Abstract
Aims:
To investigate the outcomes of the surgical management and longitudinal assessment of patients with subclinical Cushing's syndrome (SCS) and nonfunctioning adrenocortical adenoma (NFA).
Materials and Methods:
Between the years 1995 and 2008, 73 patients with asymptomatic adrenocortical adenoma were enrolled. They were informed of the risks and benefits of adrenalectomy and conservative management, and then chose the treatment.
Results:
SCS was observed in 13 patients (17.8%) and NFA in 60 patients (82.2%). Tumor size in SCS was significantly larger than that in NFA (34.6 ± 9.7 mm vs. 24.5 ± 8.0 mm in diameter, P=0.001). Of the SCS patients, 7 also suffered from hypertension (HT), 2 from diabetes mellitus (DM) and 3 from hyperlipidemia (HL). After adrenalectomy, the insulin dose could be reduced in 2 (100%) patients with DM, in 5 (71.4%) of those with HT and in 2 (66.7%) of those with HL. In the NFA surveillance group, 1 (2.6%) case developed into SCS 3 years after the initial presentation and an increase in size of the tumor was observed in 1 (2.6%), with a mean follow-up of 51.2 months.
Conclusions:
Surgical resection may be beneficial for the control of SCS and is likely to provide improvement of concomitant HT, DM and HL. Although NFA can be managed conservatively, its size and hormonal activities may change longitudinally. Thus, long-term follow-up is necessary for NFA.
Keywords: Adrenal incidentalomas, adrenalectomy, conservative management, nonfunctioning adrenocortical adenoma, subclinical Cushing's syndrome
INTRODUCTION
More than half of adrenal incidentalomas consist of subclinical Cushing's syndrome (SCS) and nonfunctioning cortical adenoma (NFA) in recent studies.[1–3] SCS and NFA are often difficult to differentiate in the clinical setting. The diagnostic criteria for SCS have not been established yet, although several different criteria were used.[3,4] In addition, there is no consensus as to whether surgery is appropriate.[5,6] It is apparent that SCS has autonomous cortisol production when it is diagnosed using proper criteria. However, it is not clear that hypertension and diabetes mellitus can be caused by SCS and the benefit of surgical treatment has not been clarified.
NFA has no endocrinological activity and can be managed conservatively. However, in a few patients, an increase of tumor size occurs. In addition, hormonal activity becomes apparent during follow-up,[7] which suggests that NFA has the potential to develop into subclinical or overt Cushing's syndrome.
In this study, we investigated the outcome of the surgical management and longitudinal assessment of patients with SCS and NFA to verify the validity of the diagnostic criteria and management strategy in our institution.
MATERIALS AND METHODS
This study included a total of 73 patients with adrenal incidentaloma who visited our institution from January 1995 to December 2008. All these patients were confirmed not to have primary aldosteronism or pheochromocytoma by evaluating the plasma renin activity, plasma aldosterone level and blood and urinary catecholamine levels. In addition, computed tomography (CT) revealed no findings suggestive of malignant tumors such as infiltration into surrounding tissue or distant metastases in the patients. Tumors with radiographic findings suggestive of myelolipoma, extraadrenal origin or concomitant active malignant disease in other organs were also excluded.
Endocrinological examinations for 73 patients consisted of serum adrenocorticotropic hormone (ACTH) and cortisol measurement in the morning, serum ACTH and cortisol circadian rhythm determination, and adrenocortical scintigraphy using 131I-6β-iodomethyl-19-norcholest-5(10)-en-3β-ol (NP-59). To determine the circadian rhythm of ACTH and cortisol, serum samples were obtained at 6:00 AM and 4:00 PM. The normal morning/evening ratio of ACTH and cortisol levels was defined as 1.33 or more. A dexamethasone suppression test was performed under oral administration of 1 mg of dexamethasone at 9:00 PM with measurement of serum cortisol at 4:00 PM before its administration and at 8:00 AM on the following day. Diagnosis of SCS was made according to the following criteria: Adrenal incidentaloma with lack of overt clinical features of Cushing's syndrome, normal basal cortisol levels, and serum cortisol levels higher than 3 μg/dL after 1 mg dexamethasone suppression. In addition, patients had to have at least one of the criteria consisting of suppression of plasma ACTH, loss of cortisol circadian rhythm, a decreased serum dehydroepiandrosterone sulfate level or unilateral visualization by adrenocortical scintigraphy.
