Abstract
Background:
Oral lichen planus (OLP), a well-known mucocutaneous lesion has been the center of debate regarding its obscure etiopathogenesis. Recent highlight has been placed on the role of autoimmunity and a sect of constitutional molecules, the native chaperones HSP70, proposed to be important in the onset and progress of disease.
Aim:
To substantiate a potential role of HSP70 in the pathogenesis of oral lichen planus.
Settings and Design:
The study involved immunohistochemical (IHC) analyses in a laboratory under monitored conditions. It was a retrospective study on clinically and histopathologically confirmed specimens.
Materials and Methods:
30 samples of confirmed cases of OLP were selected and grouped on the basis of the thickness of the epithelial layer into atrophic, normal (classical) and acanthotic. An immunohistochemical analysis of the expression of HSP70 protein was done, followed by a quantitative and qualitative analysis of the stained layers.
Statistical Analyses:
A Z test was performed to estimate the difference observed between two sample proportions. The statistics was given at 1% level of significance i.e. P<0.01.
Results:
An increased expression of HSP70 was noted in the basal and suprabasal cells of the epithelium of OLP. A higher count and intensity of HSP70 expression was seen in the basal layer of the epithelium. Greater expression was noted in the epithelium of the atrophic group.
Conclusion:
The expression pattern of HSP70 positively implicates it in the pathogenesis of OLP.
Keywords: Acanthotic lichen planus, atrophic lichen planus, HSP70
INTRODUCTION
Lichen planus is a chronic disease of the skin and mucous membrane which is relatively common and of worldwide distribution. British physician Erasmus Wilson (1869) was the first to describe this lesion, and coined the present term.[1,2]
The precise cause of Oral lichen planus (OLP) remains obscure. The current opinion suggested by Porter (1997) and Eisen et al (1999) proposes that the pathogenesis of lichen planus is a T-cell-mediated process, and there is substantial evidence to uphold this view.[3,4] Over the past decade, many apoptotic markers like bcl- 2, bax, and cell proliferation markers like Ki-67 antigen have been studied to evaluate their role in the pathogenesis of OLP.[1]
Recently, the focus has shifted onto a family of molecules, the heat shock proteins, and their role in pathogenesis. Heat shock proteins (stress proteins) are found in all organisms and are among the most conserved proteins known in phylogeny with respect to structure and function. These are expressed constitutively in low concentrations and appear to play a role in maintaining cell function.[5,6]
The classification of HSPs is based on their function and size (molecular mass). Major classes include small HSPs, HSP 40, 60, 70, 90 and 110 families.[6]
In the mammalian species, the HSP family consists of mitochondrial (mt- Hsp0 60) and cytosolic Hsp0 60. HSP0 70 family includes the constitutive cytosolic Hsp0 70, the stress-induced cytosolic Hsp0 70, the endoplasmic reticulum Bip (Grp-78), and the mitochondrial (mt Hsp0 70).[5]
Cellular expression of this antigen may take place under varied conditions, as also seen in lichen planus. Both HSP-60 and HSP70 induce the release of cytokines from the lymphocytes and contribute to the pathogenesis of autoimmune disease and chronic inflammation.
In the current study, we aim to study the expression of HSP70 molecule and their possible role in lesion chronicity.
MATERIALS AND METHODS
The present study was designed to evaluate the expression of Hsp0 70 in oral lichen planus, using immunohistochemical analysis (IHC) in paraffin-embedded tissues. Tissue specimens of 30 confirmed cases of OLP were retrospectively retrieved from the archival paraffin-embedded blocks of the Department of Oral and Maxillofacial Pathology and Microbiology, I.T.S Center for Dental Studies and Research, Muradnagar. The clinical details, case history and the light microscopic features were compiled. The selected cases were distributed into three groups as follows:
Group I: Atrophic lichen planus (<7 layers).
