Abstract
Recent technological advances have made saliva as a tool for diagnosis of many things; among them are hormone imbalances, liver function, immunodeficiency and even cancer.
Aim:
The present study was done to evaluate the levels of copper and zinc and copper/zinc ratio in saliva of premalignant and malignant lesions of oral cavity, because of the anatomical proximity of the saliva to both premalignant and malignant oral neoplasms.
Materials and Methods:
The levels of copper and zinc were estimated in the saliva of 5 patients with oral submucous fibrosis, 5 patients with oral leukoplakia, 5 patients with oral lichen planus and 10 patients with oral squamous cell carcinoma of oral cavity using inductively coupled mass spectrometry (ICP- MS). The values were compared with 6 normal age and sex matched control subjects.
Results:
There was significant difference of the mean salivary copper and zinc levels of premalignant and malignant lesions when compared to the normal controls. In oral cancer patients there was significant difference in the copper levels according the histodifferentiaton in squamous cell carcinoma. Within the premalignant group the copper levels were more in the oral sub mucous fibrosis when compared to the leukoplakia and lichen planus. Copper zinc ratio decreased in premalignant and malignant group when compared to the normal group.
Conclusions:
Saliva may be used as a potential diagnostic tool, which can be efficiently employed to evaluate the copper and zinc levels in pre malignant and malignant lesions of oral cavity. Key words: Copper, inductively couples mass spectrometry, leukoplakia, lichen planus, oral squamous cell carcinoma, oral submucous fibrosis, saliva, zinc
Keywords: Copper, inductively coupled mass spectrometry, leukoplakia, lichen planus, oral squamous cell carcinoma, oral submucous fibrosis, saliva, zinc
INTRODUCTION
Cancer of the oral cavity is the most common neoplasm in the developing countries.[1] A very high incidence of oral cancer has been reported from Kerala, South India, compared to other parts of the world. Similarly, the incidence of precancerous lesions of the oral cavity such as oral leukoplakia and oral submucous fibrosis is also very high. The etiology of this high incidence is not fully known. The high incidence was attributed to several factors such as chewing, smoking and viral infections. Whatever may be the causative factors, very little information is available on the biochemical and immunological derangements.[2] The role of certain trace metals, especially zinc in the pathology of various diseases has been the subject of a number of comprehensive reviews. Zinc and copper have been the most extensively studied of the trace elements in patients with malignant disease[3] and these elements in serum has been found to be reliable parameter as a diagnostic and prognostic index in case of craniofacial tumors.[4] Recent technological advances have made saliva as a tool for the diagnosis of many things; among them are hormone imbalances, liver function, immunodeficiency and even cancer.[5] So, the present study was undertaken to evaluate the levels of copper, zinc and copper/zinc ratio in saliva of premalignant and malignant lesions of oral cavity.
MATERIALS AND METHODS
The study sample comprised of total 31 cases, which are categorized into three groups. Group I consisted of 6 cases of healthy controls. Group II consisted of 5 oral submucous fibrosis, 5 leukoplakia, and 5 oral lichen planus. Group III consisted of 10 oral squamous cell carcinoma. All the cases for the study were selected from the department of oral and maxillofacial pathology, Sri Ramachandra Dental College, Chennai. Only histopathologically confirmed cases and the patients free from any systemic disease on clinical evaluation were selected. The individuals selected for control group are free from oral lesions. All the patients were assessed for nutritional status by questionnaire method.
The patient was instructed not to eat, drink or rinse 1 hour prior to the sample collection and should rinse with deionized water immediately before saliva collection. Each subject was asked to accumulate saliva in the mouth for 2 min and then to spit in sterilisable plastic vials. This method was carried out for 6 min to collect the whole unstimulated saliva. The samples were centrifuged at 3,000 rpm at 4°C for 5 min. This process provides a saliva sample free of large debris and of reduced viscosity, allowing more accurate and reproducible analysis. Each sample was diluted fivefold in 10 ml/L nitric acid, and the trace elements were analyzed by inductively coupled mass spectrometry (ICP- MS), Agilent 7500ce model from Japan which was carried out in SGS, TICEL Park, Tharamani [Figure 1]. The results were expressed in parts per billion (ppb) or μg/L. Statistical analysis was done using student's independent t-test to compare the mean values in two independent groups and one way analysis of variance (ANOVA) to compare the mean value in different study groups.
