Abstract
Context:
In routine histopathology, decalcification of bone and teeth is often an essential and important step during tissue processing. Various decalcifying agents have been used in the past. The rate of decalcification and the effect of decalcifying agents on the tissue and its staining characteristics are two important parameters which influence the selection of decalcifying solutions. Though some agents remove the calcium ions completely and rapidly, they adversely affect the staining characteristics and may also damage the organic components. There have been very few studies which have systematically evaluated the efficacy of these agents in decalcifying dental hard tissues.
Aims:
The present study was done to evaluate the rate of decalcification of six different decalcifying agents and also their effect on staining characteristics on dental hard tissues.
Materials and Methods:
Six decalcifying agents namely, neutral ethylene diamine tetra acetic acid (EDTA) decalcifying solution, 5% nitric acid, Perenyi's fluid, formalin–nitric acid, 5% trichloracetic acid, and 10% formic acid were used to decalcify 24 natural teeth (four in each solution). The endpoint of decalcification was evaluated by radiographic and chemical methods. The decalcified teeth were then routinely processed, sectioned, and stained with hematoxylin and eosin stains.
Results:
Neutral EDTA was the most considerate to the soft and hard tissues and 5% nitric acid was the least considerate to the tooth structure.
Conclusions:
Neutral EDTA, though being the slowest decalcifying agent among the six agents used in the study, gave excellent results for soft-tissue integrity, and best quality of both soft-tissue and hard-tissue stainings.
Keywords: 10% formic acid, 5% nitric acid, 5% trichloracetic acid, formalin–nitric acid, neutral EDTA decalcifying solution, Perenyi's fluid, pulp tissue integrity, teeth decalcification
INTRODUCTION
Pierre de Coubertin proposed the Olympic motto “citius, altius, forties” which is Latin for faster, higher, and stronger. With this basis, we too have adopted the same in our quest for a decalcifying agent.[1]
The head and neck is a complex structure of both soft and hard tissues. Soft tissues put forth little resistance to the histochemical techniques. Lesions affecting hard tissues need intricate, technique-sensitive methodology for interpretation and diagnosis. Rephrase tissue is an inherently difficult tissue to work with histologically. Technique for demonstration includes hard-tissue grinding and decalcification.[2]
The pulpal soft tissue can only be assessed in decalcified sections which otherwise in ground section is not possible as it is lost. The aim of decalcification is to remove calcium salts from mineralized tissue using chemical solutions like acids and chelating agents, while preserving the organic portions.[3]
So, an ideal decalcifying agent should
Be fast;
Be good and;
Do good.
Though some agents remove the calcium ions completely and rapidly, they inversely affect the staining characteristics and may also damage the organic components.[4]
So, we present here the comparative evaluation of different decalcifying agents with respect to rate of decalcification, the effect of decalcifying agents on the dental tissue, and its influence on the staining characteristics. The quest for at most near ideal decalcifying agent begins.
The aims and objectives of the study were:
To evaluate the fastest decalcifying agent;
To evaluate the decalcifying agents based on its effect on the organic content and integrity of the soft tissue;
To evaluate the staining characteristics of the teeth decalcified in the different decalcifying agents;
To compare and contrast between the decalcifying agents.
MATERIALS AND METHODS
Freshly extracted, noncarious, not attrited, 24 natural teeth were obtained from patients aged 40–45 years.[3–5] The teeth, fixed in 10% formalin, included incisors, canines, premolars, and molars, and were used to analyze the six different decalcifying agents namely 5% nitric acid, Perenyi's fluid, formalin-nitric acid solution, neutral ethylene diamine tetra acetic acid (EDTA) decalcifying solution, 10% formic acid, and 5% trichloroacetic acid. Each decalcifying agent was used to decalcify an incisor, a canine, a premolar, and a molar each.
Decalcifying agents were subjected to repeated agitation and replaced by freshly prepared agents as indicated by the chemical method of end point of decalcification for 5% nitric acid, Perenyi's fluid, formalin–nitric acid solution, and 5% trichloroacetic acid. For both neutral EDTA and 10% formic acid, the solutions were replaced with fresh solutions every five days.
End point of decalcification of solutions was assessed using the chemical method.[3,5]
Strong liquor ammonia is added drop by drop to 5 cm3 of decalcifying agent until it turns alkaline to litmus.
