Abstract
Background:
Oral submucous fibrosis (OSF) is a precancerous condition in which there is excessive deposition of collagen in connective tissue. The purpose of present study was to compare changes in birefringence of collagen fibers in histopathological stages of OSF.
Materials and Methods:
Collagen in connective tissue of 91 cases of OSF was studied by staining 5 μm thick sections with picrosirius red. The polarization colors of thin (0.8 μm or less) and thick (1.6-2 μm) collagen fibers were recorded.
Results:
The birefringence of thin collagen fibers showed no difference in both histopathological connective tissue stages and degrees of epithelial dysplasia. The polarization colors of thick collagen fibers showed a gradual change from predominantly yellow-orange to greenish-yellow in advancing connective tissue stages and degrees of epithelial dysplasia.
Conclusion:
The results of present study show a significant change in birefringence of collagen between connective tissue stages and between mild, moderate to severe degree of epithelial dysplasia. This change in birefringence colors and arrangement of collagen fibers may give an implication of impending neoplastic change in OSF.
Keywords: Collagen, connective tissue, oral submucous fibrosis, picrosirius red, polarizing microscopy
INTRODUCTION
Oral submucous fibrosis (OSF) is an insidious chronic disease affecting any part of the oral cavity and sometimes pharynx. It is always associated with juxta-epithelial inflammatory reaction followed by fibro-elastic change in lamina propria and epithelial atrophy; which leads to stiffness of the oral mucosa, causing trismus and inability to eat.[1] The fibro-elastic changes seen are due to excessive collagen deposition resulting in dense fibrous bands. OSF occurs predominantly in people of Indian subcontinent and South Asian ethnicity. Initially the disease was mainly found among natives of Indian subcontinent; later it was reported from many South-East Asian populations as well.[2] Even though, Schwartz[3] was the first person to describe the disease as a fibrosing condition for which he coined the term “atrophia idiopathica tropica mucosae oris”, however the disease was later renamed as “oral submucous fibrosis” by Joshi[4] and is most widely accepted.
Areca nut is the main etiologic factor of OSF and is a main ingredient in commercially available preparations such as pan masala and gutkha. As use of these preparations is increasing, prevalence of OSF is on the rise among younger population and majority of the cases occur between 20 to 40 years age group. Unfortunately, even after cessation of the causative habit, all clinical and histologic features of the disease will remain.[5]
OSF is a well-recognized precancerous condition with frequency of malignant transformation reported in the range of 7-13%.[6,7] The precancerous nature was first mentioned by Paymaster[8] who observed development of squamous cell carcinoma in one-third of his OSF patients. In South-India, Pindborg and Zachariah observed 40% of oral cancer patients had OSF.[9]
The changes in OSF are mainly due to increased collagen deposition in connective tissue, subsequent to which there are changes in epithelium. Purpose of the present study was to evaluate changes in birefringence of collagen in different histopathological stages of OSF, which may help in early detection of neoplastic changes as connective tissue changes are believed to precede epithelial changes. Further, an attempt was made to correlate changes in birefringence of collagen fibers with degrees of epithelial dysplasia in OSF.
MATERIALS AND METHODS
For the present study, 91 diagnosed cases of OSF were retrieved from files of Oral Pathology Laboratory. The diagnosis was based on clinical and histologic features. From each of paraffin embedded tissue block, two sections (serial sections) of 5 μm thickness were prepared with semi automatic microtome. One of the sections was stained with Hematoxylin and Eosin and other with modified picrosirius red procedure.[10] After deparaffinization, sections were hydrated in distilled water and were incubated in 0.1% (w/v) Sirius red F3B (C.I.35780) in saturated picric acid solution for one hour at room temperature. The sections were then rinsed with distilled water, counter stained with Mayer's hematoxylin, differentiated in 1% HCl, rinsed with tap water, dehydrated and mounted with DPX.