Figure 1.
Inductively coupled plasma mass spectrometry (ICP – MS)
RESULTS
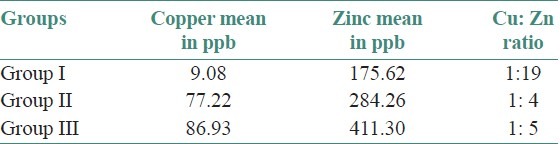
The age and sex distribution of all the subjects in the present study is given in Tables 1 and 2, [Figures 2 and 3]. Salivary copper and zinc levels in different groups are given in Table 3. Copper and zinc levels (ppb) in different groups are shown in [Figure 4]. A comparison of Salivary copper and zinc levels (ppb) between the control (Group I) with premalignant (Group II) and malignant lesions (Group III) and between Group II and Group III is given in Tables 4 and 5. Salivary copper and zinc levels (ppb) according to age in different groups is given in Table 6. Copper zinc ratio in different groups is given in Table 7. There was significant difference of the mean salivary and zinc levels of premalignant and malignant lesions when compared to the normal controls. Within the premalignant lesions the copper levels were more in the oral submucous fibrosis when compared to the leukoplakia and lichen planus. Copper zinc ratio decreased in premalignant and malignant group when compared to the normal group. There was decrease in copper levels from very well differentiated to moderately-differentiated squamous cell carcinoma.
Table 1.
Age distribution in different groups
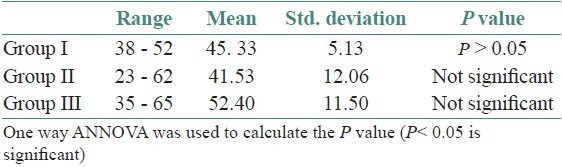
Table 2.
Sex distribution in different groups
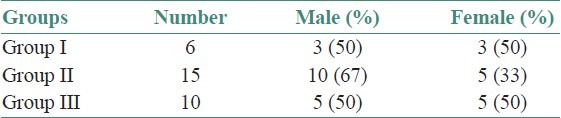
Figure 2.
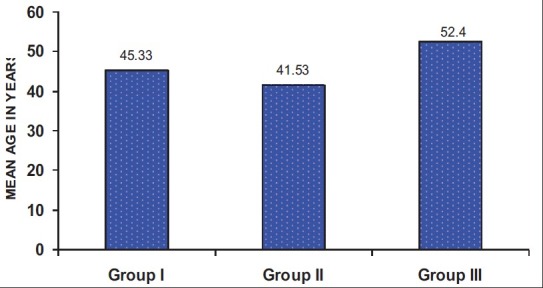
Mean age in different groups
Figure 3.
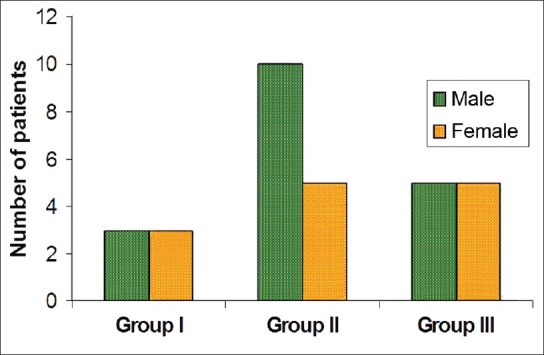
Sex distribution in different groups
Table 3.