If solution went cloudy, it indicated presence of calcium and the solution was replaced with fresh solution
If solution does not turn cloudy, 5 cm3 of saturated ammonium oxalate is added to the solution.
If solution remains clear for 30 min, it was concluded that end point of decalcification was reached and the procedure is completed.
End point of neutral EDTA was assessed radiographically wherein the opacity suggested incomplete decalcification [Figure 1]. The physical method of probing the teeth subjected to neutral EDTA with a needle was also suggestive of incomplete decalcification.
Figure 1.
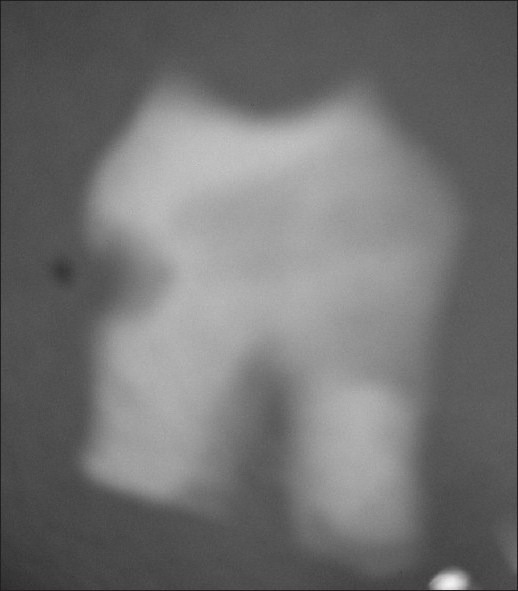
Radiograph of a molar decalcified using neutral EDTA at the end of 91 days, before processing
The speed of decalcification was graded from 1–5 [1-slowest and 5-fastest].[3–5]
All the teeth were washed under running tap water for 10 min (neutral EDTA decalcified teeth was washed for 2 h) and continued with routine processing, paraffin wax infiltration, and embedding; sectioning and staining with hematoxylin and eosin. The stained sections were observed under the microscope and were graded from 1–4 [1-poor, 2-fair, 3-good, and 4-excellent] based on the following criteria:
Ease of sectioning;
Hard-tissue staining;
Soft-tissue staining – both cytoplasmic and nuclear staining;
Soft-tissue attachment;
Soft-tissue shrinkage and;
Pulpal organization.
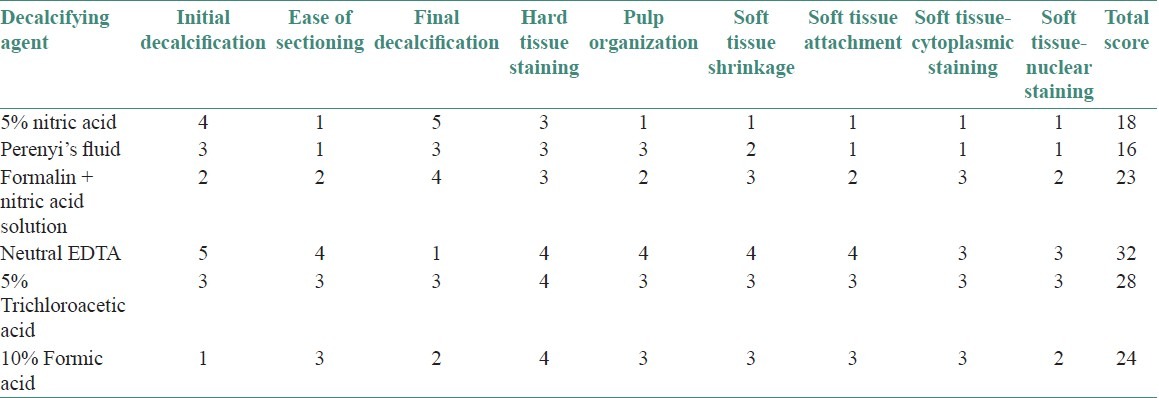
RESULTS
Parameter 1: Speed of decalcification: 5% nitric acid decalcified teeth the fastest and neutral EDTA was the slowest [Graph 1].
Parameter 2: Soft-tissue integrity: With respect to soft-tissue integrity, features like soft-tissue attachment, soft-tissue shrinkage, and pulpal organization, teeth decalcified with neutral EDTA exhibited excellent results, and the ones decalcified with 5% nitric acid scored the least [Graph 2, Figures 2 and 3].
