Abstract
Aims:
Port site metastasis (PSM) is an unwelcome consequence of laparoscopy for oncological procedures with uncertain etiology. We present the clinical evidence to prove that PSM is likely to be due to the hematogenous spread of tumor cells.
Materials and Methods:
Six cases of port site metastasis, four following laparoscopic radical nephrectomy for localized renal cell carcinoma (RCC), one after laparoscopic radical prostatectomy done in two patients and one after laparoscopic partial cystectomy for tumor at bladder dome done in two were studied. One case of metastatic RCC with bilateral gluteal masses and two cases of open radical nephrectomy, with recurrence at the drain and incision site were also studied.
Results:
During the median follow up of 59 months (range 24–120), 4/136 patients with RCC (1.47%) developed port site metastasis between 7–36 months after surgery. All six cases of PSM had advanced disease and recurrences at other sites, that is, peritoneum, omentum, bones, and lungs. None of the patients had isolated PSM. One patient of metastatic RCC, who did not have any intervention but repeated intramuscular injections of analgesics-developed bilateral gluteal masses, confirmed to be RCC on fine needle aspiration cytology. Two patients had metastasis at the incision site (one at the drain site) with distance, including cutaneous metastases.
Conclusions:
Port site metastasis did not develop in isolation. There could be a likely existence of circulating tumor cells at the time of surgical trauma of penetrating nature, that is, port site or injection site, which manifest in some patients depending upon their immune response.
Keywords: Laparoscopy, port site metastasis, recurrence
INTRODUCTION
After the first successful laparoscopic nephrectomy by Clayman et al. in 1991, there has been a rapid rise of laparoscopic procedures in urologic oncology.[1] Despite various advantages of a minimally invasive approach, oncological safety of laparoscopy has been a point of debate due to occurrence of port site metastasis (PSM) and tumor seeding.[2] Port site metastases are defined as recurrent cancerous lesions developing locally in the abdominal wall within the scar tissue at one or more trocar sites.[2]
The exact pathophysiology of PSM is not known. Multiple factors have been hypothesized and most of them are linked to laparoscopy.[3,4] Direct wound implantation, contamination of instruments, aerosolization of tumor cells, chimney effect, surgical technique, excessive manipulation of tumor, pneumoperitoneum, hematogenous spread, and local and systemic effects of the carbon dioxide pneumoperitoneum have been proposed. Even some have suggested gasless laparoscopy to reduce the incidence of PSM.[3–6]
It would be difficult to prove that the factors related to laparoscopy have a direct cause and effect relationship for the development of PSM. Most of the series have been speculative in attributing the cause for development of port site metastasis to laparoscopic factors. We hereby propose a hypothesis that laparoscopic factors are least likely to be responsible for port site metastasis, which is rather a consequence of hematogenous spread of tumor cells.
MATERIALS AND METHODS
Study subjects
Study subjects included six cases of port site metastasis; four cases following laparoscopic radical nephrectomy for localized renal cell carcinoma (RCC) and one each after laparoscopic radical prostatectomy and laparoscopic partial cystectomy for the tumor at the dome of the bladder. Study subjects also included one case of metastatic RCC who had repeated intramuscular injection at the bilateral gluteal region for pain and two cases of open radical nephrectomy, who had recurrence at the drain and incision sites.
Study period
All cases were performed between December 1999 and December 2008. Patients with recurrences were assessed with computed tomography (CT) of the ab-domen, chest x-ray, renal and liver function tests, and PET CT if required. The pattern of disease recurrence was studied along with the recurrences at the port, incision, and injection sites.
Initial laparoscopic approach
Laparoscopic nephrectomies were performed through the transperitoneal approach in 121 patients, retroperitoneal in five and combined approach (retroperitoneal renal artery clipping followed by transperitoneal nephrectomy) in ten patients. The standard three to four port approach was used. Similarly, partial cystectomy was done in two patients with localized tumor at the dome of the urinary bladder with lymphadenectomy. Laparoscopic radical prostatectomy patient was done outside our institute where the laparoscopic program was well established. Postoperative surveillance included history and physical exam, X-ray chest, ultrasonography, and blood tests 3–6 monthly and abdominal CT 1–2 yearly. Port site metastasis was diagnosed by physical examination, Contrast Enhanced Computerised Tomography (CECT), and pathological findings.