Group II: Classical/Normal lichen planus (7–14 layers)
Group III: Acanthotic lichen planus (>14 layers)
IHC procedure performed is detailed as follows. 3 μm thick sections were made and lifted on Poly-L-lysine-coated slides. Sections were dried for 16 h at 37°C followed by 1 h at 60°C. Deparaffinization of the sections was done by heating on the slide warming table at 60°C for 15–20 min and was then passed through two changes of xylene for 5 min each. The sections were rehydrated by taking them through three changes of 100% alcohol for 5 min each, followed by 90%, 70% and 50% alcohol for 5 min each. Sections were then brought to distilled water for 10 min and washed with phosphate-buffered saline (PBS pH 7.2–7.6) for 5 min. Peroxide block treatment was done for 10 min to quench endogenous peroxidase activity and subsequently washed with PBS for 5 min.
Antigen retrieval was done by placing the sections in tanks containing citrate buffer (retrieval solution) at pH 6.2–6.4 and were brought to boil in an E7 Antigen retrieval machine (Biogenex) in 2 cycles:
Cycle 1 – 95° for 10 min.
Cycle 2 – 98° for 5 min.
The sections were cooled for 30 min to bring them to room temperature, brought to distilled water for 5 min and washed in PBS for 5 min.
Power block was applied for 10 min and the sections were incubated with primary antibody for 1 h (Anti- Hsp0 70 mouse monoclonal antibody: BRM-22, in PBS with protein and preservative, Biogenex Ind. Pvt. Ltd., Hyderabad, India). Following PBS wash for 5 min, the sections were treated with super enhancer for 25 min, washed again in PBS and incubated with SS-labeled poly-HRP substrate (Poly HRP Secondary Antigen Detection System). Sections were washed thoroughly with PBS and covered with freshly prepared substrate chromogen solution for 10 min. The slides were then washed with PBS, counterstained in Harris hematoxylin (30 s), washed gently under running water, followed by distilled water and allowed to air dry. The sections were dehydrated, dipped in xylene, mounted using DPX and the slides were interpreted using light microscope.
Histopathologically confirmed case of breast carcinoma was taken as positive control for the expression of Hsp0 70 [Figure 1]. The positively stained HSP70 areas showed uptake of brown color.
Figure 1.
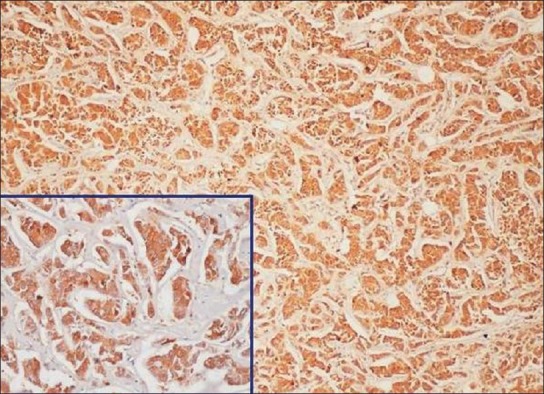
Breast carcinoma taken as positive control labeled with primary antibody (×10, Inset ×40, IHC, HSP70)
Stained sections were scanned to determine the areas that were positively stained. Areas representing the different layers to be studied, that is, basal, suprabasal and the superficial epithelial cell layers were further analyzed in high power to obtain a more cross-sectional count of the tissue. Four representative fields at 400 magnifications were selected and counted in each of the mentioned layers. Thus by selecting four random areas which were not in continuum with each other, a possible error in recounting the same cells was minimized. To negate individual bias in the methodology for counting the positively stained cells, readings of a second observer were taken and tallied for in the same regions.
A quantitative evaluation was done, the labeled cells in subsequent cell layers were counted in four successive high power fields (Magnification ×400) and tabulated. Qualitative grading was done on a four-point scale: (-) negative intensity, (+) mild intensity, (++) moderate intensity, and (+++) high intensity staining. The staining intensity was compared against HSP70-labeled normal oral mucosa [Figure 2].
