Abstract
Tumors of jaw bones are among the most uncommon of all types of neoplasms. Osteosarcoma of jaw bones represents a distinct group of lesions from the conventional type commonly occurring in long bones. Nonetheless, our present knowledge of the tumor allows us to affirm that its clinical behavior and pathologic features differ markedly from those of its homolog in the long bones. The maxillary tumors show predilection for posterior portion of the alveolar process and the antrum, whereas the body is most commonly involved in the mandible followed, by angle, symphysis, and ascending ramus. We have reviewed around 300 cases of osteosarcoma of varied racial origin from PubMed indexed journals spanning from 1967 to 2010 and present their etiology, pathogenesis, features and treatment modalities.
Keywords: Chondroblastic osteosarcoma, fibroblastic osteosarcoma, osteoblastic osteosarcoma, osteosarcoma of jaws
INTRODUCTION
Osteosarcoma referred to as osteogenic sarcoma is the most common primary malignant bone tumor excluding plasma cell tumors. It commonly involves the appendicular skeleton. It accounts for approximately 15% of all primary bone tumors confirmed at biopsy.[1]
ETIOPATHOGENESIS
There are numerous variants of osteosarcoma of jaw bones [Table 1], but these are generally classified into two types primary and secondary.[2] The etiology of primary type is unknown; may be due to genetic influence or other environmental factors. Secondary craniofacial osteogenic sarcomas occur in older patients of skeletal Paget's disease,[3] fibrous dysplasia of bone and as a late sequela to craniofacial irradiation.[4] A number of risk factors had been attributed for the cause of osteosarcoma which includes rapid bone growth[5] as the incidence increases during adolescent growth spurt and because of the typical location of tumor near the metaphyseal growth plate of the long bones. However, osteosarcoma of jaws peaks one or two decades after adolescence which excludes rapid bone growth as the major etiologic factor. Environmental factors such as ionizing radiation and chromic oxide, a radioactive scanning agent have been incriminated.