Salivary copper and Zinc levels (ppb) in different groups
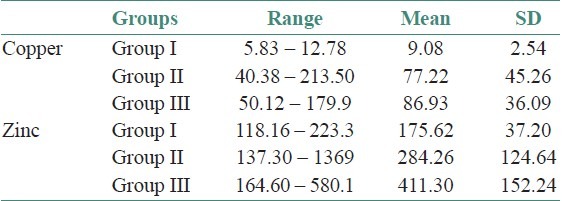
Figure 4.

Copper and zinc levels (ppb) in different groups
Table 4.
Comparison of Salivary copper levels (ppb) between the control (Group I) with Premalignant (Group II) and malignant lesions (Group III) and between Group II and Group III
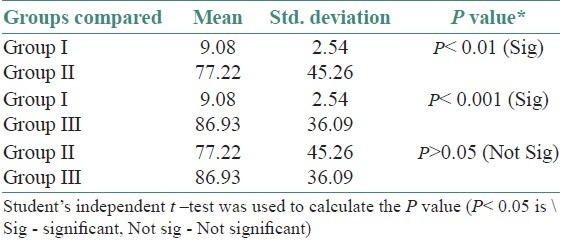
Table 5.
Comparison of salivary zinc levels (ppb) between the control (Group I) with Premalignant (Group II) and malignant lesions (Group III) and between Group II and Group III
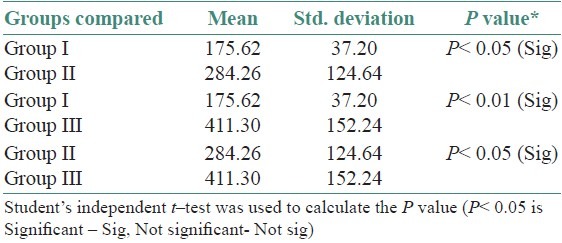
Table 6.
Salivary copper and Zinc levels (ppb) according to age in different groups
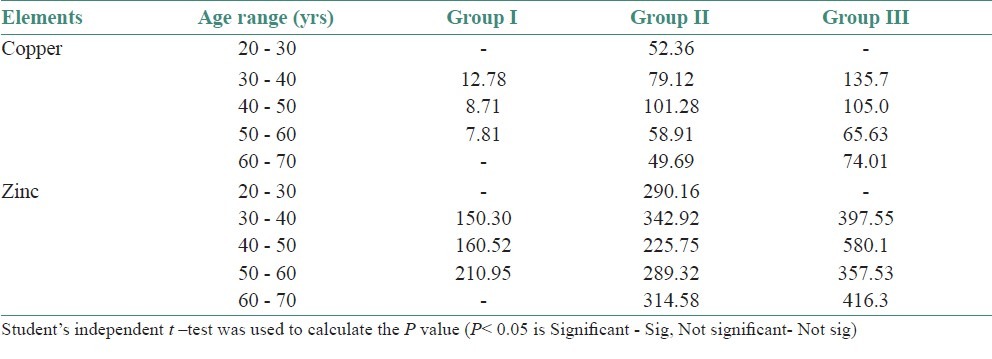
Table 7.