We recommended adrenalectomy for patients with SCS of any size, and NFA with a diameter of 4 cm or larger, which was regarded as potential malignancy. In SCS patients, postoperative cortisol substitution was routinely done with regular monitoring of serum ACTH and cortisol levels, which was discontinued when intrinsic cortisol secretion was confirmed. To those with NFA smaller than 4 cm in diameter, we presented the options of surgical treatment or conservative management with periodical examinations including imaging studies and hormonal examinations every 6-12 months.
Concomitant diseases such as hypertension, diabetes mellitus and hyperlipidemia were also assessed. In patients who underwent adrenalectomy, changes in the condition of comorbidities after surgery were evaluated.
Continuous variables were compared using the Mann Whitney U test and categorized variables were compared using Fisher's exact probability test.
RESULTS
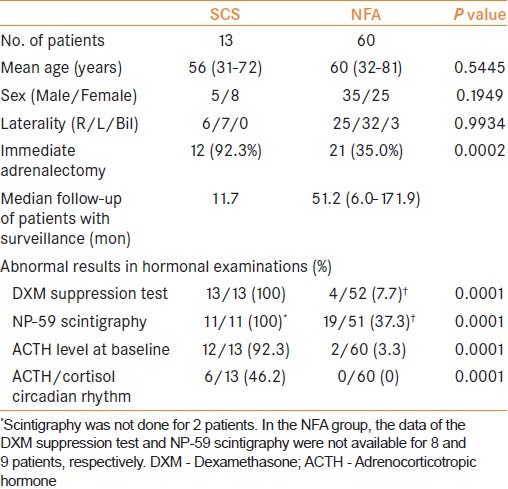
Patients’ characteristics are summarized in Table 1. Thirteen patients met our criteria for SCS. All of them underwent adrenalectomy except one patient who refused surgical treatment. Of the NFA patients, 21 (35.0%) underwent adrenalectomy. All of the adrenalectomies were performed laparoscopically with no perioperative complications. Histological examination proved no finding of malignancy in any of the resected tumors. In NFA patients who selected surveillance, the mean follow-up period was 51.2 months. There was no significant difference in age, sex, and tumor laterality between SCS and NFA. SCS showed significantly higher rates for all parameters of hormonal activity than NFA. In particular, SCS showed an extremely high positive rate for increased uptake in NP-59 scintigraphy and a low level of ACTH.
Table 1.
Characteristics of patients with subclinical Cushing's syndrome and nonfunctioning adenoma
Mean tumor size was 34.6 mm in SCS patients, which was significantly larger than that of NFA (24.5 mm, P=0.0102). In addition, tumor size in SCS was rarely smaller than 20 mm [Figure 1].
Figure 1.