Table 1.
Variants of osteosarcoma[2]
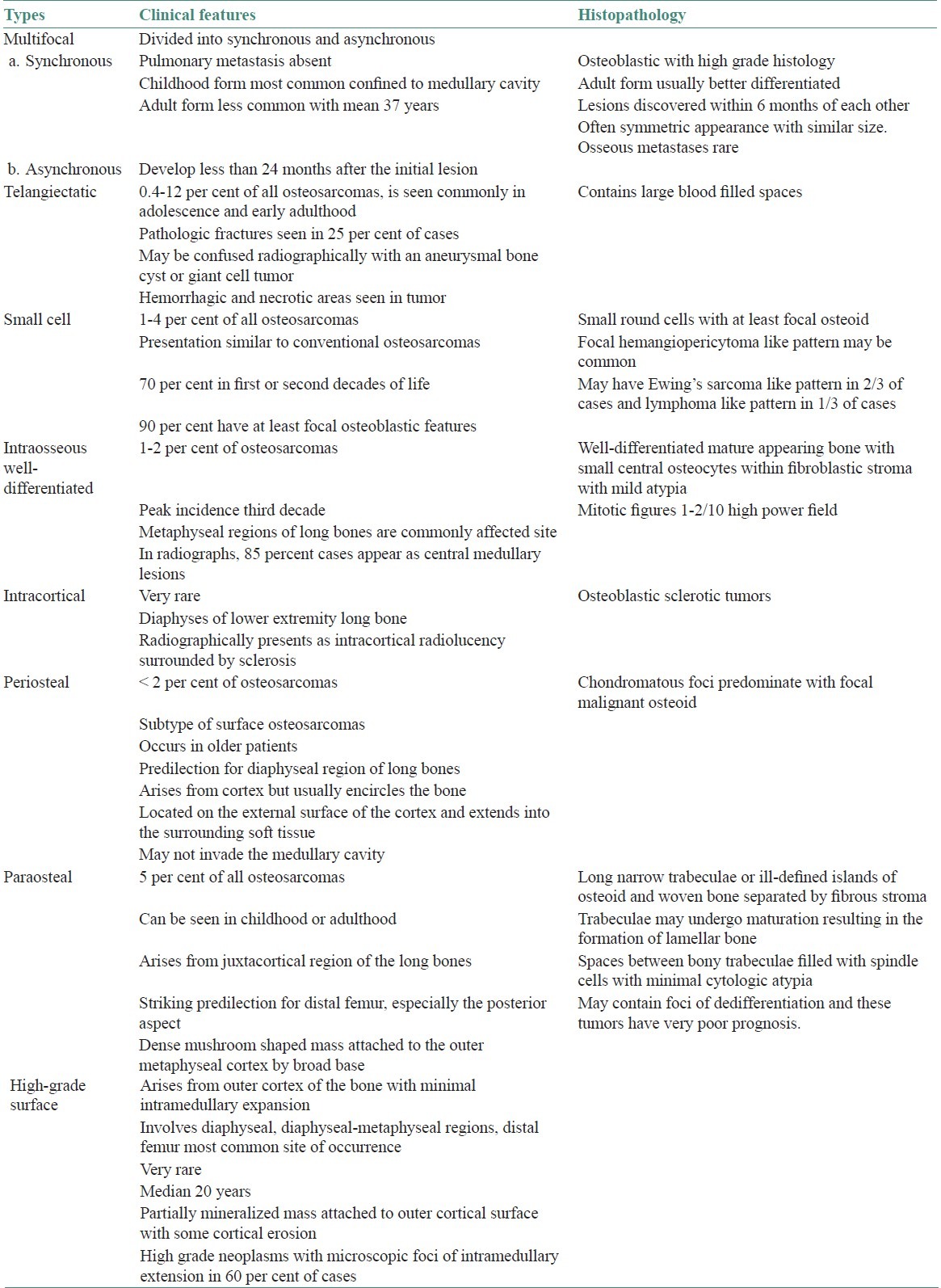
Genetic mutations in tumor suppressor gene P53 and mutated retinoblastoma gene have been claimed to be amongst other etiologic factors. In older patients, this lesion has been found secondary to benign bone lesions such as Paget's disease and fibrous dysplasia.
CLINICAL FEATURES
They affect the most rapidly growing parts of the skeleton; metaphyseal growth plates in femur, tibia and humerus being the commonest sites. Patients of primary craniofacial osteosarcomas are younger (mean age 48 years). Majority of craniofacial osteosarcomas occur in skeletally mature patients in contrast to those that affect the appendicular skeleton. Osteosarcoma of jaw bones have some distinct features such as older age at presentation, longer median survival, rare metastases and local recurrences difficult to control, typically leading to death of the patients.[1] They comprise only 6.5% of all osteosarcomas.[5] In maxilla and mandible, the presentation of the tumor at later age (around fourth decade) and its higher survival rate helps to differentiate it from osteogenic sarcomas in other locations. Mean age according to Garrington et al[5] ranges from 34 to 36 years. Distant metastases are less frequent according to some but Garrington and his colleagues reported distant metastases in approximately 50% of the cases. Men seem to be more commonly affected. August et al[6] reported gender predilection for males and found male:female ratio to be 1.1:1. In another study by Mardinger et al[7] the male:female ratio was found to be 1.2:1. This has been attributed to longer period of skeletal growth and additional volume of bone in men, though neither has been confirmed.