Copper zinc ratio in different groups
DISCUSSION
Trace elements play, directly or indirectly, an important role in various physiological metabolic processes in humans. More than 25% of the enzymes in the body need to be activated by metal ions to carry out their metabolic functions.[6] Bioelements e.g. Copper and zinc are involved in vital biochemical activities like different redox and free radical formation and in maintaining cellular proton homeostasis.[7] Copper is present in many enzymes involved in oxidation (Tyrosinase, ceruloplasmin, amine oxidase, cytochrome oxidase). Zinc is involved in carbonic acid (carbonic anhydrase), in proteolysis (carboxy peptidase, leucine amino peptidase, etc.).[4]
Many studies reported that these trace elements play a major role as either inhibitory or causative agent of cancer. Several workers studied the copper and zinc levels in the serum, plasma and tissue of premalignant and malignant lesions of oral cavity and head and neck neoplasm's. However, a systematic study in saliva is still lacking. Hence, the present study was undertaken to evaluate the copper and zinc levels in the unstimulated whole saliva of normal, premalignant and malignant lesions of the oral cavity. This is probably the first of its kind. Henkin et al, proposed that the saliva represents a useful tool in the diagnosis of some physiological and pathological changes in the body function and in understanding the important aspects of trace metal metabolism.[8] The unstimulated whole saliva is used in the present study because, the anatomical proximity of saliva to both premalignant and malignant oral neoplasm's, saliva could be ideal for screening of these lesions and highly specific and sensitive analytical methods are currently available allowing measurement of micro concentrations of various salivary components. From a logistical perspective the collection of saliva is safe i.e. No needle punctures, non-invasive and relative simple and collected repeatedly without discomfort to the patient.[9] Various authors studied the copper and zinc levels in human resting mixed saliva. Mathur et al, compared the levels of zinc in the parotid saliva and resting mixed saliva in their study and mentioned that the concentration of salivary zinc in the oral cavity should be estimated from that in the resting mixed saliva. However, the mean zinc content of resting mixed saliva was one third of that in plasma and about one fifteenth of that in whole blood.[10] Borella et al, stated the use of mixed saliva seems the most convenient and practical approach in the field studies.[11] Hence in this study, unstimulated whole saliva was used. A critical literature analysis reveals that the copper and zinc levels in the saliva has been measured by atomic absorption spectrometry, thermal neutron activation analysis, gamma ray spectrometry and the most recent method Inductively Coupled Plasma Mass Spectrometry. So, in this study Inductively Coupled Plasma Mass Spectrometry was used, which is very sensitive. Copper metabolism is profoundly altered in neoplastic disease and serum copper correlates with tumor incidence and burden, malignant progression, and recurrence in variety of human cancers. Copper plays an important role in tumor angiogenesis, especially in early stages. Copper is necessary for endothelial cell activation as it stimulates their proliferation and activation. Copper activates several angiogenic factors (VEGF, TNF-α, IL –1, b-FGF) which bind to endothelial cells switch from G0 into G1 phase and force proliferation. The level of ceruloplasmin, the principal copper transporting protein, increases four to eight folds during malignant progression, often tumors become palpable Nasulewicz et al.[12] Zinc is essential for regulation of cell cycle and cell division and also essential for DNA polymerase activity and is particularly important for rapid cell proliferation encountered in growing tumors. Bloniarz et al, compared the copper, zinc levels in saliva of patients with oral cancer to the control group and observed that these elements were significantly higher in the case group when compared to controls. They also have done a similar study in the serum and found that copper and zinc levels were higher in case group when compared to control group.[13] Toke et al, found increased level of serum copper and zinc in patients with head and neck cancer. The results in the present study also showed significant increase in the copper and zinc levels in squamous cell carcinoma, when compared to normal control group that correlates with the findings of the above authors.[14] Varghese et al, studied the serum copper and zinc levels in premalignant group that included submucous fibrosis and leukoplakia, showed decreased levels of serum copper and zinc in submucous fibrosis and there was no change in leukoplakia when compared to controls.[2]
In the present study, we observed significant increase in the salivary copper and zinc levels in the premalignant group when compared to normal control group, which comprises submucous fibrosis, leukoplakia and lichen planus. Jayadeep et al, showed elevated serum copper levels in oral leukoplakia.[1] Our study showed significant increase in salivary copper levels in leukoplakia. So far no studies have been carried out either in serum, plasma or saliva to evaluate the levels of copper and zinc in lichen planus. Copper and zinc levels are more in oral cancer when compared to premalignant group; there was no statistical difference between these two groups. The difference in the values may be attributed to various factors like habits, stage of the oral cancer, the oral status and varied sample size among the groups. Higher copper levels in sub mucous fibrosis when compared to leukoplakia and lichen planus. The possible explanation is that all the patients with the submucous fibrosis were areca nut or pan chewers and the previous studies shown that subjects chewing areca would consume over 5mg of copper per day Trivedy et al. Local factors such as site of quid placement, length of time chewed, consistency of quid, composition of quid, and whether the saliva is expelled or not can affect the salivary copper levels. The time period between the chews may also be important. It has been demonstrated that it takes 40 min for the raised salivary copper level to return to its baseline value. If quid chewing is on frequent basis, the salivary copper level may stay raised for long time. These factors may help to explain the marked variations seen in the salivary copper levels within the submucous fibrosis group.[15] However, there was no marked difference in copper levels between leukoplakia and lichen planus. The copper and zinc levels in saliva differed in consecutive samples per individual in the control group. These variations are difficult to explain and may be affected by many physiological variables.