Figure 2.
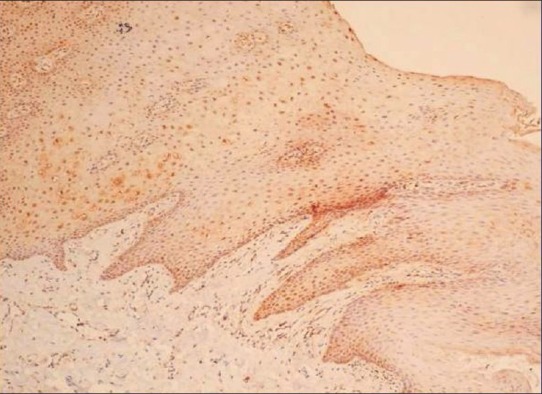
(Normal oral mucosa) Hyperplastic stratified squamous epithelium showing faint staining in the epithelium (×10, IHC, HSP70)
Both quantitative and qualitative assessments were subjected to statistical analysis (Z proportion).
RESULTS
Quantitative assessment
The IHC-labeled OLP tissue specimens revealed a higher quantitative score in the basal cell layer [Table 1]. A statistical evaluation was performed and when Zcal values for atrophic and acanthotic groups were compared against the normal group with respect to the overall percentage of stained cells in different epithelial layers, a highly significant difference was noticed [Table 2]. A full thickness HSP70 labeling was noticed, with the basal cell layer showing a greater count of labeled cells as depicted by the photomicrographs [Figures 3 and 4].
Table 1.
Quantitative assessment of HSP70 positive epithelial cells in OLP groups
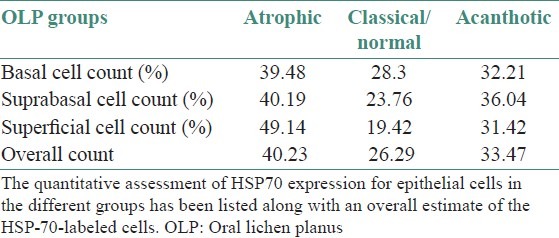
Table 2.
Zcal values for the HSP70 positive epithelial cells among pairs of OLP groups
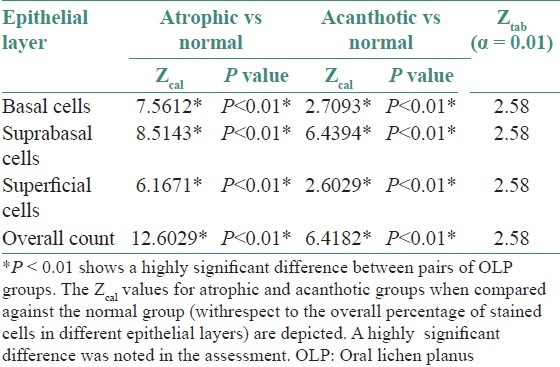
Figure 3.
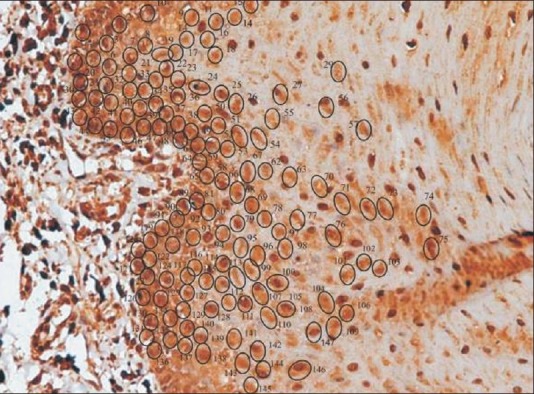
Acanthotic oral lichen planus showing labeled cells with manual tag (×40, IHC, HSP70)
Figure 4.