In a study by Forteza et al[1] on 81 cases of osteosarcoma, maxillary osteosarcomas occurred in females with the ratio of 4:1 whereas mandibular lesions occurred only in males. Few reports state even distribution of the lesion between maxilla and mandible. Clinically, osteosarcoma of long bones presents as pain during activity compared to osteosarcoma of jaw bones where swelling rather than pain is the commonest finding.[5,8] In a study by Nissanka et al[9] most patients related the occurrence of tumor to previous dental treatment, most commonly, dental extractions. The reason for this is most likely to be rapid growth of tumor immediately after tooth extraction, a phenomenon often shown by bone tumors.[10]
RADIOGRAPHIC FEATURES
Osteosarcoma shows varied radiographic appearance ranging from osteolytic to mixed to osteogenic pattern of bone. If the tumor invades the periosteum, many thin irregular spicules of new bone may develop outward and perpendicular to the surface of the lesion producing the so-called ‘sun ray appearance.’ Lindquist et al[11] reported that the widening of periodontal ligament space and inferior dental canal, together with sunburst effect are almost pathognomonic of osteosarcoma of jaw bone. Not all the lesions show such peculiar characteristics. Forteza et al[1] reported that the presence of destructive unicentric lesion with poorly defined margins and a predominantly sclerotic, lytic or mixed radiographic pattern should lead one to suspect an osteogenic sarcoma.
The preoperative diagnosis of these neoplasms is often difficult because of its nonspecific nature. The importance of special investigations such as computerized tomography (CT) and magnetic resonance imaging (MRI) lies in assessing the size of the lesion for staging, intramedullary and extramedullary involvement, tumor calcification and invasion into adjacent tissues.
HISTOPATHOLOGIC FEATURES
The varied radiographic appearance of this lesion highlights the importance of histopathologic analysis in the diagnosis of osteosarcomas. The diagnosis of osteosarcoma is based on recognition of osteoid production by tumor cells.[12] Depending upon the predominant type of extracellular matrix present, osteosarcomas are categorized histopathologically into osteoblastic, chondroblastic, fibroblastic subtypes.[13] The osteoblastic variety consists of tumor osteoid surrounded by bizarrely arranged fibroblast--like cells.
In chondroblastic osteosarcoma, tumor cells lie in the lacunae and form lobules. The center of the lobule has bony trabeculae producing a feathery appearance, and towards the periphery, the tumor becomes hypercellular. Most of the times, an area of atypical chondroid tissue is also seen with large chondrocytes. Fibroblastic osteosarcoma is the least common variant where the tumor cells are spindle-shaped and characteristically arranged in herring bone pattern typically resembling fibrosarcoma. The formation of tumor osteoid differentiates this variant of osteosarcoma from fibrosarcoma[14]
Mardinger et al[15] reported the highest prevalence for chondroblastic osteosarcoma (42%), osteoblastic osteosarcoma being lesser (33%). Histologic diversity of osteosarcomas points to the fact that histology alone is insufficient for the diagnosis of osteosarcoma. Therefore, combined clinical, radiographic and histopathologic analysis before definitive diagnosis is prudent.
STAGING AND GRADING
Cellularity is the most important criterion used for histological grading. In general, the more cellular the tumor, the higher the grade. Irregularity of the nuclear contours, enlargement and hyperchromasia of the nuclei are correlated with grade. Mitotic figures and necrosis are additional features useful in grading.
Staging incorporates the degree of differentiation as well as local and distant spread, in order to estimate the prognosis of the patient. The universal TNM staging system is not commonly used for sarcomas because of their rarity to metastasize in lymph nodes. The system used most often to formally stage bone sarcomas is known as the Enneking system.[7,10] It is based on the grade (G) of the tumor, the local extent of the primary tumor (T), and whether or not it has metastasized to regional lymph nodes or other organs (M).
The grade is divided into low grade (G1) and high grade (G2).
The extent of the primary tumor is classified as either intracompartmental (T1), meaning it has basically remained in place, or extracompartmental (T2), meaning it has extended into other nearby structures.
Tumors that have not spread to the lymph nodes or other organs are considered M0, while those that have spread are M1.
These factors are combined to give an overall stage [Table 2].
Table 2.
Grading and staging of osteosarcomas
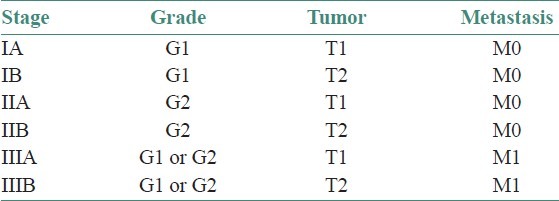
In summary, low-grade tumors are stage I, high-grade tumors are stage II, and metastatic tumors (regardless of grade) are stage III.