Parameter 3: Staining quality: Neutral EDTA and 5% trichloroacetic acid decalcified teeth stained the best, and 5% nitric acid and Perenyi's fluid stained the worst [Graph 3, Figures 4 and 5].
Overall, neutral EDTA scored over the other agents and Perenyi's fluid scored the least [Table 1].
Graph 1.
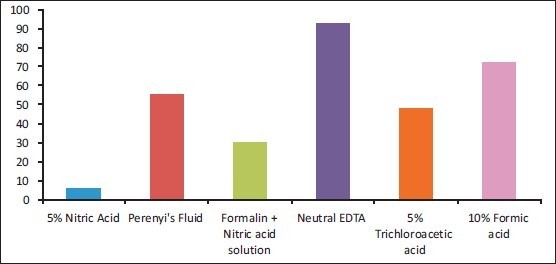
Time in days taken for decalcification
Graph 2.
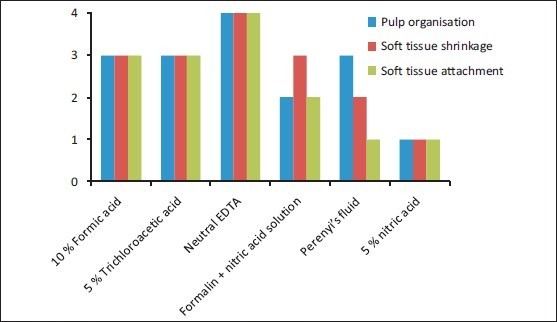
Soft-tissue integrity of the various decalcifying agents
Figure 2.
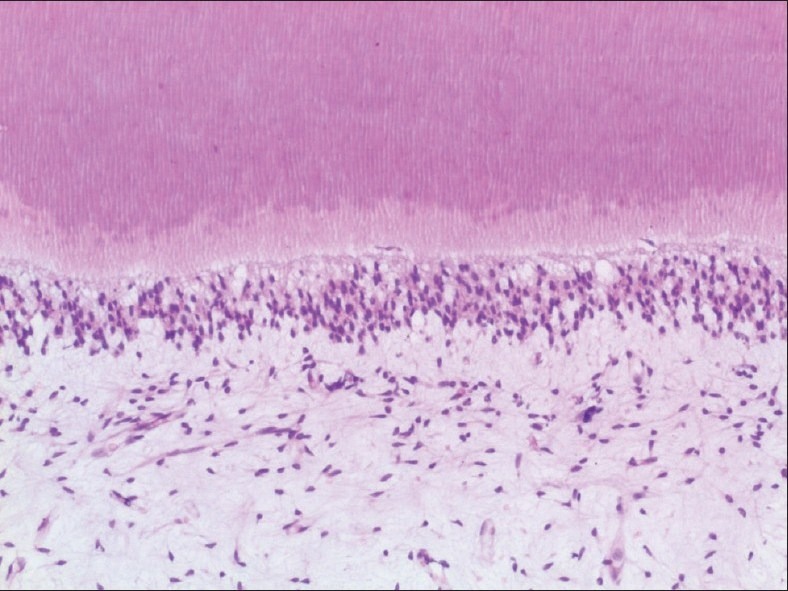
Tooth decalcified using neutral EDTA decalcifying solution under high power
Figure 3.
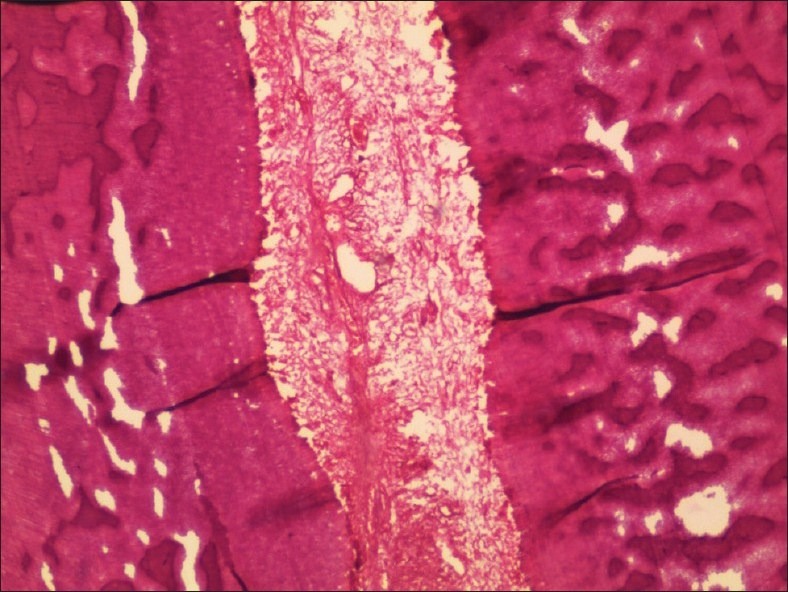
5% Nitric acid decalcified tooth in low power
Graph 3.