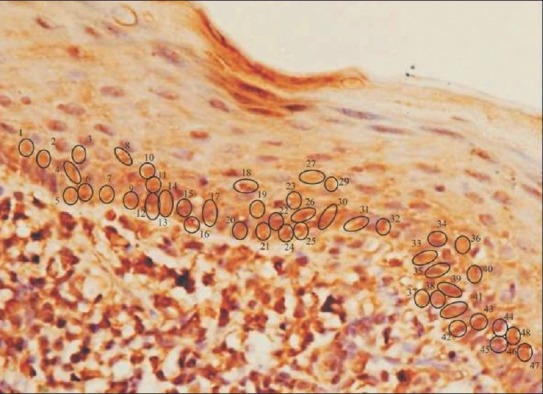
Atrophic oral lichen planus showing labeled cells with manual tag (×40, IHC, HSP70)
Qualitative assessment
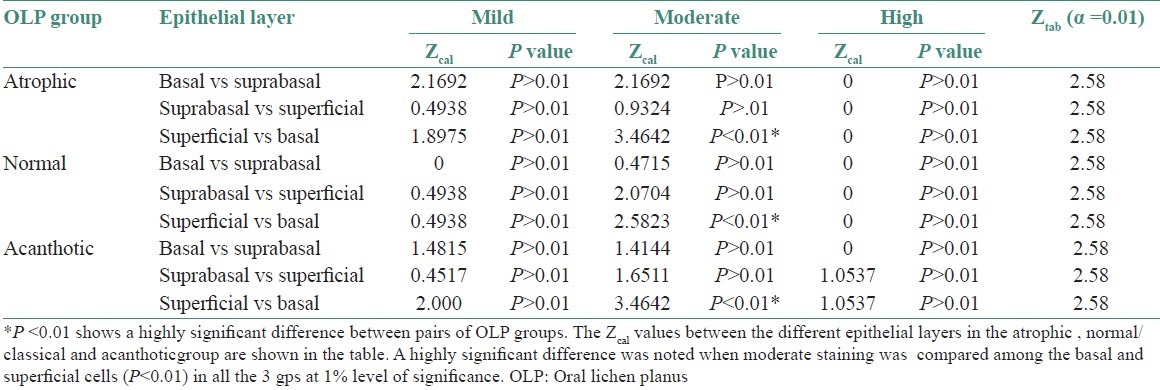
The qualitative evaluation revealed a gradation of intensities in the successive layers with the maximum intensity of stain localized in the basal layer [Table 3 and Figure 5]. A statistically significant difference was noted in the staining intensities between the basal and superficial layers in all the three groups [Table 4].
Table 3.
Qualitative assessment of HSP70 positive epithelial cells in OLP groups
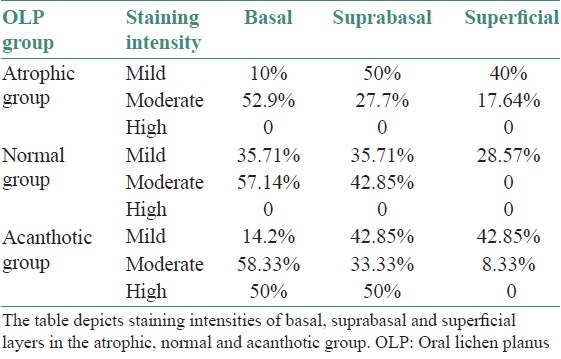
Figure 5.
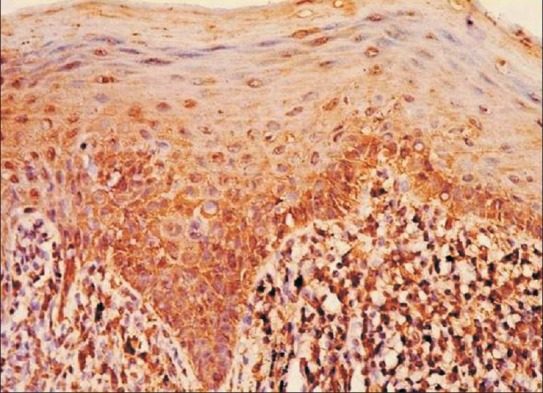
Oral lichen planus showing labeled cells with gradation in staining intensities (×40, IHC, HSP70).