There are no known specific laboratory parameters. Increase in alkaline phosphatase or lactic dehydrogenase (LDH) serum levels are observed in a considerable number of patients. Although they do not correlate reliably with disease extent, they may have negative prognostic significance.
Histopathologic grading of this neoplasm is done according to Broder's grading system developed for epitheliomas, based on degree of cellular anaplasia shown by tumor cells. Mardinger et al[15] stated that nearly 50% of the jaw osteosarcomas are low grade and according to Unni,[16] the most common form is grade II.
IMMUNOHISTOCHEMISTRY
Immunohistochemistry (IHC) plays an important role in the differentiation between chondrosarcoma and chondroblastic osteosarcoma. IHC will show chondrosarcoma to be positive for S100 and Vimentin and negative for cytokeratin and EMA (Epithelial Membrane Antigen). Chondroblastic osteosarcoma will be positive for Vimentin, EMA, S100 and rarely cytokeratin.[17]
Recently, Yoshida et al reported that the combination of MDM2 and CDK4 by immunohistochemical analysis shows 100% sensitivity and 97.5% specificity for the diagnosis of low-grade osteosarcoma. They concluded that MDM2 and CDK4 immunostains therefore reliably distinguish low-grade osteosarcoma from benign histological mimics, and their combination may serve as a useful adjunct in this difficult differential diagnosis.[18]
In a study by Hu et al, the expressions of IDH1 and p53 in formalin-fixed paraffin-embedded tissue sections from 44 osteosarcoma patients were determined by immunohistochemistry, and the correlation between them and clinicopathological features were analyzed. They concluded that osteosarcoma patients with High IDH1 expression have a very high p53 expression. Thus IDH1 may correlate with p53 and be a candidate biomarker for osteosarcoma.[19]
TREATMENT AND PROGNOSIS
Wide radical resection is the treatment of choice for osteosarcoma of jaws with clearance margins of 1.5–2 cm. Surgery and adjuvant chemotherapy and radiotherapy may be required sometimes. The presence of micro metastases decides the need of adjuvant therapy. In mandible, hemimandibulectomy is commonly preferred. Maxillectomy is difficult to perform due to the involvement of adjacent structures like maxillary sinus, pterygopalatine fossa and orbital fossa. A subtotal inferior maxillectomy for selected malignancies located on the alveolar ridge, palate and involving the antral floor have been described.[20] Obturators have been prescribed for the defect created. Obturators can be divided into three classes: surgical, post-surgical, and definitive. Surgical obturators are those that are placed at the time of surgery. Post-surgical obturators are those prostheses which are placed immediately after packing removal, used until tissue contracture is minimal, and prior to definitive obturator placement. They are designed with the use of a preoperative cast that is modified to account for resected areas. Time between packing, removal and obturator placement should be minimal, as tissue contraction and edema will quickly alter the shape of the defect, making it difficult to insert an obturator. The definitive obturator is designed when the surgical sight is stable, approximately 3-12 months after definitive surgery.[21]
The use of chemotherapy as an adjuvant for treatment of osteosarcomas of long bone was first reported by Jaffe[22] who used methotrexate as an anticancer drug. Since then most of the chemotherapeutic agents such as doxorubicin, cisplatin, adriamycin have been used. Rosen et al[23] found an increase from 15% to 60-80% in 5-year survival rate of patients suffering from osteosarcoma when chemotherapy was used as an adjuvant to surgery. According to Nissanka et al[9] the 5-year survival rate using adjuvant chemotherapy was 83.3%, whereas, Mardinger et al[15] found no significant change in prognosis. These controversial findings may be due to diversity of chemotherapeutic regimens used with different agents, dosages and intervals.