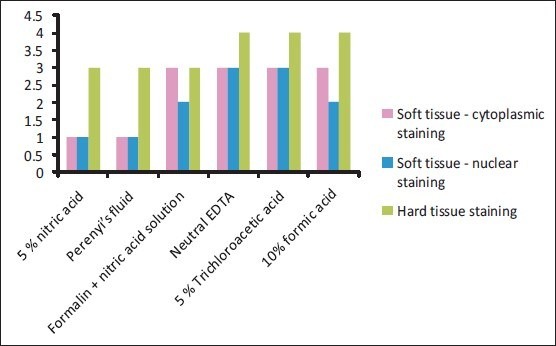
Staining characteristics of the different decalcifying agents
Figure 4.
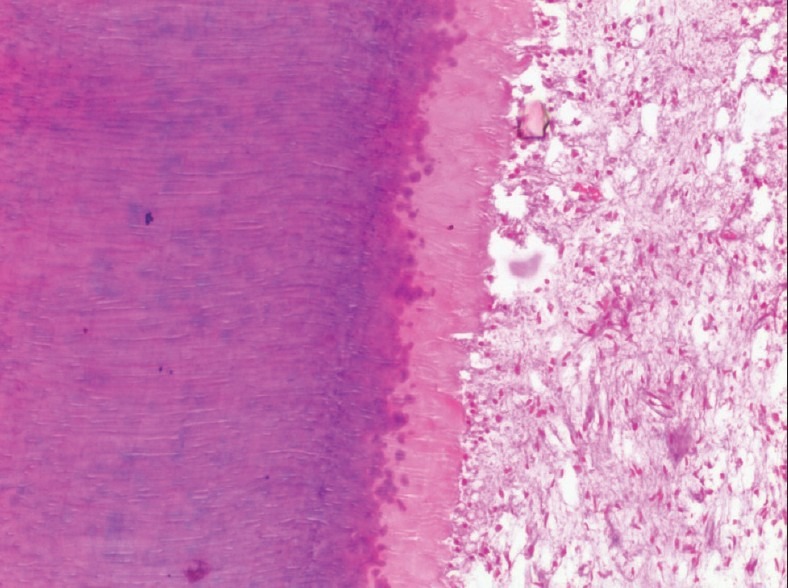
High-power view of a tooth decalcified in 5% Trichloroacetic acid
Figure 5.
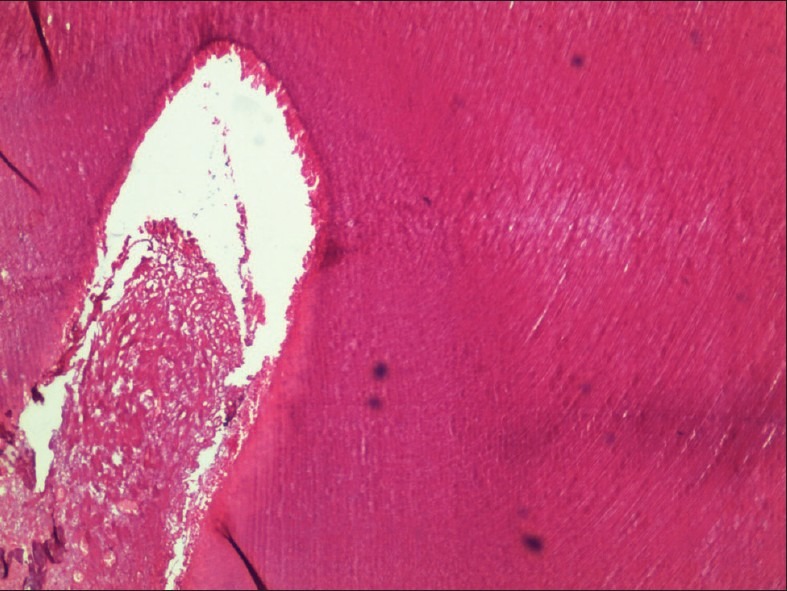
Tooth decalcified using Perenyi's fluid under low power
Table 1.