The Hematoxylin and Eosin stained sections were examined with light microscopy and cases were categorized depending on connective tissue changes into very early, early, moderately advanced and advanced stages according to Sirsat and Pindborg[11] and also into cases with no epithelial dysplasia, mild epithelial dysplasia, moderate epithelial dysplasia and severe epithelial dysplasia.[12] It is stated that atypia in OSF rarely exhibits signs of basal cell hyperplasia; instead there will be markedly irregular epithelial stratification, nuclear pleomorphism and pronounced intercellular edema.[13] Five among 91 cases showed invasion of epithelial cells into connective tissue which were categorized as OSF with carcinoma. Subsequently, picrosirius red stained sections were examined under polarizing microscope for collagen fiber arrangement in connective tissue. Areas showing epithelial ulceration and dense inflammatory cell infiltration in the connective tissue were excluded as inflammation is said to have an impact on packing of collagen fibers.[14] Areas of connective tissue devoid of inflammation and those with mild inflammatory cell infiltration were considered for the study as mild inflammation is commonly found in cases of OSF. Thickness of collagen fibers was determined with a calibrated ocular micrometer using an oil immersion ×100 objective. Polarization colors were noted for at least 100 thin (0.8 μm or less) and 100 thick collagen fibers (1.6-2.4μm) in each section. Data read independently by two of the authors was found to be comparable and analyzed statistically using Kruskal-Wallis and Mann Whitney test.
RESULTS
Polarization colors of collagen in connective tissue stages of OSF
When observed for predominance of fibers, there was increase in thick collagen fibers with advancing connective tissue stages [Table 1], which was significant (p=0.000).
Table 1.
Type of collagen fiber arrangement seen in the connective tissue stages of OSF
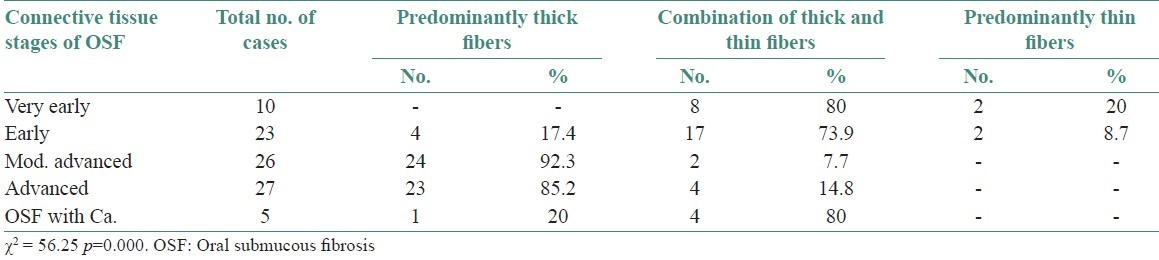
Polarization colors of most of the thin collagen fibers were greenish-yellow (84.8-92 %). The changes in polarization colors of thin collagen fibers [Figure 1] in connective tissue stages of OSF was not significant (p=0.12). Whereas, in thick collagen fibers polarization colors were found to be yellowish-orange in early stages which gradually changed to greenish-yellow in advanced cases of OSF [Figures 2 and 2–6] and was statistically significant (p<0.0001). When statistical test was applied between connective tissue stages for changes in thick collagen fibers, difference was statistically significant between early stage versus moderately advanced stage (p<0.001) and moderately advanced stage versus advanced stage (p<0.001), but was not statistically significant between very early stage versus early stage (p=0.5) and advanced stage versus OSF with carcinoma (p=0.84).
Figure 1.