Specimen retrieval
A custom-made extraction bag (made from urobag) was used in all the cases for specimen retrieval without morcellation through a 5–7 cm incision. All the necessary precautions to avoid tumor spillage were taken.
RESULTS
The median age of the patients was 54 years (range 16–74years). Out of 136 patients who underwent radical nephrectomy, laparoscopic procedures could be accomplished successfully in 103 (five by retroperitoneal approach), while in 33 patients, laparoscopy was converted to open procedure due to various reasons such as bleeding at the hilum, improper case selection, learning curve and finding lymph nodes during the surgery. There was no tumor violation or breach in the continuity as the threshold for conversion was very low at the initial stages.
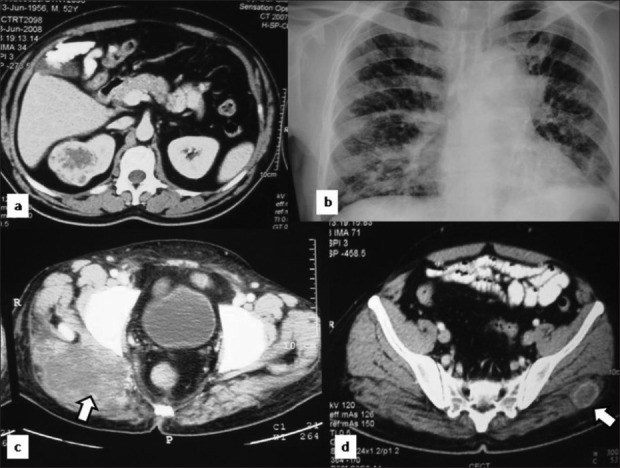
During the median follow up of 59 months (ranging between 24–120), 4/136 patients with renal cell carcinoma (1.47%) developed port site metastasis between 7–36 months following the surgery [Figures 1 and 2]. Clinical stage, histopathological characteristics, and location of the PSM are given in Table 1. One of the two patients with mucinous adenocarcinoma of the bladder, who had partial cystectomy and lymphadenectomy presented with PSM along with distant metastasis at 13 months following the surgery [Table 1]. Similarly, one patient who had laparoscopic radical prostatectomy was presented with skeletal metastasis and PSM at left working port 5 months after the surgery. [Table 1]
Figure 1.
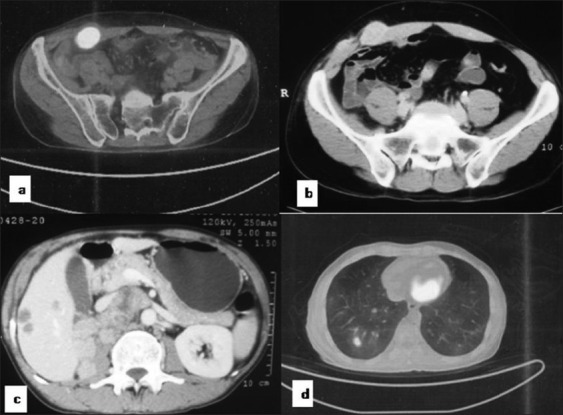
A case of renal cell carcinoma showing PSM on PET and NCCT (a, b) and distant metastasis in liver (c) and lung (d)
Figure 2.