Smeele et al[24] investigated the value of chemotherapy in the treatment of craniofacial osteosarcoma by analyzing 201 reviewed cases. They found that the over all and disease free survival rates significantly improved with chemotherapy. Raymond et al[25] reported 33% 5-year survival for patients treated with adjuvant chemotherapy and surgery and 41% 5-year disease free survival for those treated with surgery alone. Radiotherapy must be confined for the treatment of residual, recurrent and unresectable tumors.
Unni KK has reported a 40% 5-year survival for jaw osteosarcomas compared to conventional osteosarcomas (20.3%).[9] Clark et al[26] attributed this to occurrence of predominantly chondroblastic low grade osteosarcomas in the jaws.
In one study, bone marrow and peripheral blood samples from 60 patients with suspected bone sarcoma were examined for the presence and number of micrometastatic osteosarcoma cells by a sensitive immunomagnetic detection assay, using in parallel two osteosarcoma associated antibodies. Forty-nine of the patients had osteosarcoma, and of these, as many as 31 (63%) had tumor cells in bone marrow, in many cases with a high number of cells. Only four (8%) were positive also in blood. The data showed the clinical potential of this immunomagnetic method in detection of metastasis in osteosarcoma.[27]
A number of potential prognostic factors have been identified which include the expression of HER2/CerbB2, tumor cell ploidy, and specific chromosome gains or losses, loss of heterozygosity of the RB gene, loss of heterozygosity of the p53 locus, and increased expression of p-glycoprotein. The only feature that consistently predicts outcome is the degree of histologic necrosis following induction chemotherapy. Patients with more than 95% necrosis in the primary tumor after induction chemotherapy have a better prognosis than those with smaller amounts of necrosis.[28–40]
The prognosis for patients with metastatic disease appears to be determined largely by the site(s), the number of metastases, as well as the surgical resectability of the metastatic disease. The most common site for the metastases is lung accounting for almost 20%. Prognosis appears more favorable for patients with unilateral rather than bilateral pulmonary metastases, and for patients with fewer nodules rather than many nodules. The degree of necrosis in the primary tumor after induction chemotherapy remains prognostic in metastatic osteosarcoma. Patients with skip metastases (≥2 discontinuous lesions in the same bone) have been reported to have inferior prognoses. Patients with multifocal osteosarcoma (>1 bone lesion at diagnosis) have a poor prognosis.[41–46]
CONCLUSION
Osteosarcoma is an ancient disease many aspects of which are still incompletely understood. It is a malignancy of mesenchymal cells that have the ability to produce osteoid or immature bone. Excluding hematopoeitic neoplasms, osteosarcoma is the most common type of malignancy to originate within bone. There have been a plethora of discussions and also controversies about the nature, aggressiveness, behavior, and various treatment modalities of this entity. We have reviewed more than 300 cases from PUBMED of this perilous lesion, and made a humble attempt to include cases of varied ethnic origins as well as follow the changing trends in the management of this tumor. However, for purposes of management, emphasis should be laid on the aggressiveness of this lesion which warrants an early identification and diagnosis of the lesion followed by prompt treatment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Forteza G, Colmenero B, Lopez-Barea F. Osteogenic sarcoma of maxilla and mandible. Oral Surg Oral Med Oral Pathol. 1986;62:179–84. doi: 10.1016/0030-4220(86)90042-3. [DOI] [PubMed] [Google Scholar]