Determination of salivary copper and zinc levels depending upon the age range and sex was done in - Group I, Group II and Group III. Although, statistically no significant difference was observed in the levels depending on the age range and sex, similar to the study done by Cieslak et al.[16] We noticed that the copper levels were decreased and the zinc levels were increased as age increases in normal healthy individuals. Results of previous studies Schwartz showed decline in serum zinc levels with increasing age and women demonstrated lower levels than males, which are contradictory to our findings.[2]
The copper and zinc levels were analyzed according to the histopathological differentiation in cases of oral cancer. We noticed a steady decrease in copper levels and increase in zinc levels from very well differentiated to moderately differentiated squamous cell carcinoma, with more decline of levels as the cell becomes more anaplastic. The decrease in copper levels may be due to high vascularity in well- differentiated tumors when compared to the poorly differentiated tumors and the vascularity for the growth of the tumor in turn is dependent on the copper. This finding can be further supported with the findings of Rodallec et al, who assessed vascular density in pancreatic tumors and showed that poorly differentiated were less vascularized than well differentiated tumors and carcinomas.[17] Solid tumors are dependent on vascular supply to grow beyond spheres of about 0.5–2 mm in diameter i.e. angiogenesis diameter Hannen 2004. Copper plays an important role in tumor angiogenesis especially at its early stages, furthermore, the cellular deposition of copper is also altered in tumor tissues – from cytoplasm in normal tissue to intranuclear and perinuclear zones in tumors. Similarly, increase in zinc levels according to histodifferentiation, may be due to their important role in the rapid cell proliferation as encountered in growing tumors.[18] While the copper zinc ratio increased from very well differentiated to moderately differentiated squamous cell carcinoma.
We also observed increase in copper levels from mild OSMF to severe cases which are graded histopathologically. The probable reason is that copper has been implicated in tissue fibrinogenesis via the copper –dependent enzyme lysal oxidase, which has a crucial role in the cross linking of collagen and elastin. This enzyme appears to be an intrinsic protein of connective tissue that is induced at detectable levels during fibrinogenesis. Likewise, the copper levels also increased in leukoplakia according to the degree of keratosis. Among the lichen planus group the highest copper levels was seen in the reticular type followed by vesiculobullous, hyperplastic, and erosive type.