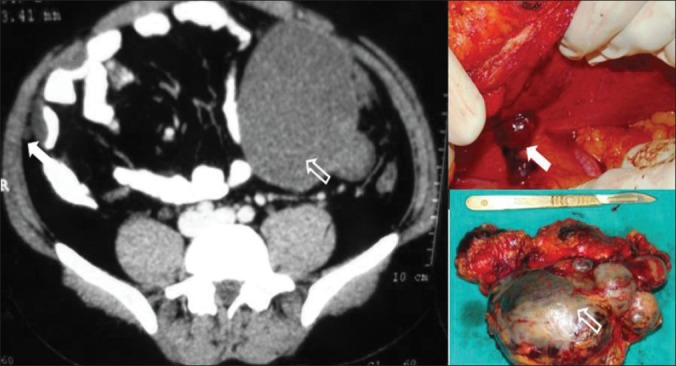
A case of RCC showing large mesenteric mass (arrow) and port site metastasis (solid white arrow)
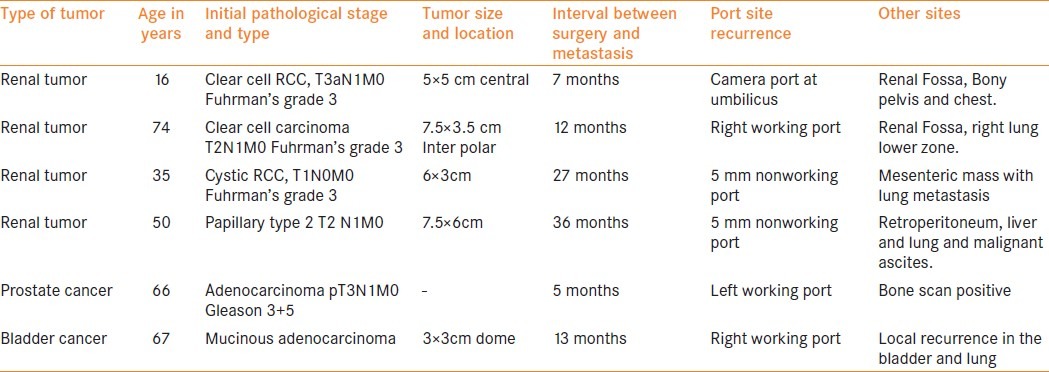
Table 1.
Port site metastasis following laparoscopic surgery
All six cases of port site metastasis had advanced disease and recurrences at other sites too, that is, peritoneum, abdominal wall, omentum, bony pelvis, and lung. None of the patients had isolated port site metastasis. Four of six had metastasis within a year of initial laparoscopic surgery and rest two patients with RCC presented at 27 and 36 months each following laparoscopic radical nephrectomy.
Metastatic pattern in nonlaparoscopic procedure
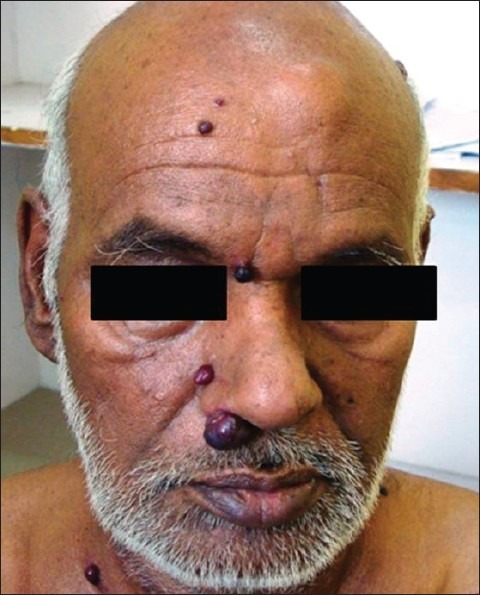
Of 225 patients of renal cell carcinoma operated during the same period with open radical nephrectomy, we picked up two cases of incision site metastasis [Table 2]. This may not represent the exact incidence in this group as attention was paid to look for incision site since 2008. One of those two patients had an incision and drain site recurrence along with other site metastatic lesions [Figure 3]. Another patient had multiple cutaneous metastases apart from recurrence at the incision site [Figure 4]. One patient of metastatic RCC, who did not have any intervention but repeated intramuscular injection in the gluteal region for backache, had bilateral gluteal masses, which were confirmed to be clear cell RCC on fine needle aspiration cytology [Figure 5].
