Abstract
Aim:
To assess p53 and Cyclin D1 expression using Immunohistochemistry in normal mucosa and oral squamous cell carcinoma.
Materials and Methods:
Twenty cases of Oral squamous cell carcinoma (OSCC) and 10 normal mucosa were used and the primary antibodies used were p53 (DAKO-DO7) and Cyclin D1 Mouse Anti human Cyclin D1 (DCS-6) 1: 100 dilution. Statistical analysis: Labelling index was calculated and mean LI and SD were calculated, using Descriptive Statistics and t-test was used to compare mean LI between antibodies used in OSCC. Percentage positivity was done by Chi-Square test. Comparison of LI between p53 and Cyclin D1 was studied using t test.
Results:
p53 was positive in 30% and Cyclin D1 in40% of normal cases and 65% and 95% of OSCC were positive for p53 and Cyclin D1 respectively. Mean LI of p53 and Cyclin D1 were found to be statistically significant between the normal mucosa and OSCC. The correlation of mean LI of p53 and Cyclin D1 in OSCC was found to be statistically significant. LI of p53 was found to be higher than Cyclin D1 in OSCC.
Conclusion:
In the present study, increased p53 and Cyclin D1 expression were seen in OSCC when compared to the normal mucosa and a positive correlation was seen between increased p53 and Cyclin D1 expression in OSCC.
Keywords: Cyclin D1, IHC, oral squamous cell carcinoma, p53
INTRODUCTION
Oral squamous cell carcinoma (OSCC) constitutes the sixth most common cancer worldwide and the third most common cancer in the developing countries.[1] Squamous cell carcinoma occurs due to multiple genetic changes leading to formation of either abnormal proteins or increased amount of normal proteins, leading to loss of control of the cell cycle. The interaction between the activated oncogene and the mutations in the tumor suppressor genes appear to be the driving force directing normal cells to uncontrolled growth and invasion.[2]
The most studied tumor suppressor gene is the p53 gene. P53 is located on chromosome 17p. The p53 protein participates in cell cycle control and also plays a role in apoptosis of cells with DNA damage. Mutations in p53 abrogate its transactivating activity leading to uncontrolled cell growth.[2]
In addition to suppressor genes, other genes controlling the entry of cells into the cell cycle have been implicated in cancer development. One such gene is the Cyclin D1 gene, which has been too mapped to 11q13.[2]
The function of Cyclin D1 gene is negatively regulated by the p53 genes, which acts through p21/WAF1.[3] Studies have suggested that approximately half of all OSCC contain p53 mutation, and about a third contain mutations in Cyclin D1. Therefore, understanding the molecular mechanisms involved in the initiation and progression to malignancy of oral cancer will help to improve its prognosis and in the elaboration of new forms of treatment. The present study was undertaken to study the expression of cyclin D1 and p53 in OSCC as well as to identify the correlation between cyclin D1 and p53 expression using immunohistochemistry.
MATERIALS AND METHODS
The study material comprised of archival formalin fixed paraffin embedded specimens of patients from the Department of Oral and Maxillofacial Pathology, Ragas Dental College and Hospital. Twenty cases of oral squamous cell carcinoma and 10 cases of normal controls constituted the study group.
OSCC were histologically graded as well differentiated as moderately differentiated and poorly differentiated according to the differentiation of cells and the resemblance of neoplastic cells to that of epithelial cells. The normal controls were from the buccal mucosa obtained during the extraction of the lower third molars.