- 2.Rajendran R. Benign and Malignant tumors of the oral cavity. In: Rajendran R, Sivapathasundharam B, editors. Shafer's textbook of Oral Pathology. India: Elsevier, a Division of Reed Elsevier India Private Limited; 2006. pp. 113–308. [Google Scholar]
- 3.Brackenridge CJ. A statistical study of sarcoma complicating Paget's disease of bone in three countries. Br J Cancer. 1979;40:194–200. doi: 10.1038/bjc.1979.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huvos AG, Woodard HQ, Cahan WG, Higinbotham NL, Stewart FW, Butler A, et al. Postradiation osteogenic sarcoma of bone and soft tissues.A pathological study of 66 patients. Cancer. 1985;55:1244–55. doi: 10.1002/1097-0142(19850315)55:6<1244::aid-cncr2820550617>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Garrington GE, Scofield HH, Cornyn J, Hooker SP. Osteosarcoma of jaws, analysis of 56 cases. Cancer. 1967;20:377–91. doi: 10.1002/1097-0142(1967)20:3<377::aid-cncr2820200306>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.August M, Magennis P, Dewitt D. Osteogenic sarcoma of the jaws: Factors influencing prognosis. Int J Oral Maxillofac Surg. 1997;26:198–204. doi: 10.1016/s0901-5027(97)80819-3. [DOI] [PubMed] [Google Scholar]
- 7.Staging of musculoskeletal neoplasms. Musculoskeletal Tumor Society. Skeletal Radiol. 1985;13:183–94. doi: 10.1007/BF00350572. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman S, Jakoway JR, Krolls SO. intraosseous and Parosteal tumors of jaws. Atlas of tumor pathology. Washington, D.C: AFIP; 1987. Malignant odontogenic tumors of jaws; pp. 170–80. [Google Scholar]
- 9.Nissanka EH, Amartunge EA, Tilakaratne WM. Clinicopathological analysis of osteosarcoma of jaw bones. Oral Dis. 2007;13:82–7. doi: 10.1111/j.1601-0825.2006.01251.x. [DOI] [PubMed] [Google Scholar]
- 10.Adekeye EO, Chan KK, Edwards MB, Williams HK. Osteosarcoma of the jaws: A series from Kaduna Nigeria. Int J Oral Maxillofac Surg. 1987;16:205–13. doi: 10.1016/s0901-5027(87)80132-7. [DOI] [PubMed] [Google Scholar]
- 11.Lindquist C, Teppo L, Sane J, Holmstrom T, Wolf J. Osteosarcoma of mandible: Analysis of 9 cases. J Oral Maxillofac Surg. 1986;44:759–64. doi: 10.1016/0278-2391(86)90149-7. [DOI] [PubMed] [Google Scholar]
- 12.Schajowicz F. 2nd ed. Berlin: Springer-Verlag; 1993. Histological typing of bone tumours. [Google Scholar]
- 13.Frei C, Bornstein MM, Stauffer E, Iizuka T, Buser D. Osteosarcoma of the maxilla and the maxillary sinus: A case report. Quintessence Int. 2004;35:228–33. [PubMed] [Google Scholar]
- 14.Neville BW, Damm DD, Allen CM, Bouquot JE. Bone Pathology. In: Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Bone Pathology, editors. Oral and Maxilofacial Pathology. Philadelphia: Saunders, an imprint of Elsevier; 2002. pp. 574–7. [Google Scholar]
- 15.Mardinger O, Givol N, Talmi YP, Taicher S. Osteosarcoma of the jaw- The Chaim Sheba Medical Center Experience. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2001;91:445–51. doi: 10.1067/moe.2001.112330. [DOI] [PubMed] [Google Scholar]
- 16.Unni KK, Dahlin DC. Grading of bone tumors. Semin Diag Pathol. 1984;1:165–72. [PubMed] [Google Scholar]
- 17.Akpolat N, Yildirim H, Poyraz K. Sacral chondroblastic osteosarcoma misdiagnosed as chondrosarcoma and chordoma. Turk J Med Sci. 2007;37:243–9. [Google Scholar]