Table 2.
Metastatic pattern in nonlaparoscopic surgery for renal cell carcinoma
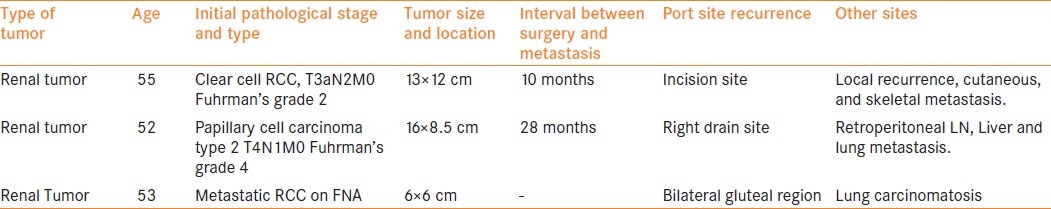
Figure 3.
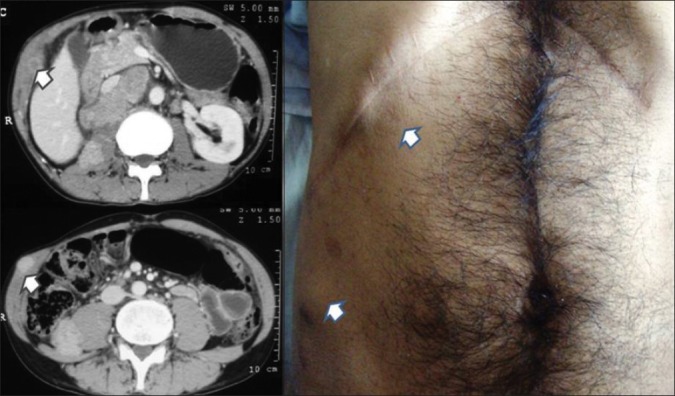
A case of open radical nephrectomy showing incisions site and drain site metastasis (marked with arrow)
Figure 4.
A case of open radical nephrectomy showing multiple cutaneous metastases
Figure 5.
A case of metastatic RCC showing bilateral gluteal masses at the injection sites
DISCUSSION
After the first reported case of PSM in 1978 by Debronte,[7] the incidence in the published surgical literature has been reported in a wide range between 0.6% and 21%.[8,9]
In urologic oncology, PSM was first reported in 1994 after laparoscopic lymphadenectomy for bladder cancer.[10] The incidence in the contemporary urologic literature accruing a large number of patients of various urological malignancies has been reported from 0.09% to 0.73%.[11,12]
The occurrence of port site tumor seeding after laparoscopy for malignant disease is a real concern. The initial high incidence reported in laparoscopic surgery done for gastrointestinal surgery and gynecology has not been seen in the same magnitude as in laparoscopic surgery done for urologic oncology. The exact pathophysiology of port site metastasis is not known, but various clinical and experimental studies have proposed factors such as natural tumor behavior, local wound factors, immune, and stress response of an individual and laparoscopy related factors.[13]
Various factors related to laparoscopy have been studied but their role remains controversial. Though initially aerosolization of tumor cells and peri-port gas leakage (chimney effect) have been extensively reported to be the possible mechanism that can lead to tumor cell dissemination and PSM, recent literature has a contrary report as the quantity of tumor cells needed for port site metastasis formation was extremely high.[14,15]
The moot question is whether port site recurrence is a result of implantation from the tumor being resected or it is an outcome of hematogenous spread. Local tumor cell implantation theory has not been proven and evidence supporting this mechanism is tenuous. Why would a properly removed T2/T3 renal cell cancer with no breach of tumor per se (proven by negative margin) have PSM? Why would one expect a PSM after diagnostic laparoscopy where tumor manipulation was absent?