IHC procedure
All the slides were APES (3 amino propyl tri ethoxy silane) coated. Tissue sections of 0.5-micron thickness were made in a rotary manual microtome. The ribbons of tissue sections were transferred on to the APES coated slide from the tissue float bath such that two tissue bits come on to the slide with a gap in between. One of the tissue sections was labelled positive (P) and the other negative (N) control. The slides with tissue sections were treated with two changes of xylene to remove paraffin wax. They were then put in descending grades of alcohol that helps in dehydration of the tissue. Slides were then treated with 0.3% hydrogen peroxide for 30 min to quench endogenous peroxidase activity of cells that would otherwise result in nonspecific staining. Then the slides were transferred to citrate buffer and autoclaved for antigen retrieval at 15 lbs pressure for 15 min. Then the slides were dipped in 2 changes of PBS for 5 min each. Then primary antibody Mouse Anti-Human Cyclin D1 (DCS-6) 1:100 dilution was added to P tissue on the slides and then to the N tissue, PBS added and left in the refrigerator overnight, while staining for Cyclin D1 and while staining for p53 the slides were flooded with PBS and left in fridge. Following day primary antibody p53 (DAKO-DO7) was added on P tissue and on the N section PBS was added and incubated for 1 h. Then the slides were washed in PBS for 5 min. Then a drop of Biotinylated link from the secondary antibody kit (DAKO LSAB PLUS KIT) was added and the slides were incubated for 30 min. Later slides were washed in cold PBS. Then a drop of streptavidin (DAKO LSAB PLUS KIT) was added and incubated for 30 min. Then the slides were then washed in cold PBS. Then a drop of freshly prepared DAB (3”-Diaminobenzidine Tetra hydrochloride-a substrate chromogen) was added and were counter stained with hematoxylin. Slides were transferred to 70% alcohol, 100% alcohol and 2 changes of xylene in a sequential order. The tissue sections were mounted with DPX. Slides were then observed under microscope. Counting of positive cells was done on all slides. One thousand epithelial cells were counted on the positive section and the number of positive cells was noted.
Statistical analysis
Labeling Index (LI) for each slide was calculated by dividing the number of positive cells by the total number of cells counted in the slides and multiplying by 100. One thousand cells were counted in each slide.
![]()
Mean LI and standard deviation was calculated using descriptive statistics. Data was entered and analyzed in SPSS version 10.0.5.
t-test was used to compare mean LI between antibodies used in oral squamous cell carcinoma.
Percentage positivity comparison of p53 between OSCC and normal mucosa and Percentage positivity of Cyclin D1 between normal mucosa and OSCC was done by using Chi-Square test.
Comparison of LI between p53 and Cyclin D1 was studied using t-test.
RESULTS
p53
Both normal and OSCC slides stained with p53 antibody. P53 staining was nuclear with no cytoplasmic staining.
Normal group
Of the 10 cases studied, only 3% cases were positive for p53 antibody. The percentage positivity was found to be 30%.[Table 1] The intensity of staining was found to be moderate in all positive cases. Staining was mostly found in the basal layer of the epithelium. Mean LI was found to be 1.7 ± 0.36 [Table 2, Graph 3, Figure 1].
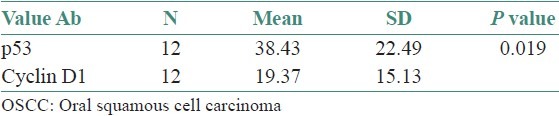
Table 1.
Labeling index of p53 and Cyclin D1 in normal and OSCC t-test

Table 2.
Percentage Positivity of p53 and Cyclin D1 – OSCC & normal mucosa Ab – antibodies

Graph 3.

Mean labeling index of Cyclin D1 and p53 in normal controls
Figure 1.
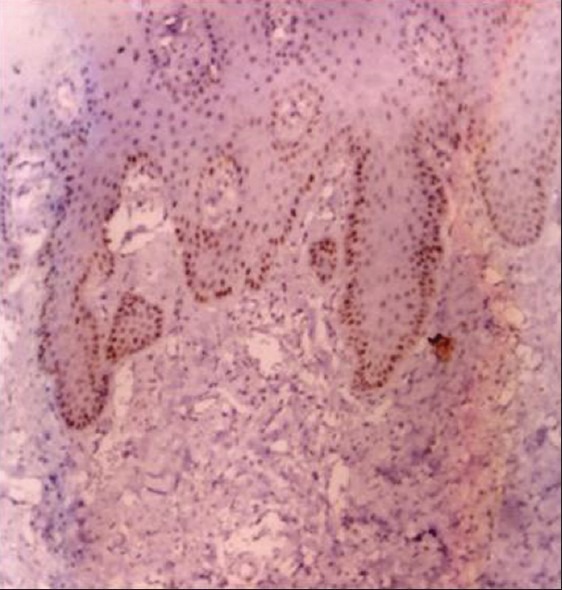
Photo micrograph of p53 expression in normal mucosa (10×)
OSCC
Of the 20 samples of OSCC studied, 13 cases were positive for p53 antibody. Staining was found to be in the basal and suprabasal layers of the epithelium. Two samples showed positivity throughout the epithelial islands [Figures 2 and 3]. The percentage positivity was found to be 65%. The mean LI was found to be 36.08 ± 23.15 [Table 2]. The percentage positivity between normal and OSCC groups was not statistically significant (p=0.122) [Table 1].
