% of recurrences are caused by bacteria that are isogenic to the original strain.[33–39] Furthermore, each IBC is clonal, that is, invasion of a single UPEC is sufficient to support all of the growth observed within a single epithelial cell.[40] In addition, a single epithelial cell can support at least 105 UPEC individuals,[41] indicating that invasion of a single bacterium is sufficient to initiate an acute infection. This new paradigm for recurrence parallels clinical observations and changes the management for treatment of these infections from a “hygienic problem” to an antibiotic-insensitive latent infection.
In summary, researchers are beginning to uncover the molecular details that underlie UTIs. Specifically, new diagnostic and therapeutic approaches based on the combination of host genetic factors, innate immunity, and bacterial virulence factors are needed to identify the patients most prone to UTIs to avoid the cost and potential side-effects of treatment.[42] Fortunately, advances being made in both basic and clinical scientific research of the urinary tract in patients and animal models are providing some explanations and insight into the clinical problems that remain with the management of UTIs. Improved knowledge of the genetics, uropathogen virulence factors, and host immune responses to UTIs will enhance the ability of clinicians to more readily distinguish high-risk patients from uncomplicated patients, which is necessary to prevent major sequelae in these high-risk patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wiles TJ, Kulesus RR, Mulvey MA. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol. 2008;85:11–9. doi: 10.1016/j.yexmp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatt JK, Rather PN. Role of bacterial biofilms in urinary tract infections. Curr Top Microbiol Immunol. 2008;322:163–92. doi: 10.1007/978-3-540-75418-3_8. [DOI] [PubMed] [Google Scholar]
- 3.Macejko AM, Schaeffer AJ. Asymptomatic bacteriuria and symptomatic urinary tract infections during pregnancy. Urol Clin North Am. 2007;34:35–42. doi: 10.1016/j.ucl.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Lapides J, Woodburne RT. Configuration of ureteral lumen during peristalsis. J Urol. 1972;108:234–7. doi: 10.1016/s0022-5347(17)60698-0. [DOI] [PubMed] [Google Scholar]
- 5.Ottolini MC, Shaer CM, Rushton HG, Majd M, Gonzales EC, Patel KM. Relationship of asymptomatic bacteriuria and renal scarring in children with neuropathic bladders who are practicing clean intermittent catheterization. J Pediatr. 1995;127:368–72. doi: 10.1016/s0022-3476(95)70065-x. [DOI] [PubMed] [Google Scholar]
- 6.Zegers B, Uiterwaal C, Kimpen J, van Gool J, de Jong T, Winkler- Seinstra P, et al. Antibiotic Prophylaxis for Urinary Tract Infections in Children With Spina Bifida on Intermittent Catheterization. J Urol. 2011;186:2365–70. doi: 10.1016/j.juro.2011.07.108. [DOI] [PubMed] [Google Scholar]
- 7.Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol. 1967;97:723–38. doi: 10.1016/0002-9378(67)90458-9. [DOI] [PubMed] [Google Scholar]
- 8.Covert bacteriuria in schoolgirls in Newcastle upon Tyne: A 5-year follow-up. Newcastle Covert Bacteriuria Research Group. Arch Dis Child. 1981;56:585–92. [PMC free article] [PubMed] [Google Scholar]
- 9.Schlager TA, Dilks S, Trudell J, Whittam TS, Hendley JO. Bacteriuria in children with neurogenic bladder treated with intermittent catheterization: Natural history. J Pediatr. 1995;126:490–6. doi: 10.1016/s0022-3476(95)70477-9. [DOI] [PubMed] [Google Scholar]
- 10.Boscia JA, Abrutyn E, Kaye D. Asymptomatic bacteriuria in elderly persons: treat or do not treat? Ann Intern Med. 1987 May;106(5):764–6. doi: 10.7326/0003-4819-106-5-764. [DOI] [PubMed] [Google Scholar]
- 11.Ragnarsdottir B, Fischer H, Godaly G, Gronberg-Hernandez J, Gustafsson M, Karpman D, et al. TLR- and CXCR1-dependent innate immunity: Insights into the genetics of urinary tract infections. Eur J Clin Invest. 2008;38(Suppl 2):12–20. doi: 10.1111/j.1365-2362.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 12.Storm DW, Patel AS, Koff SA, Justice SS. Novel management of urinary tract infections. Curr Opin Urol. 2011;21:328–33. doi: 10.1097/MOU.0b013e328346d4ee. [DOI] [PubMed] [Google Scholar]
- 13.Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol. 2010;64:203–21. doi: 10.1146/annurev.micro.112408.134258. [DOI] [PubMed] [Google Scholar]