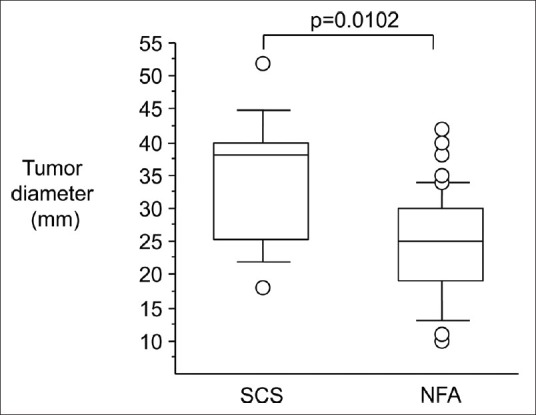
Tumor diameters with subclinical Cushing's syndrome and nonfunctioning adenoma. They are indicated in a box and whisker diagram. The ends of each box are the interquartile ranges 25% and 75%. The line in each box is the statistical median
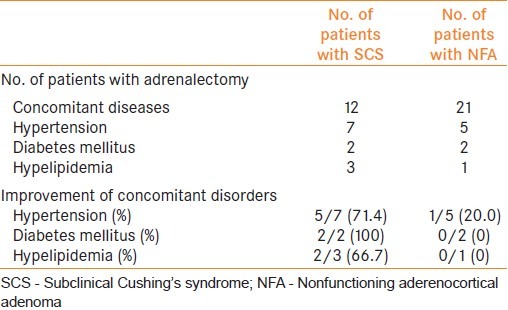
Of the patients with SCS, hypertension was found in 7, diabetes in 2 and hyperlipidemia in 3. Among the patients with hypertension, the antihypertensive drug was withdrawn in one and dose reduction of the drug was successful in 4 after adrenalectomy. In all those with diabetes, glucose tolerance became improved after the operation. One of the patients became free of insulin injection and reduction of its dose was successful for another patient. Similarly, 60% of patients having hyperlipidemia showed improvement in control of low-density lipoprotein cholesterol and triglyceride. Postoperative cortisol substitution was done for all patients and could be discontinued in a mean period of 14.6 months (1.0-144.0) after the adrenalectomy.
On the other hand, for NFA, hypertension was improved only in one patient and no one showed any improvement in diabetes or hyperlipidemia [Table 2]. The improvement rate was significantly lower than for those with SCS.
Table 2.
Improvement of concomitant disorders after surgery
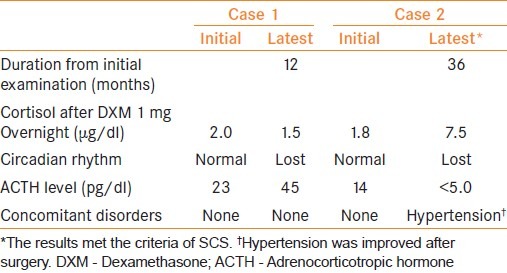
During surveillance of NFA patients, 2 (5.1%) patients developed hormonal abnormalities [Table 3]. In Case 1, the latest examination showed a loss of circadian rhythm of serum cortisol, which was not observed at the initial examination 12 months earlier. In Case 2, examination 36 months after the initial presentation revealed suppression of cortisol after DXM 1 mg overnight and loss of circadian rhythm of serum cortisol with suppression of serum basal ACTH, which were confirmed by repeated measurement. In addition, hypertension became apparent. The patient was diagnosed as developing SCS; then underwent laparoscopic adrenalectomy with subsequent improvement of the hypertension.
Table 3.
NFA patients developing endocrine abnormalities
Among the NFA patients who underwent conservative management, an increase in size of the tumor occurred in 1 (2.6%). The tumor grew from 26 mm to 46 mm, 10 years after the first examination, and the patient then underwent adrenalectomy. Histological examination revealed cortical adenoma without any findings suggestive of malignancy.