When we looked at the magnitude of the problem of PSM, reported incidence from a single centre is 0.18% in 1098 patients, who had undergone laparoscopic procedures for urologic malignancies between 1992 and 2002.[11] Similarly in an international survey by Micali et al., on a total of 10 912 procedures done for various cancers the incidence of PSM was reported as 0.09%.[12]
Unlike laparoscopic surgery, there is not much attention given to the pattern of recurrence after open radical nephrectomy. In one series the incidence of scar metastases after open radical nephrectomy has been reported as 0.4%.[16] This incidence is much higher than the cumulative incidence reported by Micali et al. and raises an important point about the association of laparoscopy per se with the occurrence of PSM.
Tumor recurrence at the incision site following open surgery has been reported in GI malignancy. In one study, the incidence of recurrence at the port and at the incision site was same, that is, 13 of 1650 (0.79%) laparoscopic procedures and 9 of 1040 (0.86%) laparotomies.[17] In addition, reports have described tumor implantation in or near surgical sites in laparotomy scars, pelvic drain sites, and episiotomy scars in gynecologic malignancies.[18–20]
Once viable tumor cells release into the circulation, disseminated cancer cells called “seeds,” would only colonize in an organ where microenvironments (soil) is conducive for their growth.[21,22] The trauma caused at port insertion site leads to release of growth factors creating a premetastatic niche or fertile soil for the tumor cells to lodge there and grow. Tumor cells implant in much better way in the early stages of wound healing as they benefit from the release of growth factors.[23,24]
It is a common parlance in urologist fraternity that if hematogenous spread is the reason for PSM then more often than not we should be seeing incision site metastasis following open radical surgery. Port site metastasis in laparoscopy is probably a bias as incision site metastasis is seen after open oncological surgeries also but are not talked about as often. A plausible reason for port site being a favored site for PSM could be that penetrating wounds are more prone to home circulating tumor cells as we saw in two of our cases, that is, drain site metastasis and metastases in the gluteal region.
Another reason to support PSM to be the consequence of hematogenous spread of tumor cells is that none of our patients had isolated port site recurrence and all six had disseminated disease. Similar observations were made by various authors who found that PSM as a solitary site of recurrence was rare and most probably it reflected the aggressive biology of malignant disease and the level of immunosuppression of an individual rather than the technical aspects of the laparoscopic approach.[11,25,26] In one study on colon cancer surgery, it was found that laparotomy scar tumor implantation occurred in 13 of 1600 cases, of which the majority were accompanied by carcinomatosis. Isolated tumor deposits were noted in only 0.2% of their patients.[27]
Moreover, in an isolated PSM, it is difficult to prove the presence of micrometastasis or emerging metastasis somewhere else. A follow up study would facilitate a further understanding of this concept of PSM being a result of hematogenous spread. In another interesting review of 31 cases of PSM in urologic malignancy, reported in English literature till 2008, it was found that the majority of the cases had higher stage and grades of tumors and most of them had widespread metastases too.[28] Among the isolated PSM, where follow up was available in the reported cases, majority of them died within 1 year of the development of PSM.[28]
Clinical evidence presented in this study favors hematogenous seeding of circulating cells, which home in at the site of injury and depending on the biological nature of the tumor cells, tumor cell load, and individual immune response, the process of homing and subsequent growth of the tumor cells results. However, one should not only continue to take necessary precautions during the laparoscopy for cancer surgery, but also should keep a very low threshold for conversion to open surgery. Choosing the right patient of cancer for laparoscopy and further understanding of mechanism of PSM would allay the anxiety of PSM after laparoscopic surgery. This hypothesis, that PSM is a consequence of hematogenous spread of tumor cells, can prospectively be studied by documenting circulating tumor cells and by doing proper follow up of all the cases that develop PSM. This would further shed some light on the exact mechanism of occurrence of PSM.