- 18.Yoshida A, Ushiku T, Motoi T, Shibata T, Beppu Y, Fukayama M, et al. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol. 2010;23:1279–88. doi: 10.1038/modpathol.2010.124. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Yu AX, Qi BW, Fu T, Wu G, Zhou M, et al. The expression and significance of IDH1 and p53 in osteosarcoma. J Exp Clin Cancer Res. 2010;29:43. doi: 10.1186/1756-9966-29-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pogrel MA. Inferior hemi-maxillectomy for treatment of palatal tumors. J Oral Maxillofac Surg. 1988;46:85–7. doi: 10.1016/0278-2391(88)90309-6. [DOI] [PubMed] [Google Scholar]
- 21.Taylor Thomas D. Illinois, USA: Quintessence Publishing Co; 2000. Clinical maxillofacial prosthetics. [Google Scholar]
- 22.Jaffe N. Recent advances in the chemotherapy of metastatic osteogenic sarcoma. Cancer. 1972;30:1627–31. doi: 10.1002/1097-0142(197212)30:6<1627::aid-cncr2820300631>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, et al. Preoperative chemotherapy for osteogenic sarcoma: Selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to the preoperative chemotherapy. Cancer. 1982;49:1221–30. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Smeele LE, Kostense PJ, van der waal I, Snow GB. Effect of Chemotherapy on survival of craniofacial osteosarcoma: A systematic review of 201 patients. J Clin Oncol. 1997;15:363–7. doi: 10.1200/JCO.1997.15.1.363. [DOI] [PubMed] [Google Scholar]
- 25.Raymond AK, Spires J, Ayala A, Chawla S, Lee YY, Benjamin RS, et al. Osteosarcoma of head and neck. Lab Invest. 1989;60:76a. [Google Scholar]
- 26.Clark JL, Unni KK, Dahlin DC, Devine KD. Osteosarcoma of the jaw. Cancer. 1983;51:2311–6. doi: 10.1002/1097-0142(19830615)51:12<2311::aid-cncr2820511224>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.Bruland OS, Høifødt H, Saeter G, Smeland S, Fodstad O. Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res. 2005;11:4666–73. doi: 10.1158/1078-0432.CCR-05-0165. [DOI] [PubMed] [Google Scholar]
- 28.Gorlick R, Huvos AG, Heller G, Aledo A, Beardsley GP, Healey JH, et al. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J Clin Oncol. 1999;17:2781–8. doi: 10.1200/JCO.1999.17.9.2781. [DOI] [PubMed] [Google Scholar]
- 29.Onda M, Matsuda S, Higaki S, Iijima T, Fukushima J, Yokokura A, et al. ErbB-2 expression is correlated with poor prognosis for patients with osteosarcoma. Cancer. 1996;77:71–8. doi: 10.1002/(SICI)1097-0142(19960101)77:1<71::AID-CNCR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Kilpatrick SE, Geisinger KR, King TS, Sciarrotta J, Ward WG, Gold SH, et al. Clinicopathologic analysis of HER-2/neu immunoexpression among various histologic subtypes and grades of osteosarcoma. Mod Pathol. 2001;14:1277–83. doi: 10.1038/modpathol.3880474. [DOI] [PubMed] [Google Scholar]
- 31.Kusuzaki K, Takeshita H, Murata H, Hirata M, Hashiguchi S, Ashihara T, et al. Prognostic value of DNA ploidy response to chemotherapy in human osteosarcomas. Cancer Lett. 1999;141:131–8. doi: 10.1016/s0304-3835(99)00092-0. [DOI] [PubMed] [Google Scholar]
- 32.Ozaki T, Schaefer KL, Wai D, Buerger H, Flege S, Lindner N, et al. Genetic imbalances revealed by comparative genomic hybridization in osteosarcomas. Int J Cancer. 2002;102:355–65. doi: 10.1002/ijc.10709. [DOI] [PubMed] [Google Scholar]
- 33.Feugeas O, Guriec N, Babin-Boilletot A, Marcellin L, Simon P, Babin S, et al. Loss of heterozygosity of the RB gene is a poor prognostic factor in patients with osteosarcoma. J Clin Oncol. 1996;14:467–72. doi: 10.1200/JCO.1996.14.2.467. [DOI] [PubMed] [Google Scholar]