Cumulative scores of the decalcifying agents based on the various parameters
DISCUSSION
The process of decalcification is done to study the structure of tooth, pulp calcifications, and also to evaluate the biological response of dental pulp to restorative materials.[6]
For many years, scientists have tried to introduce new decalcifying substances or tried to modify known decalcification agents,[5] in order to meet the criteria of a good decalcifying agent which
Ensures complete removal of calcium;
Causes minimal damage to cells and tissues;
Causes non impairment to subsequent staining and;
Decalcifies at reasonable speed.[7]
Most authors have compared two to four decalcifying agents, sometimes varying the methods used and by employing a lot of permutations and combinations of the methods and agents, mainly to decalcify bone.[2,8–10]
In the present study, we attempted to compare the efficacy of six decalcifying agents, its rate of decalcification, its effect on organic and inorganic components of teeth, and staining characteristics.
The speed factor of the decalcifying agents was the highest with 5% nitric acid and lowest by neutral EDTA decalcifying solution, which was in accordance with literature. Also noted, based on chalky white appearance of enamel, was the initial rate of decalcification 4 days after the start of the procedure. Contrary to the overall increased time taken by neutral EDTA, it was noted that teeth decalcified in it had lost its enamel faster than 5% nitric acid.[3,6,8,10,11]
When sectioning it was noted that there was crumbling of tissue decalcified in 5% nitric acid and Perenyi's fluid which also contains nitric acid. Also, tissue was indistinct when observed under microscope as also noted by Zappa et al.[11] with respect to 7% nitric acid. Teeth decalcified with neutral EDTA responded the best to microtome knife, hence deceiving the physical and radiological methods of testing end point of decalcification with respect to neutral EDTA.
In terms of efficacy of agents with respect to soft-tissue integrity and hard- and soft-tissue staining, excellent results were actually obtained with the slowest decalcifying agent, i.e., neutral EDTA.[10,11] Even 5% trichloroacetic acid also showed good staining characteristics.
Soft-tissue attachment and soft-tissue shrinkage, as reported by Zappa et al.,[11] suggest that formic acid and nitric acid produce worst results in contrast to the results obtained from our study, wherein formic acid gave good results as it showed minimal soft-tissue shrinkage and minimal loss of tissue [Figure 6]. The pulp organization with its extra-cellular matrix and histological zones were clearly distinct and excellent in teeth decalcified with neutral EDTA and 5% trichloroacetic acid.
Figure 6.
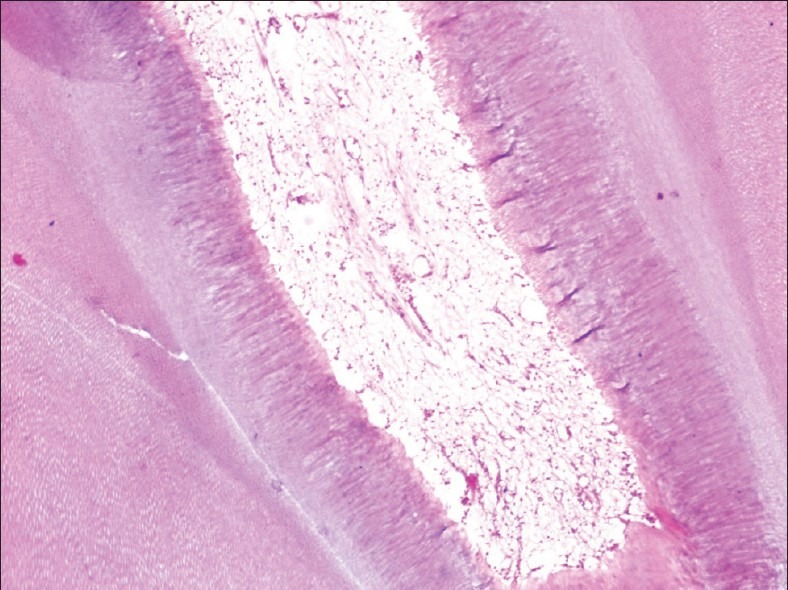
10% Formic acid decalcified tooth in low power