CONCLUSION
Port site metastasis after laparoscopic procedures for malignancy may not be the outcome of the laparoscopic technique as such. Most of the time it is associated with an advance stage of the malignancy and portends a poor prognosis. Based on the clinical evidence we present, we suggest that port site metastasis is an expression of an aggressive malignancy disease. Its occurrence is a result of homing in the circulating tumor cells at the site of injury and subsequent growth, depending upon the biological nature of the malignancy and local host factors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Clayman RV, Kavoussi LR, Soper NJ, Dierks SM, Merely S, Darcy MD. Laparoscopic nephrectomy: Initial case report. J Urol. 1991;146:278–82. doi: 10.1016/s0022-5347(17)37770-4. [DOI] [PubMed] [Google Scholar]
- 2.Schneider C, Jung A, Reymond MA, Tannapfel A, Balli J, Franklin ME, et al. Efficacy of surgical measures in preventing port site recurrences in a porcine model. Surg Endosc. 2001;15:121–5. doi: 10.1007/s004640010069. [DOI] [PubMed] [Google Scholar]
- 3.Jones DB, Goo LW, Reinhardt MK, Super NJ, Philpot GW, Connett J, et al. Impact of pneumoperitoneum on trocar site implantation of colon cancer in hamster model. Dis Colon Rectum. 1995;38:1182–8. doi: 10.1007/BF02048334. [DOI] [PubMed] [Google Scholar]
- 4.Mathew G, Watson DI, Ellis T, De Young N, Rofe AM, Jamieson GG. The effect of laparoscopy on the movement of tumor cells and metastasis to surgical wounds. Surg Endosc. 1997;11:1163–6. doi: 10.1007/s004649900561. [DOI] [PubMed] [Google Scholar]
- 5.Bouvy ND, Marquet RL, Jeekel H, Bonjer HJ. Impact of gas(less) laparoscopy and laparotomy on peritoneal tumor growth and abdominal wall metastases. Ann Surg. 1996;224:694–700. doi: 10.1097/00000658-199612000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson DI, Mathew G, Ellis T, Baigrie CF, Rofe AM, Jamieson GG. Gasless laparoscopy may reduce the risk of port-site metastases following laparoscopic tumor surgery. Arch Surg. 1997;132:166–8. doi: 10.1001/archsurg.1997.01430260064014. [DOI] [PubMed] [Google Scholar]
- 7.Dobronte Z, Wittmann T, Karacsony G. Rapid development of malignant metastases in the abdominal wall after laparoscopy. Endoscopy. 1978;10:127–30. doi: 10.1055/s-0028-1098280. [DOI] [PubMed] [Google Scholar]
- 8.Wexner SD, Cohen SM, Ulrich A, Reissman P. Laparoscopic colorectal surgery—are we being honest with our patients? Dis Colon Rectum. 1995;38:723–7. doi: 10.1007/BF02048029. [DOI] [PubMed] [Google Scholar]
- 9.Berends FJ, Kazemier G, Bonjer HJ, Lange JF. Subcutaneous metastases after laparoscopic colectomy. Lancet. 1994;344:58. doi: 10.1016/s0140-6736(94)91079-0. [DOI] [PubMed] [Google Scholar]
- 10.Stolla V, Rossi D, Bladou F, Rattier C, Ayuso D, Serment G. Subcutaneous metastasis after coelioscopic lymphadenectomy for vesical urothelial carcinoma. Eur Urol. 1994;26:342–3. doi: 10.1159/000475412. [DOI] [PubMed] [Google Scholar]
- 11.Rassweiler J, Tsivian A, Kumar AV, Lymberakis C, Schulze M, Seeman O, et al. Oncological safety of laparoscopic surgery for urological malignancy: Experience with more than 1,000 operations. J Urol. 2003;169:2072–5. doi: 10.1097/01.ju.0000067469.01244.5c. [DOI] [PubMed] [Google Scholar]
- 12.Micali S, Celia A, Bove P, De Stefani S, Sighinolfi MC, Kavoussi LR, et al. Tumor seeding in urological laparoscopy: An international survey. J Urol. 2004;171:2151–4. doi: 10.1097/01.ju.0000124929.05706.6b. [DOI] [PubMed] [Google Scholar]