Table 4.
Ztab values for different intensities of staining among epithelial layers in OLP groups
DISCUSSION
Although lesions of lichen planus have some rather distinct clinical and histopathological features, these have proven insufficient to explain the etiology and pathogenesis of the disease. The etiology of OLP is unknown and diverse exogenous agents including systemic medications, mechanical trauma, viral infection, contact sensitivity reactions and tobacco are often associated with the initial onset and the subsequent exacerbations of OLP lesions.[7,8] Since the onset of oral lichen planus is known to exacerbate by such a large plethora of exogenous agents, it was pertinent to study the altered expression of HSP in OLP.
In the present study, 30 clinically and histopathologically confirmed cases of OLP were included, which were grouped as atrophic, classical/normal and acanthotic based on epithelial thickness.
The expression of Hsp0 70 was evaluated in the study sections. A quantitative evaluation of the labeled cells revealed the expression to be present in all the layers of the epithelium.
Sugerman et al, drew comparisons in HSP staining among OLP, dysplastic OLP, normal oral mucosa and non-specific oral ulceration. They noted a full thickness expression of HSP in 94% of their OLP cases.[8] Our findings concur with the above study as we also noted a full thickness expression in all but six of the cases. This finding can be justified by appreciating the fact that HSP, a molecular chaperone, is synthesized in all the cells and is an essential protein folding tool in the cellular machinery, as also stated by Deuerling and Bukau and Beere.[9,10] The normal, non-diseased mucosa (free of any pathology/ source of irritation) of the oral cavity shows a faint expression of heat shock proteins in the epithelium. This is supported by Bramanti et al and Seoane et al, who concluded in their study that the expression of HSPs is seen in normal oral mucosa.[11,12] Literature reveals that the expression of HSPs was noted to be altered in OLP.[13]
The current study went a step further in trying to evaluate the labeling differences between the atrophic, normal and acanthotic groups of lichen planus. Such a detailed comparison between the atrophic, normal and acanthotic types has not been done previously in the literature and we attempted to throw some light on the variations in the expression of HSP among the three histological groups.
The labeled cells were assessed in the basal, suprabasal and the superficial layers of the groups. A highly significant difference (P<0.01) was noted in the count of stained cells in these layers, when atrophic and acanthotic groups were compared with normal. On an overall assessment there was a higher count of stained cells in the atrophic group, followed by the acanthotic and the normal group. The maximum count of stained cells was noted in the basal cells layer in all the groups. A similar finding of varied tissue distribution, with basal keratinocyte staining occurring significantly more frequently, has been noted in a previous study by Sugerman.[8] This finding can be substantiated by the fact that, in OLP, it is the basal region which is the apparent target for destruction by the subepithelial T lymphocytic population.[8,10,13,14] Thus, basal keratinocytes are vulnerable to the “stress of dying” and thus the raised labeling index.