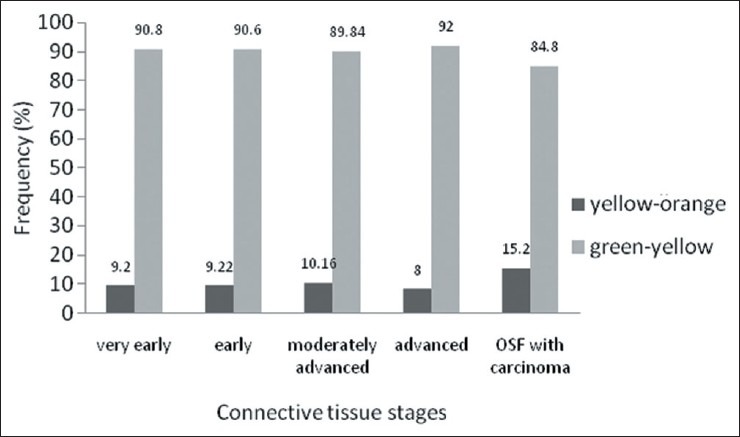
Polarization colors of thin fibers in connective tissue stages of OSF. Kruskal-Wallis Test p=0.12
Figure 2.
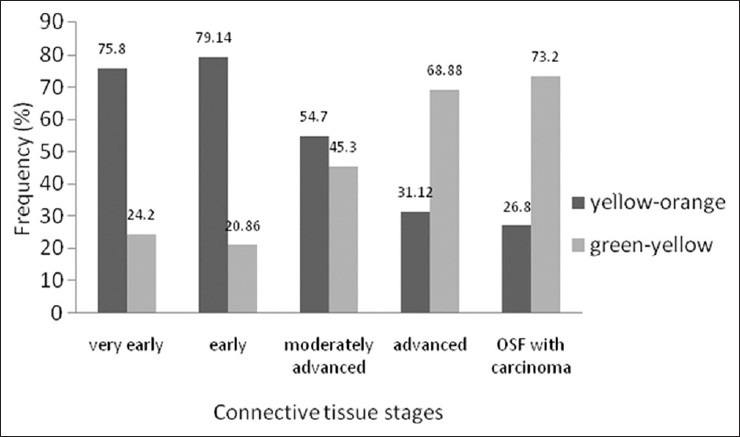
Polarization colors of thick fibers in connective tissue stages of OSF. Kruskal-Wallis test p<0.0001. Mann Whitney test - Very early versus early p=0.5, early versus moderately advanced p<0.001, moderately advanced versus advanced p<0.001, advanced versus OSF with carcinoma p=0.84
Figure 6.
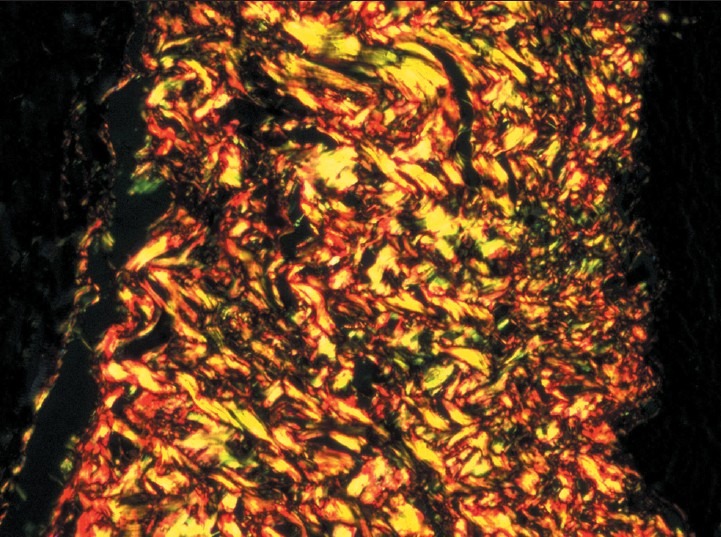
Advanced stage - Increase in number of thick collagen fibers seen in connective tissue with both thin fibers and thick fibers showing green and green-yellow birefringence by polarizing microscopy. (Picrosirius red stain, ×200)
Figure 3.