Figure 2.
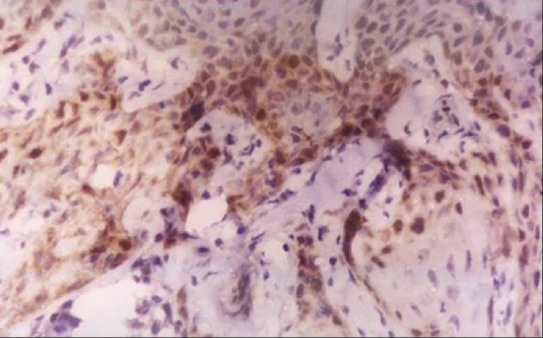
Photo micrograph of p53 In Oscc (10×) (Well Diff)
Figure 3.

Photo micrograph of p53 expression in OSCC (10×) (Mod Diff)
Mean LI of p53 was found to be statistically significant between the normal and OSCC groups. (p=0.00) [Table 2, Graph 1].
Graph 1.

Mean labelling Index of p53 in normal and OSCC
Cyclin D1
The normal and OSCC slides stained with Cyclin D1 antibody showed positive staining with a variable number of positive cells. Staining was both nuclear and cytoplasmic.
Cytoplasmic staining in the absence of nuclear staining was considered negative.
Normal groups
Of the 10 samples studied, 4% cases were positive for Cyclin D1 [Figure 4]. The intensity of staining was found to be moderate in all the 4 cases. The % positivity was found to be 40% [Table 1]. Mean LI was 4.77 ± 4.7 [Table 2, Graph 3].
Figure 4.
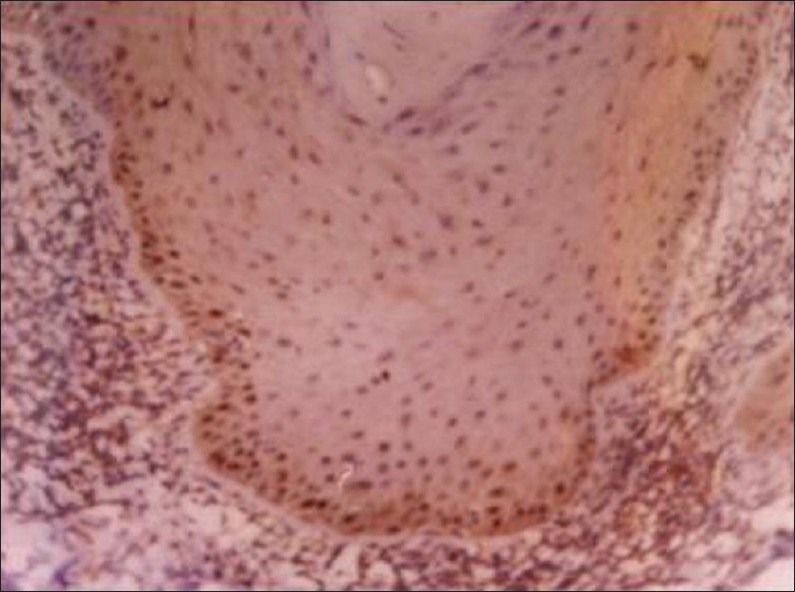
Photo micrograph of cyclin D1 expression in normal mucosa (40 X)
OSCC
Staining was both nuclear and cytoplasmic [Figure 5]. Of the 20 cases studied, 19% were positive for Cyclin D1. Moderate staining was seen in most of the cases. The percentage positivity was found to be 95% [Table 1]. The mean LI was 18.88 ± 13.70 [Table 2].