DISCUSSION
Improvements in abdominal imaging techniques and technologies have increased the detection of adrenal incidentalomas,[1] and SCS and NFA account for about half of them.[8] The diagnostic criteria for SCS have not been established, so a differential diagnosis of SCS and NFA is often difficult in the clinical setting. Moreover, some cases of NFA show changes in hormonal activity during longitudinal assessment and may have the potential to develop into SCS.[7] The dexamethasone suppression test is commonly employed as a screening examination for adrenal cortical function in incidentally discovered adrenal adenomas,[8,9] in addition to measurement of the basal serum levels and circadian rhythm of serum ACTH and cortisol. The American Association of Clinical Endocrinologist and American Association of Endocrine Surgeons (ACCE/AAES) guideline recommends a cutoff point of 5 μg/dl for the dexamethasone suppression test.[9] We used the cutoff point of 3 μg/dl in this study, and the specificity of the test may have been lower. However, our diagnostic criteria included other parameters that supported the accuracy of the final diagnosis. The efficacy of NP-59 scintigraphy has also been reported[10] and it is widely used. Grumbach et al.[1] recommended that all patients with adrenal incidentaloma undergo the dexamethasone suppression test, irrespective of tumor size. On the other hand, of those with tumor sizes smaller than 20 mm, only one showed autonomous cortisol secretion in our series. In addition, most of those with SCS had a low ACTH level at baseline, with a cutoff level of 10 pg/ml in the morning. Exceptionally, 1 patient with a tumor 20 mm in diameter had an ACTH level of 12 pg/ml, but abnormal uptake in NP-59 scintigraphy. Our results suggest that we can rule out hormonally active tumors among those with sizes smaller than 20 mm by assessing the serum ACTH and cortisol levels at baseline and NP-59 scintigraphy in the outpatient setting. To conclude that, further studies with large numbers of patients are needed. Libe et al.[11] reported that only 40% of SCS patients had a low ACTH level, with a cutoff level of 0.7 pmol/L (3.18 pg/ml). Its low sensitivity may be due to the strict criterion. In patients with adrenal incidentaloma, after pheochromocytoma and primary aldosteronism are ruled out, those with tumor sizes of 20 mm or larger in diameter or serum ACTH less than 10 pg/ml at baseline should undergo further detailed examinations such as the dexamethasone suppression test and determination of the circadian rhythm of serum ACTH and cortisol.
In treatment of SCS, the definite advantage of adrenalectomy over conservative management has not been determined.[1,8] However, several studies indicated that the surgery improved conditions associated with hypercortisolism such as hypertension, impaired glucose tolerance, and hyperlipidemia in some patients.[12,13] Moreover, some patients with SCS showed deterioration of these conditions during conservative management.[6] Tauchmanovà et al. pointed out that chronic mild excess cortisol secretion might lead to an increased risk of cardiovascular disease.[14] Our results also support the clinical benefits of adrenalectomy for SCS. In addition, adrenalectomy can be performed laparoscopically with very minimal invasiveness.[15] Although postoperative steroid substitution is needed, it can be ceased within one year without any complications.[2] On the other hand, in NFA patients, less benefit for cardiovascular and metabolic status was provided by surgical treatment. This may indicate the validity of our criteria to diagnose SCS.
For large nonfunctioning adrenal masses, surgical treatment is recommended due to their possible malignancy, but not for those smaller than 4 cm in diameter.[1,8] Our series included no adrenocortical carcinoma even in those with masses 4 cm or larger. In addition to the low incidence, adrenocortical carcinoma tends to have clinically overt hormonal activity or metastatic lesions,[16] which may account for the rarity of adrenocortical carcinoma in incidentalomas. In the short term, surgical treatment seems to be less beneficial for NFA because there was little contribution to improvement of concomitant disease in our series. However, some of them showed growth and development of autonomous cortisol secretion subsequently.[11,17,18] Long-term periodic radiographic and endocrinological examinations may be essential,[8] although a definite interval and the time of completion of follow-up are difficult to establish.