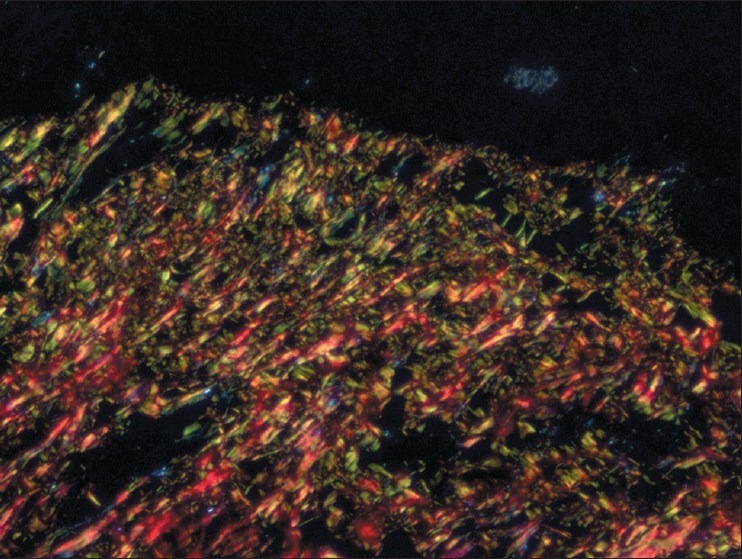
Very early stage - Both thin and thick collagen fibers seen in connective tissue with thin fibers showing green and green-yellow and thick fibers showing yellow-orange and orange red birefringence by polarizing microscopy. (Picrosirius red stain, ×200)
Figure 4.
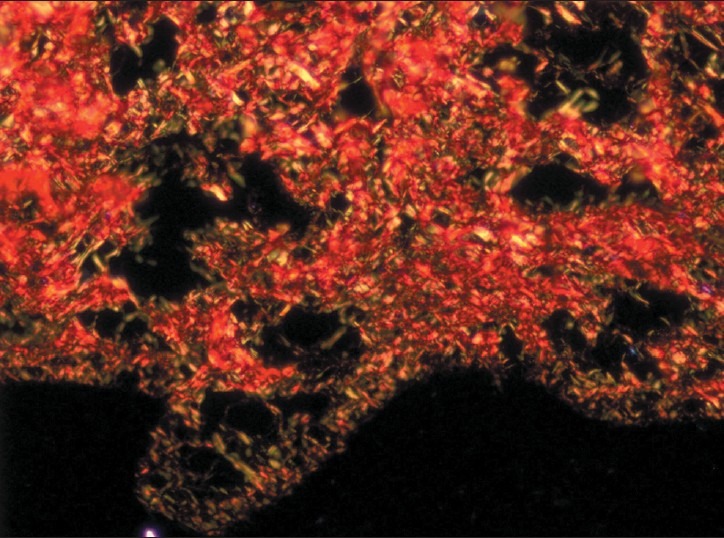
Early stage - Both thin and thick collagen fibers seen in connective tissue with thin fibers showing green and green-yellow and thick fibers showing yellow-orange and orange red birefringence by polarizing microscopy. (Picrosirius red stain, ×200)
Figure 5.