Figure 5.

Photo micrograph of cyclin D1 expression in oscc (40×)
The percentage positivity between normal and OSCC was found to be statistically significant. (p=0.009) [Table 1]. Mean LI was found to be statistically significant between normal and OSCC (p=0.003) [Table 2, Graph 2].
Graph 2.
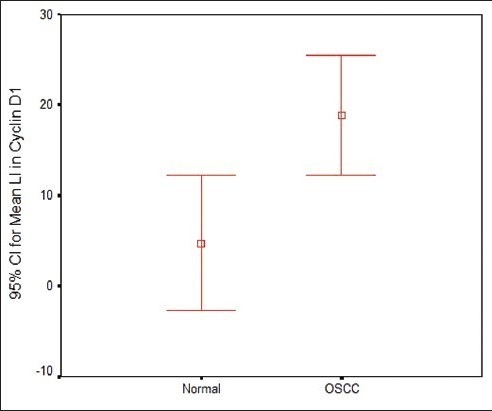
Labelling index of Cyclin D1 in normal and OSCC
p53 and Cyclin D1 IN OSCC
Both p53 and Cyclin D1 were positive in 12 (60) of the 20 cases of OSCC. In OSCC, the mean LI of Cyclin D1 was found to be 18.8 ± 13.7 and mean LI of p53 was 36.1 ± 23.6. The correlation of mean LI of p 53 and Cyclin D1 in OSCC was found to be statistically significant (p=0.019) [Table 3, Graph 4]. LI of p53 was found to be higher than Cyclin D1 in OSCC.
Table 3.
Comparison of labeling index between p53 and Cyclin D1 in OSCC t-test Ab – antibodies
Graph 4.
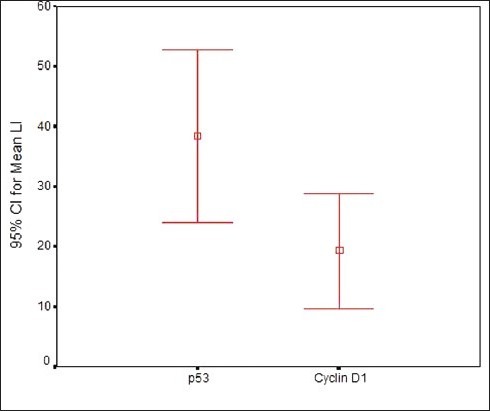
Mean labelling index of Cyclin D1 and p53 in OSCC
DISCUSSION
The development of OSCC is a multi-step genetic process involving specific alterations in oncogene and tumor suppressor genes. In the present study, the tumor suppressor gene p53 was positive in 30% of normal mucosa and staining was confined to the basal layer of the epithelium.
Kerdpon et al 1997, in their study found that all cases of normal oral mucosa studied by IHC were p53 negative.[4] In contrast Sauter et al and Shin et al, found p53 positivity in 5% and 21% of the normal mucosa respectively.[4] Our study was similar to Yanomoto et al, who reported 20% positivity for p53 in normal mucosa.[5] In addition to p53 mutation, the detection of p53 by IHC in the normal epithelium has been attributed to the physiological stabilization of the wild type p53 due to genotoxic stress caused by UV radiation, hypoxia and viral proteins leading to increased half life of p53 protein and therefore detection by IHC.