- 34.Goorin A, Baker A, Gieser P. No evidence for improved event free survival (EFS) with presurgical chemotherapy (PRE) for non-metastatic extremity osteogenic sarcoma (OGS): preliminary results of randomized Pediatric Oncology Group [POG] trial 8651. Proc Am Soc Clin Oncol. 1995;15:444. [Google Scholar]
- 35.Goto A, Kanda H, Ishikawa Y, Matsumoto S, Kawaguchi N, Machinami R, et al. Association of loss of heterozygosity at the p53 locus with chemoresistance in osteosarcomas. Jpn J Cancer Res. 1998;89:539–47. doi: 10.1111/j.1349-7006.1998.tb03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serra M, Maurici D, Scotlandi K, Barbanti-Brodano G, Manara MC, Benini S, et al. Relationship between P-glycoprotein expression and p53 status in high-grade osteosarcoma. Int J Oncol. 1999;14:301–7. doi: 10.3892/ijo.14.2.301. [DOI] [PubMed] [Google Scholar]
- 37.Hornicek FJ, Gebhardt MC, Wolfe MW, Kharrazi FD, Takeshita H, Parekh SG, et al. P-glycoprotein levels predict poor outcome in patients with osteosarcoma. Clin Orthop Relat Res. 2000;373:11–7. doi: 10.1097/00003086-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Serra M, Scotlandi K, Reverter-Branchat G, Ferrari S, Manara MC, Benini S, et al. Value of P-glycoprotein and clinicopathologic factors as the basis for new treatment strategies in high-grade osteosarcoma of the extremities. J Clin Oncol. 2003;21:536–42. doi: 10.1200/JCO.2003.03.144. [DOI] [PubMed] [Google Scholar]
- 39.Pakos EE, Ioannidis JP. The association of P-glycoprotein with response to chemotherapy and clinical outcome in patients with osteosarcoma. A meta-analysis. Cancer. 2003;98:581–9. doi: 10.1002/cncr.11546. [DOI] [PubMed] [Google Scholar]
- 40.Goorin AM, Shuster JJ, Baker A, Horowitz ME, Meyer WH, Link MP. Changing pattern of pulmonary metastases with adjuvant chemotherapy in patients with osteosarcoma: results from the multiinstitutional osteosarcoma study. J Clin Oncol. 1991;9:600–5. doi: 10.1200/JCO.1991.9.4.600. [DOI] [PubMed] [Google Scholar]
- 41.Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer. 1999;86:1602–8. doi: 10.1002/(sici)1097-0142(19991015)86:8<1602::aid-cncr31>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 42.Bacci G, Briccoli A, Ferrari S, Saeter G, Donati D, Longhi A, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with synchronous lung metastases: treatment with cisplatin, adriamycin and high dose of methotrexate and ifosfamide. Oncol Rep. 2000;7:339–46. doi: 10.3892/or.7.2.339. [DOI] [PubMed] [Google Scholar]
- 43.Bacci G, Briccoli A, Ferrari S, Longhi A, Mercuri M, Capanna R, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity: long-term results of the Rizzoli's 4th protocol. Eur J Cancer. 2001;37:2030–9. doi: 10.1016/s0959-8049(01)00229-5. [DOI] [PubMed] [Google Scholar]
- 44.Bacci G, Briccoli A, Mercuri M, Ferrari S, Bertoni F, Gasbarrini A, et al. Osteosarcoma of the extremities with synchronous lung metastases: long-term results in 44 patients treated with neoadjuvant chemotherapy. J Chemother. 1998;10:69–76. doi: 10.1179/joc.1998.10.1.69. [DOI] [PubMed] [Google Scholar]
- 45.Sajadi KR, Heck RK, Neel MD, Rao BN, Daw N, Rodriguez-Galindo C, et al. The incidence and prognosis of osteosarcoma skip metastases. Clin Orthop Relat Res. 2004;426:92–6. doi: 10.1097/01.blo.0000141493.52166.69. [DOI] [PubMed] [Google Scholar]
- 46.Longhi A, Fabbri N, Donati D, Capanna R, Briccoli A, Biagini R, et al. Neoadjuvant chemotherapy for patients with synchronous multifocal osteosarcoma: results in eleven cases. J Chemother. 2001;13:324–30. doi: 10.1179/joc.2001.13.3.324. [DOI] [PubMed] [Google Scholar]