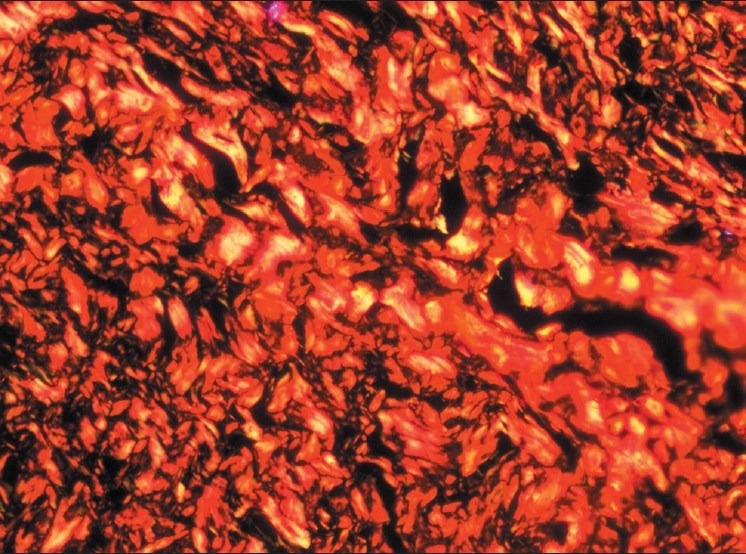
Moderately advanced stage – Increase in number of thick collagen fibers seen in connective tissue with thin fibers showing green and green-yellow and thick fibers showing yellow-orange and orange red birefringence by polarizing microscopy. (Picrosirius red stain, ×200)
Polarization colors of collagen in degrees of epithelial dysplasia in OSF
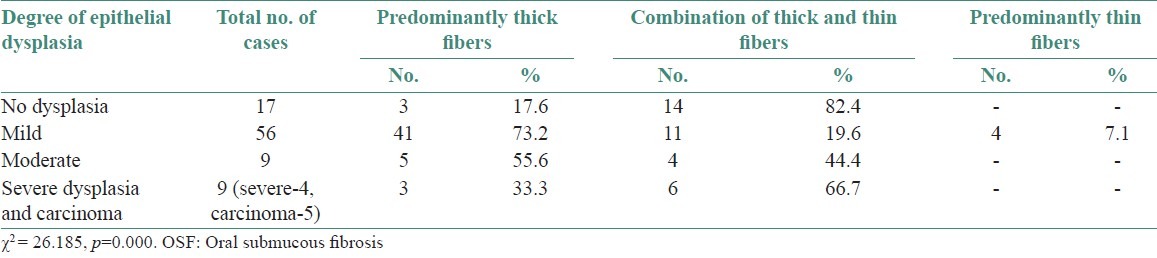
When observed for predominance of fibers, there was increase in thick collagen fibers with advancing degrees of epithelial dysplasia [Table 2], which was significant (p=0.000).
Table 2.
Type of collagen fiber arrangement seen in grades of epithelial dysplasia in OSF
Polarization colors of most of the thin collagen fibers were greenish-yellow (84.8-94.4 %). The changes in polarization colors in degrees of epithelial dysplasia was not statistically significant for both thin collagen fibers [Figure 7] (p=0.21) as well as thick collagen fibers [Figure 8] (p=0.14). However, when statistical test was applied between degrees of epithelial dysplasia for changes in polarization colors of thick collagen fibers, difference was statistically significant between mild epithelial dysplasia versus moderate epithelial dysplasia (p<0.001) and moderate epithelial dysplasia versus severe epithelial dysplasia (p<0.001). But, not statistically significant between no epithelial dysplasia versus mild epithelial dysplasia (p=0.5) and severe epithelial dysplasia versus OSF with carcinoma (p=0.11) which might be due to lower number of carcinoma cases in the present study.
Figure 7.
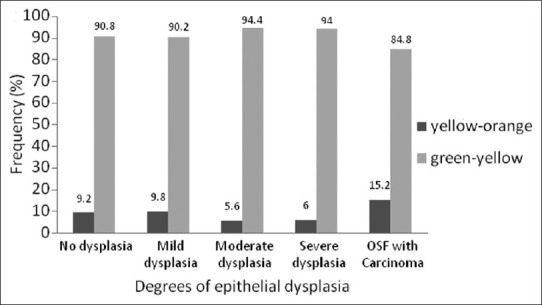
Polarization colors of thin fibers in degrees of epithelial dysplasia in OSF. Kruskal-Wallis test p=0.21
Figure 8.