A qualitative analysis of the staining intensities was also done in the OLP groups. Basal, suprabasal and superficial cell layers were graded on the intensity scale as mild, moderate or high. A highly significant difference was noted when moderate staining intensity was compared among the basal and superficial cells (P<0.01) at 1% level of significance. A majority of cells staining for moderate intensity were noted in the basal cell layer. The assessment of an increased staining in the basal cell layer is similar to that made by Sugerman et al, thus reiterating the fact that focus of stressed cells is in the basal region.[8]
According to Bramanti et al and Sugerman et al, OLP represents an autoimmune response, directed towards the basal cell antigens or a hypersensitive response to antigens shared by basal cells and a microbial agent. HSPs represent antigenic proteins that may potentially be involved in the initiation or the persistence of the lymphocytic response of lichen planus.[11,14]
Previous studies provide both supporting and opposing evidence for the participation of HSP70 in OLP. Sugerman et al found a significant increase in HSP70 staining intensity and distribution of positively stained cells in OLP as compared with normal mucosa.[8] Their findings supported a possible involvement of HSPs in the pathogenesis of OLP. In contrast to this, Chaiyarit et al confirmed that the expression of HSP60 in the basal layer of OLP was significantly higher than in oral fibromas implicating it in the pathogenesis of OLP, but found no significant difference in the expression of HSP70 between the two groups.[15] Bramanti et al reported that HSP70 expression was reduced in OLP in comparison with normal oral mucosa, which prompted them to conclude that HSPs do not play a significant role in the pathogenesis of OLP.[11] Seaone concluded that differences in HSP70 expression among OLP and normal mucosa were slight and inconclusive.[12]
The findings of the current investigation are in consistence with those of Sugerman.[7] It is hypothesized that, in OLP cases, diverse exogenous agents may cause an upregulated expression of HSP by the basal keratinocytes. A reaction of the cytotoxic lymphocytes against these activated keratinocytes could then result in cell death and tissue destruction, characteristic of OLP lesions.[16]
Once initiated, such a cytotoxic immunological reaction, or cytokines such as gamma interferon and tumor necrosis factor released from the activated T lymphocytes could further upregulate HSP expression by the neighboring keratinocytes, thus propagating lesion chronicity. This is also coherent with the views of Bramanti et al, Sugerman et al, Roopshree et al and Lavanya et al, who mention that HSPs are expressed as auto-antigens OLP and have a role to play in the persistence or chronicity of the disease.[11,14,17,18]
The immunohistochemical evaluation of every case of OLP using Hsp0 70 is not recommended for, since it is not a diagnostic aid (upregulated in other inflammatory conditions and pathologies). However, the revelation that Hsp0 70 is involved in the pathogenesis of OLP could, in future be the basis for using it in therapeutics, as has been discussed in relation to other disease processes.[19–21]
CONCLUSION
The current study was carried out with an aim of assessing the overall altered expression of HSP70 (quantitative and qualitative) in oral lichen planus, localizing the expression and comparing the variations (if any) in labeling between atrophic, normal/classical and acanthotic histological subgroups. A comparison in the HSP70 labeling and intensity profiles has been attempted among the histological subtypes of OLP, which has not been previously done. The count of HSP70-labeled cells among the atrophic and acanthotic subgroups was found to be significantly higher than the classical group. A higher count was noted in the basal third of the epithelium, especially in the atrophic group.
This implies that a possible correlation exists between the histological nature/state of the epithelium and aggravation in the disease process, as reflected by the increase in the Hsp0 70 profile in the basal keratinocytes.