- 13.Whelan RL, Lee SW. Review of investigations regarding the etiology of port site tumor recurrence. J Laparoendosc Adv Surg Tech A. 1999;9:1–16. doi: 10.1089/lap.1999.9.1. [DOI] [PubMed] [Google Scholar]
- 14.Kazemier G, Bonjer HJ, Berends FJ, Lange JF. Port site metastases after laparoscopic colorectal surgery for cure of malignancy. Br J Surg. 1995;82:1141–2. doi: 10.1002/bjs.1800820850. [DOI] [PubMed] [Google Scholar]
- 15.Wittich P, Marquet RL, Kazemier G, Bonjer HJ. Port-site metastases after CO(2) laparoscopy. Is aerosolization of tumor cells a pivotal factor? Surg Endosc. 2000;14:189–92. doi: 10.1007/s004649900098. [DOI] [PubMed] [Google Scholar]
- 16.Uson AC. Tumor recurrence in the renal fossa and/or abdominal wall after radical nephrectomy for renal cell cancer. Prog Clin Biol Res. 1982;100:549–60. [PubMed] [Google Scholar]
- 17.Stenson R, Jacobs AJ, Janney CG, Schmidt DA. Incisional recurrence of squamous cell cervical carcinoma following operative staging. Gynecol Oncol. 1990;39:232–5. doi: 10.1016/0090-8258(90)90440-v. [DOI] [PubMed] [Google Scholar]
- 18.Copas PR, Spann CO, Thoms WW, Horowitz IR. Squamous cell carcinoma of the cervix metastatic to a drain site. Gynecol Oncol. 1995;56:102–4. doi: 10.1006/gyno.1995.1018. [DOI] [PubMed] [Google Scholar]
- 19.Copeland LJ, Saul PB, Sneige N. Cervical adenocarcinoma tumor implantation in the episiotomy sites of two patients. Gynecol Oncol. 1987;28:230–5. doi: 10.1016/0090-8258(87)90219-8. [DOI] [PubMed] [Google Scholar]
- 20.Stewart GD, Tolley DA. What are the oncological risks of minimal access surgery for the treatment of urinary tract cancer? Eur Urol. 2004;46:415–20. doi: 10.1016/j.eururo.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Fidler IJ. The pathogenesis of cancer metastasis: The seed and soil hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 22.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3. [PubMed] [Google Scholar]
- 23.Murthy SM, Goldschmidt RA, Rao LN, Ammirati M, Buchmann T, Scanlon EF. The influence of surgical trauma on experimental metastasis. Cancer. 1989;64:2035–44. doi: 10.1002/1097-0142(19891115)64:10<2035::aid-cncr2820641012>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Tseng LN, Berends FJ, Wittich P, Bouvy ND, Marquet RL, Kazemier G, et al. Port-site metastases, Impact of local tissue trauma and gas leakage. Surg Endosc. 1998;12:1377–80. doi: 10.1007/s004649900862. [DOI] [PubMed] [Google Scholar]
- 25.Pearlstone DB, Feig BW, Mansfield PF. Port site recurrence after laparoscopy for malignant disease. Semin Surg Oncol. 1999;16:307–12. doi: 10.1002/(sici)1098-2388(199906)16:4<307::aid-ssu5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Savalgi RS. Port-site metastasis in the abdominal wall: Fact or fiction? Semin Surg Oncol. 1998;15:189–93. doi: 10.1002/(sici)1098-2388(199810/11)15:3<189::aid-ssu8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Hughes ES, McDermott FT, Polglase AL, Johnson WR. Tumour recurrence in the abdominal wall scar tissue after large–bowel cancer surgery. Dis Colon Rectum. 1983;26:571–2. doi: 10.1007/BF02552962. [DOI] [PubMed] [Google Scholar]
- 28.Castillo OA, Vitagliano G. Port site metastasis and tumor seeding in oncologic laparoscopic urology. Urology. 2008;71:372–8. doi: 10.1016/j.urology.2007.10.064. [DOI] [PubMed] [Google Scholar]