As number of studies has showed that the copper zinc ratio have a prognostic and diagnostic value and reliable parameter, rather than an individual copper and zinc in premalignant and malignant lesions. So, we analyzed the copper zinc ratio in different groups. The results showed decrease in copper zinc ratio in premalignant and malignant group when compared to normal group. Therefore salivary levels of copper and zinc in premalignant and malignant lesions of the oral cavity may be used as a potential diagnostic tool. Further research is required on larger subjects to determine the importance of these levels in saliva.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Jayadeep A, Pillai KR, Kannan S, Nalinakumari KR, Mathew B, Nair MK, et al. Serum levels of copper, zinc and ceruloplasmin in oral leukoplakia and squamous cell carcinoma. J Exp Clin Cancer Res. 1997;16:295–300. [PubMed] [Google Scholar]
- 2.Varghese I, Sugathan CK, Balasubramoniyam G, Vijayakumar T. Serum copper and zinc levels in premalignant and malignant lesions of the oral cavity. Oncology. 1987;44:224–7. doi: 10.1159/000226482. [DOI] [PubMed] [Google Scholar]
- 3.Capel ID, Pinnock MH, Williams D C, Hanham IW. The serum levels of some trace and bulk elements in cancer patients. Oncology. 1982;39:38–41. doi: 10.1159/000225602. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MK. Role of trace elements in cancer. Cancer Res. 1975;35:3481–7. [PubMed] [Google Scholar]
- 5.Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69–76. doi: 10.1034/j.1601-0825.2002.1o834.x. [DOI] [PubMed] [Google Scholar]
- 6.Chin-Thin W, Wei-Tun C, Tzu-Ming P, Ren-Tse W. Blood concentrations of selenium, zinc, iron, copper and calcium in patients with hepatocellular carcinoma. Clin Chem Lab Med. 2002;40:1118–22. doi: 10.1515/CCLM.2002.196. [DOI] [PubMed] [Google Scholar]
- 7.Paul RR, Chaterjee J, Das AK, Cervera ML, de la Guardia M, Chaudhuri K. Altered elemental profile as indicator of homeostatic imbalance in pathogenesis of oral submucous fibrosis. Biological Trace Element Research. 2002;87:45–56. doi: 10.1385/BTER:87:1-3:045. [DOI] [PubMed] [Google Scholar]
- 8.Olmez I, Gulovali MC, Gordan GE, Henkin RI. Trace elements in human parotid saliva. Biol Trace Elem Res. 1988;17:259–70. doi: 10.1007/BF02795462. [DOI] [PubMed] [Google Scholar]
- 9.Hofman LF. Human Saliva as a diagnostic specimen. J Nutr. 2001;131:1621S–5S. doi: 10.1093/jn/131.5.1621S. [DOI] [PubMed] [Google Scholar]
- 10.Mathur A, Wallenius K, Abdulla M. Relation between zinc content in saliva and blood in healthy human adults. Scan J Clin Lab Invest. 1977;37:469–72. [PubMed] [Google Scholar]
- 11.Borella P, Fantuzzi G, Aggazzotti G. Trace elements in saliva and dental caries in young adults. Sci Total Environ. 1994;153:219–24. doi: 10.1016/0048-9697(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 12.Nasulewicz A, Wietrzyk J, Opolski A. The role of Copper in Tumour angiogenesis. Cell Mol Biol Lett. 2002;7(Suppl):308. [Google Scholar]
- 13.Bloniarz J, Rahnama M, Zareba S, Swiatkowski W. The influence of carcinogenesis in the oral cavity on the level of zinc, copper and iron in serum. Rocz Panstw Zakl Hig. 2004;55:235–41. [PubMed] [Google Scholar]
- 14.Toke GB, Dhamne BK. A study of copper, serum zinc and Cu/Zn ratio as diagnostic and prognostic index in cases of head and neck and face tumors. Indian J Pathol Microbiol. 1990;33:171–4. [PubMed] [Google Scholar]
- 15.Trivedy S, Warnakulasuriya KA, Peters TT, Senkus R, Hazarey VK, Jhonson NW. Raised tissue copper levels in oral submucous fibrosis. J Oral Path Med. 2000;29:241–8. doi: 10.1034/j.1600-0714.2000.290601.x. [DOI] [PubMed] [Google Scholar]
- 16.Cieslak M, Jedrzejewska T, Zgirski A. Determinations of magnesium, iron and copper in the saliva of healthy subjects. Czas Stomtol. 1990;43:202–6. [PubMed] [Google Scholar]
- 17.Rodallec M, Vilgrain V, Couvelard A, Rufat P, O’Toole D, Barrau V, et al. Endocrine pancreatic tumours and helical CT: contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology. 2006;6:77–85. doi: 10.1159/000090026. [DOI] [PubMed] [Google Scholar]
- 18.Hannen EJ, Riediger D. The quantification of angiogenesis in relation to metastasis in oral cancer: A review. Int J Oral Maxillofacial Surg. 2004;33:2–7. doi: 10.1054/ijom.2003.0433. [DOI] [PubMed] [Google Scholar]