CONCLUSIONS
The results of this study suggest that we can omit routine detailed assessment other than basal hormonal examination in patients with adrenocortical adenoma smaller than 20 mm in diameter, which is unlikely to have autonomous cortisol secretion. For SCS, surgical resection is beneficial for the control of concomitant hypertension, diabetes and hyperlipidemia. Although NFA can be managed conservatively, long-term follow-up with periodic examinations may be necessary.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, et al. Management of the clinically inapparent adrenal mass (“incidentaloma”) Ann Intern Med. 2003;138:424–9. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 2.Emral R, Uysal AR, Asik M, Gullu S, Corapcioglu D, Tonyukuk V, et al. Prevalence of subclinical Cushing's syndrome in 70 patients with adrenal incidentaloma: Clinical, biochemical and surgical outcomes. Endocr J. 2003;50:399–408. doi: 10.1507/endocrj.50.399. [DOI] [PubMed] [Google Scholar]
- 3.Terzolo M, Reimondo G, Bovio S, Angeli A. Subclinical Cushing's syndrome. Pituitary. 2004;7:217–23. doi: 10.1007/s11102-005-4024-6. [DOI] [PubMed] [Google Scholar]
- 4.Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, et al. Subclinical Cushing's syndrome in patients with adrenal incidentaloma: Clinical and biochemical features. J Clin Endocrinol Metab. 2000;85:1440–8. doi: 10.1210/jcem.85.4.6515. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell IC, Auchus RJ, Juneja K, Chang AY, Holt SA, Snyder WH, 3rd, et al. “Subclinical Cushing's syndrome” is not subclinical: Improvement after adrenalectomy in 9 patients. Surgery. 2007;142:900–5. doi: 10.1016/j.surg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Toniato A, Merante-Boschin I, Opocher G, Pelizzo MR, Schiavi F, Ballotta E. Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: A prospective randomized study. Ann Surg. 2009;249:388–91. doi: 10.1097/SLA.0b013e31819a47d2. [DOI] [PubMed] [Google Scholar]
- 7.Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273–85. doi: 10.1530/eje.0.1490273. [DOI] [PubMed] [Google Scholar]
- 8.Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: Update in diagnosis and management. Endocr Rev. 2004;25:309–40. doi: 10.1210/er.2002-0031. [DOI] [PubMed] [Google Scholar]
- 9.Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, et al. American Association of Clinical Endcocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1–20. doi: 10.4158/EP.15.S1.1. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez-Gadea L, Diez L, Piedrola-Maroto G, Crespo A. Scintigraphic diagnosis of subclinical Cushing's syndrome in patients with adrenal incidentalomas. Nucl Med Commun. 1996;17:29–32. doi: 10.1097/00006231-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Libè R, Dall’Asta C, Barbetta L, Baccarelli A, Beck-Peccoz P, Ambrosi B. Long-term follow-up study of patients with adrenal incidentalomas. Eur J Endocrinol. 2002;147:489–94. doi: 10.1530/eje.0.1470489. [DOI] [PubMed] [Google Scholar]
- 12.Reincke M. Subclinical Cushing's syndrome. Endocrinol Metab Clin North Am. 2000;29:43–56. doi: 10.1016/s0889-8529(05)70115-8. [DOI] [PubMed] [Google Scholar]
- 13.Bernini G, Moretti A, Iacconi P, Miccoli P, Nami R, Lucani B, et al. Anthropometric, haemodynamic, humoral and hormonal evaluation in patients with incidental adrenocortical adenomas before and after surgery. Eur J Endocrinol. 2003;148:213–9. doi: 10.1530/eje.0.1480213. [DOI] [PubMed] [Google Scholar]
- 14.Tauchmanovà L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri EA, et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002;87:4872–8. doi: 10.1210/jc.2001-011766. [DOI] [PubMed] [Google Scholar]
- 15.Mutoh M, Takeyama K, Nishiyama N, Kunishima Y, Matsukawa M, Takahashi S, et al. Systemic inflammatory response syndrome in open versus laparoscopic adrenalectomy. Urology. 2004;64:422–5. doi: 10.1016/j.urology.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 16.Nishida S, Itoh N, Sasao T, Masumori N, Taguchi K, Tsukamoto T. Adrenocortical carcinoma: Retrospective study of 14 patients experienced at a single institution over 34 years. Int J Urol. 2007;14:581–4. doi: 10.1111/j.1442-2042.2007.01792.x. [DOI] [PubMed] [Google Scholar]
- 17.Barzon L, Scaroni C, Sonino N, Fallo F, Paoletta A, Boscaro M. Risk factors and long-term follow-up of adrenal incidentalomas. J Clin Endocrinol Metab. 1999;84:520–6. doi: 10.1210/jcem.84.2.5444. [DOI] [PubMed] [Google Scholar]
- 18.Grossrubatscher E, Vignati F, Possa M, Lohi P. The natural history of incidentally discovered adrenocortical adenomas: A retrospective evaluation. J Endocrinol Invest. 2001;24:846–55. doi: 10.1007/BF03343941. [DOI] [PubMed] [Google Scholar]