In OSCC, of the 20 samples studied, 65% were positive for p53 similar to reports by Girold et al, (54%), Kaur et al, (75%), and Kerdpon et al, (95%).[4] Their studies indicated that p53 expression increased from hyperplasia to dysplasia to OSCC. Kerdpon et al, 2001 showed 70% of cases of OSCC positive for p53 in Southern Thailand[5] and Thongusakai et al, 2001 found p53 positivity in 38.5% of OSCC from Thailand.[6]
Schoelch et al, in their study observed 50% of OSCC expressing p53 expression and it increased as lesions progressed from keratosis to dysplasia to carcinoma.[7] Lam et al, observed 78% positivity for p53 in OSCC from buccal mucosa, floor of mouth and tongue.[3] Cruz et al, found supra basal p53 expression in the non-malignant mucosa adjacent to p53 positive carcinomas, suggesting that p53 alterations can occur in early carcinogenesis.[8] In the present study the mean LI of OSCC was found to be significantly higher than normal controls. The difference in the mean LI was found to be statistically significant between OSCC and normal controls. This indicates that there is increased p53 mutation as reported by the previous studies.
Of the 10 normal samples studied, 40% were positive for Cyclin D1. Staining was confined to the basal layer of the epithelium. It could be attributed to the proliferating activity of the basal layer of the cells, as Cyclin D1 is a positive regulator of the transition from G1 phase to S phase in cell cycle progression.[9] Mean LI was found to be 4.8 ± 4.7. Rousseau et al, investigated the expression of Cyclin D1 in normal mucosa and they observed scattered cells showing nuclear Cyclin D1 protein expression in the suprabasal and basal epithelial layers and their mean LI for normal mucosa was found to be 5.7 ± 0.9.[10]
In our study the frequency of Cyclin D1 expression was found to be 95%. Michalides et al, have reported 33% Cyclin D1 expression in OSCC[3] and Xu et al, 38%,[2] van Oijen et al, 71%,[3] Takes et al, 29%,[11] Kuo et al, 83%,[12] Mineta et al, 19%[13] and Lam et al, reported Cyclin D1 expression in 63% of OSCC. Thus the results of our study are comparable with the previous studies. The over expression of Cyclin D1 suggests as expected, that there is increased proliferation in OSCC. The mean LI was significantly higher in OSCC than normal mucosa, which suggests that over expression of Cyclin D1 increases in OSCC.
Clinical studies have found a correlation between p53 and Cyclin D1 over expression in OSCC. In our study, p53 was positive in 13/20 cases and Cyclin D1 in 19/20 cases and both p53 and Cyclin D1 were positive in 12 of the 20 cases studied. Co-expression of Cyclin D1 and p53 was noted in 68% OSCC as reported by Lam et al,[3] and Mineta et al, have reported a statistically significant positive correlation between Cyclin D 1 and p53 gene alterations in head and neck carcinomas.[13] In the present study, Cyclin D1 and p53 expressions were positively related to each other in 60% of OSCC, comparable with the study of Lam et al, who found co expression of p53 and Cyclin D1 in 68% of OSCC. It has been shown that p53 negatively regulates cell cycle progression through its action on WAF-1 /p21, an inhibitor of cyclin dependent kinases (CDKs). P21 associates with and inhibits multiple CDKs, and so prevents the cell from exiting G1. In tumors, loss of wild type p53 function deactivates this growth control pathway and is considered to play a role in the uncontrolled growth of tumor cells.[2] In contrast, Cyclin D1 acts as a positive regulator of the transition from G1 phase to S phase by association with and activation of CDKs. Consequently, loss of wild type p53 function and over expression of Cyclin D1 has a similar effect on cell cycle, namely abrogation of G1 growth control.[2] Both p53 and Cyclin D1 were over expressed in OSCC in the present study. However, further studies on larger number of cases and analysis using methods such as single strand conformation polymorphism would throw further light on the exact alteration of p53 and Cyclin D1in OSCC in our population. The results of this study suggest that p53 and Cyclin D1 IHC has the potential to be used as the marker of malignant transformation in oral mucosa.