The overall superior results obtained with neutral EDTA may be attributed to the mechanism of capturing metallic ions like calcium which binds to the chelating agent. This means that the calcium ions from the external layer of the apatite crystals will be removed. When all calcium ions from the outer layer of apatite crystals are removed, they will be replaced by ions from the deeper layers. In this way, the crystal size decreases gradually, producing an excellent preservation of tissue components.[4–6]
The quality of decalcified sections and rate of decalcification depends on factors like fixation concentration of decalcifying agent used, temperature, pressure, agitation, electric current, microwave radiation, tissue suspension, and size and type of tissue.[4–6,9]
In a study by Waerhaug, bone and teeth were decalcified rapidly under vacuum. The time taken for decalcification was reduced to one-tenth.[12] Changes in temperature at which decalcification occurs also varies the time taken for complete decalcification. Vongsavan et al., in his study on cat and rat teeth, reported a faster process of decalcification in microwave oven at 37 ± 2°C than in room temperature or conventional oven.[13] Another study by Pitol et al. showed that microwave-aided decalcification showed to be more effective than the traditional methods in aspects like reduction of time period for decalcification, good morphology of bone tissue, and an increase of calcium release using microwaving.[14]
Warshawsky and Moore studied the effects of decalcifying agents, namely nitric acid, formic acid, and EDTA, on number and stainabilty of gram-positive bacteria. The reductions in count in EDTA were fewer.[15] The number of studies using EDTA was greater than the other decalcifying agents, especially when the need was for academic interests and research.[16–19]
To conclude, harder the structure, hardest to decalcify. Slower the process, better the results.
Here we propose, in cases of urgent requirement, use of acids may be employed although with poor tissue integrity. For preservation and presentation, when time is not a factor, use of neutral EDTA may be advocated for its excellent soft-tissue integrity and quality of staining.
An agent that seemed to balance both of the factors of time and tissue integrity was formalin-nitric acid solution [Figure 7].
Figure 7.
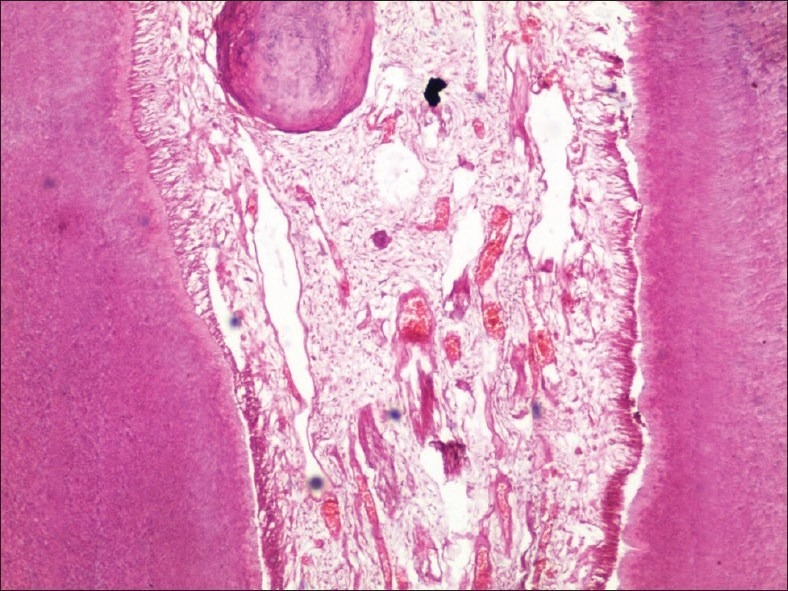
Low-power view of a tooth decalcified in formic acid-nitric acid solution
Regardless of the solution used, methods of decalcification share their characteristic of being accelerated when additional factors are employed. The present study was done with respect to different decalcifying agents only and none of the factors were employed, thus standardizing the procedure. Future studies in which the factors can be varied and decalcifying agent can be kept constant, might bring us to the near ideal decalcifying agent.