Thus, as seen in this study HSP70 could be used as a tool in hypothetically validating the general perception of alteration in the antigenic profiles within keratinocytes in OLP.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Tyldesley WR. Oral lichen planus. Br J Oral Surg. 1974;11:187–206. doi: 10.1016/0007-117x(74)90101-2. [DOI] [PubMed] [Google Scholar]
- 2.Pullon PA. Ultrastructure of oral lichen planus. Oral Surg Oral Med Oral Pathol. 1969;28:365–71. doi: 10.1016/0030-4220(69)90231-x. [DOI] [PubMed] [Google Scholar]
- 3.Porter SR, Kirby A, Olsen I, Barrett W. Immunologic aspects of dermal and oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:358–66. doi: 10.1016/s1079-2104(97)90244-4. [DOI] [PubMed] [Google Scholar]
- 4.Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:431–6. doi: 10.1016/s1079-2104(99)70057-0. [DOI] [PubMed] [Google Scholar]
- 5.Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004;286:C739–44. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- 6.Zugel U, Kaufmann S. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyden G, Arwill T, Gisslen H. Histochemical studies on lichen planus. Oral Surg Oral Med Oral Pathol. 1974;37:239–48. doi: 10.1016/0030-4220(74)90419-8. [DOI] [PubMed] [Google Scholar]
- 8.Sugerman PB, Savage NW, Xu LJ, Walsh LJ, Seymour GJ. Heat shock protein expression in oral lichen planus. J Oral Pathol Med. 1995;24:1–8. doi: 10.1111/j.1600-0714.1995.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 9.Deuerling E, Bukau B. Chaperone-assisted folding of newly synthesized proteins in the cytosol. Crit Rev Biochem Mol Biol. 2004;39:261–77. doi: 10.1080/10409230490892496. [DOI] [PubMed] [Google Scholar]
- 10.Beere HM. “The stress of dying”: The role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–51. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- 11.Bramanti TE, Dekker NP, Lozada-Nur F, Sauk JJ, Regezi JA. Heat shock (stress) proteins and γδ T lymphocytes in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:698–704. doi: 10.1016/s1079-2104(05)80254-9. [DOI] [PubMed] [Google Scholar]
- 12.Seoane J, Ramírez JR, Romero MA, Varela-Centelles P, Garcia-Pola MJ. Expression of heat shock protein (HSP70) in oral lichen planus and non-dysplastic oral leukoplakia. Clin Otolaryngol Allied Sci. 2004;29:191–6. doi: 10.1111/j.0307-7772.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 13.Cruchley AT, Williams DM, Farthing PM, Lesch CA, Squier CA. Regional variation in Langerhans cell distribution and density in normal human oral mucosa determined using monoclonal antibodies against CD1, HLADR, HLADQ and HLADP. J Oral Pathol Med. 1989;18:510–6. doi: 10.1111/j.1600-0714.1989.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 14.Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 15.Chaiyarit P, Kafrawy AH, Miles DA, Zunt SL, Van Dis ML, Gregory RL. Oral lichen planus: An immunohistochemical study of heat shock proteins (HSPs) and cytokeratins (CKs) and a unifying hypothesis of pathogenesis. J Oral Pathol Med. 1999;28:210–5. doi: 10.1111/j.1600-0714.1999.tb02026.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhou XJ, Sugerman PB, Savage NW, Walsh LJ, Seymour GJ. Intra-epithelial CD8+ T cells and basement membrane disruption in oral lichen planus. J Oral Pathol Med. 2002;31:23–7. doi: 10.1046/j.0904-2512.2001.10063.x. [DOI] [PubMed] [Google Scholar]
- 17.Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus--a review. J Oral Pathol Med. 2010;39:729–34. doi: 10.1111/j.1600-0714.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 18.Lavanya N, Jayanthi P, Rao Umadevi K, Ranganathan K. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127–32. doi: 10.4103/0973-029X.84474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pockley AG. Heat shock proteins in health and disease: Therapeutic targets or therapeutic agents? Expert Rev Mol Med. 2001;3:1–21. doi: 10.1017/S1462399401003556. [DOI] [PubMed] [Google Scholar]
- 20.Margulis BA, Sandler S, Eizirik DL, Welsh N, Welsh M. Liposomal delivery of purified heat shock protein hsp0 70 into rat pancreatic islets as protection against interleukin 1 beta-induced impaired beta-cell function. Diabetes. 1991;40:1418–22. doi: 10.2337/diab.40.11.1418. [DOI] [PubMed] [Google Scholar]
- 21.Hansen JE, Sohn W, Kim C, Chang SS, Huang NC, Santos DG, et al. Antibody-mediated Hsp0 70 protein therapy. Brain Res. 2006;1088:187–96. doi: 10.1016/j.brainres.2006.03.025. [DOI] [PubMed] [Google Scholar]