SUMMARY AND CONCLUSION
Based on our results it is justified to conclude that mutant p53 expression increases as normal mucosa undergoes malignant transformation and Cyclin D1 is amplified and is over expressed in oral squamous cell carcinoma and a positive correlation is seen between increased mutant p53 expression and Cyclin D1 expression in OSCC.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Carlos de Vicente J, Junquera Gutiérrez LM, Zapatero AH, Fresno Forcelledo MF, Hernández-Vallejo G, López Arranz JS. Prognostic significance of p53 expression in oral squamous cell carcinoma: Without neck node metastases. Head Neck. 2004;26:22–30. doi: 10.1002/hed.10339. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Gimenez-Conti IB, Cunningham JE, Collet AM, Luna MA, Lanfranchi HE, et al. Alterations of p53, Cyclin D1, Rb and H-ras in human oral carcinomas related to tobacco use. Cancer. 1998;83:204–12. doi: 10.1002/(sici)1097-0142(19980715)83:2<204::aid-cncr2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Lam KY, Ng IO, Yuen AP, Kwong DL, Wei W. Cyclin D1 expression in oral squamous cell carcinomas: Clinicopathological relevance and correlation with p53 expression. J Oral Pathol Med. 2000;29:167–72. doi: 10.1034/j.1600-0714.2000.290404.x. [DOI] [PubMed] [Google Scholar]
- 4.Kerdpon D, Rich AM, Reade PC. Expression of p53 in oral mucosal hyperplasia, dysplasia and squamous cell carcinoma. Oral Dis. 1997;3:86–92. doi: 10.1111/j.1601-0825.1997.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 5.Kerdpon D, Sriplung H, Kietthubthew S. Expression of p53 in oral squamous cell carcinoma and its association with risk habits in southern Thailand. Oral Oncol. 2001;37:553–7. doi: 10.1016/s1368-8375(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 6.Thongsuksai P, Boonyaphiphat P. Lack of association between p53 expression and betel nut chewing in oral cancers from Thailand. Oral Oncol. 2001;37:276–81. doi: 10.1016/s1368-8375(00)00127-5. [DOI] [PubMed] [Google Scholar]
- 7.Schoelch ML, Regezi JA, Dekker NP, Ng IO, McMillan A, Ziober BL, et al. Cell cycle proteins and the development of oral squamous cell carcinoma. Oral Oncol. 1999;35:333–42. doi: 10.1016/s1368-8375(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 8.Cruz IB, Meijer CJ, Snijders PJ, Snow GB, Walboomers JM, van Der Waal I. P53 immunoexpression in non-malignant oral mucosa adjacent to oral squamous cell carcinoma: Potential consequences for clinical management. J Patho. 2000;191:132–7. doi: 10.1002/(SICI)1096-9896(200006)191:2<132::AID-PATH605>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Shintani S, Mihara M, Nakahara Y, Kiyota A, Ueyama Y, Matsumura T, et al. Expression of cell cycle control proteins in normal epithelium, premalignant and malignant lesions of oral cavity. Oral Oncol. 2002;38:235–43. doi: 10.1016/s1368-8375(01)00048-3. [DOI] [PubMed] [Google Scholar]
- 10.Rousseau A, Lim MS, Lin Z, Jordan RC. Frequent Cyclin D1 gene amplification and protein over expression in oral epithelial dysplasias. Oral Oncol. 2001;37:268–75. doi: 10.1016/s1368-8375(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 11.Takes RP, Baatenburg de Jong RJ, Wijffels K, Schuuring E, Litvinov SV, Hermans J, et al. Expression of genetic markers in lymph node metastases, compared with their primary tumors in head and neck cancer. J Pathol. 2001;194:298–302. doi: 10.1002/1096-9896(200107)194:3<298::AID-PATH900>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 12.Kuo MY, Lin CY, Hahn LJ, Cheng SJ, Chiang CP. Expression of cyclin D1 is correlated with poor prognosis in patients with areca quid chewing- related oral squamous cell carcinomas in Taiwan. J Oral Pathol Med. 1999;28:165–9. doi: 10.1111/j.1600-0714.1999.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 13.Mineta H, Borg A, Dictor M, Wahlberg P, Wennerberg J. Correlation between p53 mutation and Cyclin D1 amplification in head and neck squamous cell carcinoma. Oral Oncol. 1997;33:42–6. doi: 10.1016/s0964-1955(96)00039-5. [DOI] [PubMed] [Google Scholar]