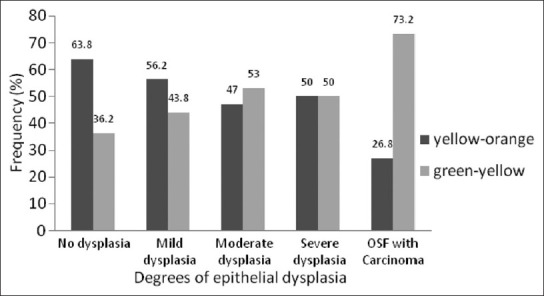
Polarization colors of thick fibers in degrees of epithelial dysplasia in OSF. Kruskal-Wallis test p=0.14. Mann Whitney test - No dysplasia versus mild dysplasia p=0.5, mild dysplasia versus moderate dysplasia p < 0.001, moderate dysplasia versus severe dysplasia p <0.001, severe dysplasia versus OSF with carcinoma p=0.11
DISCUSSION
Usually, thin normal collagen fibers in picrosirius red stained sections show green to greenish yellow polarization colors, whereas thick fibers show yellowish-orange through orange to red polarization colors. It is stated that in both thin and thick fibers green to greenish-yellow colors suggest that the collagen is poorly packed and orange red color originates from tightly packed fibers.[15,16]
In present study, there was increase in amount of thick collagen fiber bundles with advancing stages of OSF which was evident by observing the collagen fiber arrangement. This increase in amount of collagen fiber bundles results in diminished vascularity, thought to be responsible for atrophy of overlying epithelium, which becomes susceptible to carcinogenic agents. The polarization colors of thin collagen fibers were mostly greenish-yellow in all stages, whereas that of the thick collagen fibers showed a gradual change from predominantly yellow-orange to greenish-yellow with advancing connective tissue stages of OSF which was significant. A similar change in polarization colors was seen in the connective tissue of OSF cases with different degrees of epithelial dysplasia. Hence, this correlation of change in polarization colors of collagen fibers between connective tissue changes and epithelial changes may implicate that the connective tissue changes too, can be indicative of neoplastic transformation.
The green to greenish yellow polarization color of thick fibers is reported in pathologic conditions like odontogenic keratocyst, ameloblastic fibroma and central odontogenic fibroma.[15,17,18] This change in polarization colors of the thick fibers from yellow-orange to greenish-yellow is considered due to loosely packed fibers which might be composed of procollagens, intermediates or pathologic collagen rather than normal tight packed fibers.[10]
It is stated that transformation of tissue from preneoplastic state into carcinomas is associated with an increase in collagenolytic enzyme activity. Cancer cells produce collagenases as type I and III collagen are the most abundant component in extracellular matrix of dermal and oral submucosal connective tissue. Hence, it is likely that ability to degrade collagen is essential for invasion and metastasis of neoplastic cells. By increased formation of collagenases, the invading tumor cells are capable of dissolving collagen in connective tissue obstructing its course.[19] The loss of tight packing of collagen fibers seen in advanced stages of present study may be, due to increased collagenolytic enzyme activity during transformation of tissue from preneoplastic hyperplasia into carcinoma.
Emphasis in this study has been on collagen in connective tissue stages of OSF as fibrosis starts primarily in connective tissue and the epithelium is likely to be damaged subsequently. Even though pathology of OSF is in connective tissue, malignancy develops from the overlying epithelium. Hence, correlation of collagen changes is done even with the degrees of epithelial dysplasia.
It was found that there was enhanced birefringence of collagen in Picrosirius Red stained sections, which enabled to differentiate between thin and thick fibers, and studying the compactness of collagen by change in polarization colors. The present study indicated a significant change of collagen fiber arrangement from early stages of OSF to advanced stages which coincided with the changes found in degrees of epithelial dysplasia. As there was a gradual change observed in the polarization colors of thick collagen fibers from initial connective tissue changes of OSF to advanced stages and also from mild, moderate to severe epithelial dysplasia, study of these changes may be used to demonstrate neoplastic changes. As connective tissue changes precede epithelial changes in OSF, the connective tissue changes may give indication of neoplastic change earlier than epithelial changes.