ACKNOWLEDGMENTS
The authors wish to acknowledge the support extended by Mrs. Dorothy Anita, Senior Technician, Vydehi Institute of Dental Sciences, Ms. Priyadarshini, Junior Technician, Vydehi Institute of Dental Sciences, and Dr. Sudeendra U.S, Professor, Dept of Oral Pathology and Microbiology, People's College of Dental Sciences, Bhopal.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Olympic charter published by International Olympic committee, Lausanne, Switzerland. 2011:9–21. [Google Scholar]
- 2.Cook SF, Ezra-Cohn HE. A comparison of methods for decalcifying bone. J Histochem Cytochem. 1962;10:560–63. [Google Scholar]
- 3.Drury RA, Wallington EA. 5th ed. Oxford: Oxford University Press; 1980. Carleton's Histological Technique; pp. 199–205. [Google Scholar]
- 4.Mattuella LG, Bento LW, Vier-Pelisser FV, Araiyo FB, Fossati AC. Comparative analysis of two fixating and two decalcifying solutions for processing of human primary teeth with inactive dentin carious lesion. Rev Odonto Ciênc. 2007;22:99–105. [Google Scholar]
- 5.Callis MG. Bone. In: Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. 6th ed. Philadelphia: Churchill Livingstone; 2008. pp. 333–63. [Google Scholar]
- 6.Singh S, Sircar K. Evaluation of efficacy of various chemicals for decalcification of dental hard tissues - An in-vitro study. J Orofac Sci. 2010;1:5–10. [Google Scholar]
- 7.Culling CF, Allison RT, Barr WT. 4th ed. London: Butterworths; 1985. Cellular pathology technique; pp. 408–30. [Google Scholar]
- 8.Verdenius HH, Alma L. A quantitative study of decalcification methods in histology. J Clin Pathol. 1958;11:229–36. doi: 10.1136/jcp.11.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birkedal-Hansen H. Kinetics of acid demineralization in histologic technique. J Histochem Cytochem. 1974;22:434–41. doi: 10.1177/22.6.434. [DOI] [PubMed] [Google Scholar]
- 10.Mawhinney WH, Richardson E, Malcolm AJ. Control of rapid nitric acid decalcification. J Clin Pathol. 1984;37:1409–13. doi: 10.1136/jcp.37.12.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zappa J, Cieslik-Bielecka A, Adwent M, Cieslik T, Sabat D. Comparison of different decalcification methods to hard teeth tissues morphological analysis. Dent Med Probl. 2005;42:21–6. [Google Scholar]
- 12.Waerhaug J. Decalcification of bone and teeth under vacuum- a rapid method for producing hard tissue preparations. J Dent Res. 1949;28:525. doi: 10.1177/00220345490280051601. [DOI] [PubMed] [Google Scholar]
- 13.Vongsavan N, Matthews B, Harrison GK. Decalcification of teeth in a microwave oven. Histochem J. 1990;22:377–80. doi: 10.1007/BF01003173. [DOI] [PubMed] [Google Scholar]
- 14.Pitol DL, Caetano FH, Lunardi LO. Microwave-induced fast decalcification of rat bone for electron microscopic analysis: An ultrastructural and cytochemical study. Braz Dent J. 2007;18:153–7. doi: 10.1590/s0103-64402007000200013. [DOI] [PubMed] [Google Scholar]
- 15.Wijnbergen M, van Mullem PJ. Effect of histological decalcifying agents on number and stainability of gram-positive bacteria. J Dent Res. 1987;66:1029–31. doi: 10.1177/00220345870660050701. [DOI] [PubMed] [Google Scholar]
- 16.Bourque WT, Gross M, Hall BK. A histological processing techniq ue that preserves the integrity of calcified tissues (bone, enamel), yolky amphibian embryos, and growth factor antigens in skeletal tissue. J Histochem Cytochem. 1993;41:1429–34. doi: 10.1177/41.9.7689084. [DOI] [PubMed] [Google Scholar]
- 17.Coleman EJ, Desalva SJ. Rapid decalcification for histochemistry. J Dent Res. 1966;45:1237. doi: 10.1177/00220345660450044701. [DOI] [PubMed] [Google Scholar]
- 18.Warshawsky H, Moore G. A technique for the fixation and decalcification of rat incisors for electron microscopy. J Histochem Cytochem. 1967;15:542–9. doi: 10.1177/15.9.542. [DOI] [PubMed] [Google Scholar]
- 19.Smith CE. Effect of glutaraldehyde and decalcifying agents on acid phosphomonoester hydrolase activity in the enamel organ of the rat incisor: A biochemical study comparing enamel organ with liver. J Histochem Cytochem. 1980;28:689–99. doi: 10.1177/28.7.6771324. [DOI] [PubMed] [Google Scholar]