- 14.Sivick KE, Mobley HL. Waging war against uropathogenic Escherichia coli: Winning back the urinary tract. Infect Immun. 2010;78:568–85. doi: 10.1128/IAI.01000-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–7. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 16.Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMPregulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–30. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 17.Eto DS, Sundsbak JL, Mulvey MA. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell Microbiol. 2006;8:704–17. doi: 10.1111/j.1462-5822.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 18.Eto DS, Gordon HB, Dhakal BK, Jones TA, Mulvey MA. Clathrin, AP-2, and the NPXY-binding subset of alternate endocytic adaptors facilitate FimHmediated bacterial invasion of host cells. Cell Microbiol. 2008;10:2553–67. doi: 10.1111/j.1462-5822.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- 19.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–9. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166:1148–55. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- 21.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, et al. From the cover: Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–8. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justice SS, Hunstad DA, Seed PC, Hultgren SJ. Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc Natl Acad Sci U S A. 2006;103:19884–9. doi: 10.1073/pnas.0606329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen DA, Pinkner JS, Jones JM, Walker JN, Clegg S, Hultgren SJ. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect Immun. 2008;76:3337–45. doi: 10.1128/IAI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath DJ, Jr, Li B, Casper T, Partida-Sanchez S, Hunstad DA, Hultgren SJ, et al. Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microbes Infect. 2011;13:426–37. doi: 10.1016/j.micinf.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song J, Bishop BL, Li G, Grady R, Stapleton A, Abraham SN. TLR4- mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci U S A. 2009;106:14966–71. doi: 10.1073/pnas.0900527106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaye D. Antibacterial activity of human urine. J Clin Investig. 1968;47:2374–90. doi: 10.1172/JCI105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garofalo CK, Hooton TM, Martin SM, Stamm WE, Palermo JJ, Gordon JI, et al. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect Immun. 2007;75:52–60. doi: 10.1128/IAI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlager TA, Hendley JO, Peters CA. Absence of bacterial reservoirs in the bladder epithelium of patients with chronic bacteriuria due to neurogenic bladder. J Urol. 2009;182(4 Suppl):1714–9. doi: 10.1016/j.juro.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 30.Elliott TS, Reed L, Slack RC, Bishop MC. Bacteriology and ultrastructure of the bladder in patients with urinary tract infections. J Infect. 1985;11:191–9. doi: 10.1016/s0163-4453(85)92997-4. [DOI] [PubMed] [Google Scholar]
- 31.Blango MG, Mulvey MA. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother. 2010;54:1855–63. doi: 10.1128/AAC.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprimsulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun. 2002;70:7042–9. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brauner A, Jacobson SH, Kuhn I. Urinary Escherichia coli causing recurrent infections--a prospective follow-up of biochemical phenotypes. Clin Nephrol. 1992;38:318–23. [PubMed] [Google Scholar]
- 34.Czaja CA, Stamm WE, Stapleton AE, Roberts PL, Hawn TR, Scholes D, et al. Prospective cohort study of microbial and inflammatory events immediately preceding Escherichia coli recurrent urinary tract infection in women. J Infect Dis. 2009;200:528–36. doi: 10.1086/600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikaheimo R, Siitonen A, Heiskanen T, Karkkainen U, Kuosmanen P, Lipponen P, et al. Recurrence of urinary tract infection in a primary care setting: Analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22:91–9. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson SH, Kuhn I, Brauner A. Biochemical fingerprinting of urinary Escherichia coli causing recurrent infections in women with pyelonephritic renal scarring. Scand J Urol Nephrol. 1992;26:373–7. doi: 10.3109/00365599209181229. [DOI] [PubMed] [Google Scholar]
- 37.Karkkainen UM, Ikaheimo R, Katila ML, Siitonen A. Recurrence of urinary tract infections in adult patients with community-acquired pyelonephritis caused by E.coli: A 1-year follow-up. Scand J Infect Dis. 2000;32:495–9. doi: 10.1080/003655400458767. [DOI] [PubMed] [Google Scholar]
- 38.Mulvey MA. Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol. 2002;4:257–71. doi: 10.1046/j.1462-5822.2002.00193.x. [DOI] [PubMed] [Google Scholar]
- 39.Russo TA, Stapleton A, Wenderoth S, Hooton TM, Stamm WE. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis. 1995;172:440–5. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz DJ, Chen SL, Hultgren SJ, Seed PC. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun. 2011;79:4250–9. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson GG, Dodson KW, Hooton TM, Hultgren SJ. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 2004;12:424–30. doi: 10.1016/j.tim.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Ragnarsdottir B, Lutay N, Gronberg-Hernandez J, Koves B, Svanborg C. Genetics of innate immunity and UTI susceptibility. Nat Rev Urol. 2011;8:449–68. doi: 10.1038/nrurol.2011.100. [DOI] [PubMed] [Google Scholar]