However, a study with follow-up will give insight whether premalignant potential remains progressive after cessation of causative habit and study of collagenase production by the cells in different stages can also be done to predict malignant transformation.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1966;22:764–79. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 2.Gupta PC, Sinor PN, Bhonsle RB, Pawar VS, Mehta HC. Oral submucous fibrosis in India: A new epidemic? Natl Med J India. 1998;11:113–6. [PubMed] [Google Scholar]
- 3.Schwartz J. Canniff JP, Harvey W, Harris M, editors. Atrophia idiopathica (tropica) mucosae Oris. Demonstrated at the Eleventh International Dental Congress. London, 1952. Br Dent J. 1986;160:429–34. doi: 10.1038/sj.bdj.4805876. [DOI] [PubMed] [Google Scholar]
- 4.Joshi SG. Submucous fibrosis of the palate and pillars. Indian J Otolaryngol. 1953;4:1–4. [Google Scholar]
- 5.Seedat HA, van Wyk CW. Submucous fibrosis in ex-betel nut chewers: A report of 14 cases. J Oral Pathol. 1988;17:226–9. doi: 10.1111/j.1600-0714.1988.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 6.Thilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006;42:561–8. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Murti PR, Bhonsle RB, Pindborg JJ, Daftary DK, Gupta PC, Mehta FS. Malignant transformation rate in oral submucous fibrosis over a 17-year period. Community Dent Oral Epidemiol. 1985;13:340–1. doi: 10.1111/j.1600-0528.1985.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 8.Paymaster JC. Cancer of the buccal mucosa; a clinical study of 650 cases in Indian patients. Cancer. 1956;9:431–5. doi: 10.1002/1097-0142(195605/06)9:3<431::aid-cncr2820090302>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Pindborg JJ, Poulsen HE, Zachariah J. Oral epithelial changes in Indians with oral cancer and submucous fibrosis. Cancer. 1967;20:1141–6. doi: 10.1002/1097-0142(196707)20:7<1141::aid-cncr2820200717>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M. Are the polarization colors of picrosirius red-strained collagen determined only by the diameter of the fibers? Histochemistry. 1989;93:27–9. doi: 10.1007/BF00266843. [DOI] [PubMed] [Google Scholar]
- 11.Sirsat SM, Pindborg JJ. Subepithelial changes in oral submucous fibrosis. Acta Pathol Microbiol Scand. 1967;70:161–73. doi: 10.1111/j.1699-0463.1967.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 12.Neville BW, Damm DD, Allen CM, Bouquot JE. 2nd ed. Philadelphia: Elsevier; 2004. Oral and maxillofacial Pathology; pp. 343–4. [Google Scholar]
- 13.Pindborg JJ, Mehta FS, Daftary DK. Occurrence of epithelial atypia in 51 Indian villagers with oral submucous fibrosis. Br J Cancer. 1970;24:253–7. doi: 10.1038/bjc.1970.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirshberg A, Lib M, Kozlovsky A, Kaplan I. The influence of inflammation on the polarization colors of collagen fibers in the wall of odontogenic keratocyst. Oral Oncol. 2007;43:278–82. doi: 10.1016/j.oraloncology.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Hirshberg A, Sherman S, Buchner A, Dayan D. Collagen fibers in the wall of odontogenic keratocysts: A study with picrosirius red and polarizing microscopy. J Oral Pathol Med. 1999;28:410–2. doi: 10.1111/j.1600-0714.1999.tb02112.x. [DOI] [PubMed] [Google Scholar]
- 16.Junqueira LC, Montes GS, Sanchez EM. The influence of tissue section thickness on the study of collagen by the Picrosirius-polarization method. Histochemistry. 1982;74:153–6. doi: 10.1007/BF00495061. [DOI] [PubMed] [Google Scholar]
- 17.Nyska A, Dayan D. Ameloblastic Fibroma in a young cat. J Oral Pathol Med. 1995;24:233–6. doi: 10.1111/j.1600-0714.1995.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 18.Hirshberg A, Buchner A, Dayan D. The central odontogenic fibroma and hyperplastic dental follicle: Study with picrosirius red and polarizing microscopy. J Oral Pathol Med. 1996;25:125–7. doi: 10.1111/j.1600-0714.1996.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 19.Johansson N, Airola K, Grénman R, Kariniemi AL, Saarialho-Kere U, Kähäri VM. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol. 1997;151:499–508. [PMC free article] [PubMed] [Google Scholar]


