Abstract
Toluidine blue is a basic thiazine metachromatic dye with high affinity for acidic tissue components, thereby staining tissues rich in DNA and RNA. It has found wide applications both as vital staining in living tissues and as a special stain owing to its metachromatic property. Toluidine blue has been used in vivo to identify dysplasia and carcinoma of the oral cavity. Use of toluidine blue in tissue sections is done with the aim to highlight components, such as mast cells granules, mucins, and cartilage. This article provides an overview on chemistry, technique, and the various applications of toluidine blue.
Keywords: Metachromasia, toluidine blue, vital staining
INTRODUCTION
Toluidine blue (also known as tolonium chloride) is an acidophilic metachromatic dye that selectively stains acidic tissue components (sulfates, carboxylates, and phosphate radicals).[1] Toluidine blue (TB) has an affinity for nucleic acids, and therefore binds to nuclear material of tissues with a high DNA and RNA content.[2] It is a member of the thiazine group and is partially soluble in both water and alcohol.[3] Toluidine blue has been known for various medical applications since its discovery by William Henry Perkin in 1856, after which it was primarily used by the dye industry. Also known as methylanaline or aminotoluene, it basically has 3 isoforms, namely, ortho-toluidine, para-toluidine, and meta-toluidine. Toluidine blue has been extensively used as a vital stain for mucosal lesions and also has found applications in tissue sections to specifically stain certain components owing to its metachromatic property.
Vital staining is the staining of cells or tissues in the living state. The earliest technique developed by Paul Ehrlich in 1885 involved the immersion of freshly removed tissue in methylated blue. There are two techniques of vital staining, namely, intravital staining in the living body (in vivo) and supravital staining outside the body usually applied to slide preparation of detached cell.[4] TB was first applied for in vivo staining by Reichart in 1963 for uterine cervical carcinoma in situ.[5]
During 1960s suggestion was made that TB may stain malignant epithelia of the mucous membrane in vivo, whereas normal tissue failed to retain the dye. TB detects relative rather than absolute differences between normal and malignant cells and tissue. They can be used as 1% or 2% oral rinse or an application either in aqueous form or as weak acid solution or of undefined formulation. Only about 5% of dye by weight is retained in oral cavity following expectoration.[6] Its use in vivo is based on the fact that dysplastic and neoplastic cells may contain quantitatively more nucleic acids than normal tissues. Also malignant epithelium may contain intracellular canals that are wider than the normal epithelium, which may facilitate penetration of the dye.[1]
Vital staining of the oral epithelium has been suggested as a means of surveillance in patients who are at a risk of developing oral cancer and for those who had confirmed neoplasms of other parts of aerodigestive tract.[6] TB has been used as a vital stain to highlight potentially malignant oral lesions and may identify early lesions, which could be missed out on clinical examination. Moreover, it can outline the full extent of dysplastic epithelium or carcinoma prior to excisions,[7] and can detect multicentric or second tumors and can help in the followup of patients with oral cancer. It is useful in obtaining the marginal control of carcinoma and in selecting the biopsy sample site in premalignant lesions. Loss of heterozygosity may be detected in TB-stained lesions. TB-stained tissue may appear dark royal blue or pale royal blue color.[3]
Majority of the dyes stain tissues in differing degrees of intensity of the same color, however, certain tissue components, which in the presence of certain basic dyes of the coal tar group, will stain a color other than that of the dye. Such staining reaction is known as metachromasy and the tissue is said to exhibit metachromasia and the dye as a metachromatic dye. Among the principal tissue components that exhibit metachromasia are mucin, cartilage, and mast cell granules. The dyes exhibiting metachromatic properties are mainly of thiazine group, thionine, TB, azure A, azure B, methyl violet, safranin, and acridine orange.[4] TB is one such dye, which has been used in tissue sections for identification of various cells.
TISSUE STAINING
Principle
TB stains tissues based on the principle of metachromasia. The dye reacts with the tissues to produce a color different from that of the original dye and from the rest of the tissue. Metachromasia was discovered in 1875 by Cornil, Jurgens, and Ranvier. Metachromasia is important as it is highly selective and only certain tissue structures can stain metachromatically. It is a phenomenon whereby a dye may absorb light at different wavelengths depending on its concentration and surroundings and it has the ability to change its color without changing its chemical structure. The physical changes that bring about this color change are a specialized, orderly form of dye aggregation. For metachromasia to occur there must be free electronegative groups on the surface of tissues.[8]
Metachromasia is attributed to stacking of dye cations at the sites of high density of anionic groups in the tissue. Stacking shortens the wavelength of maximum absorption, a hypsochromic shift, so that the maximum wavelength in the spectrum of the transmitted light is longer making the observed color red instead of blue.[9] Substances that can be stained in this way are called chromotropes and they include mucins, mast cells, and so on.[10] The chromotropes carry acidic groups with a minimum density of not more than 0.5 nm between adjacent negatively charged groups. These alter the color of metachromatic dyes. Principally, van der Waals forces hold the dye together to form dimers, trimers, or polymers. Other forms that play a lesser role are hydrogen and hydrophobic bonding. The dye exists in a normal monomeric (orthochromatic) form to a potential polymeric (metachromatic) form. The negative charges on the chromotropes attract the positively charged polar groups on the dye leading to dye-to-dye aggregation in a specialized orderly form forming a polymeric form. There are three forms of metachromasia alpha (α), beta (β), and gamma (g) giving a range of colors [Table 1].[4]
Table 1.
Various forms of metachromasia
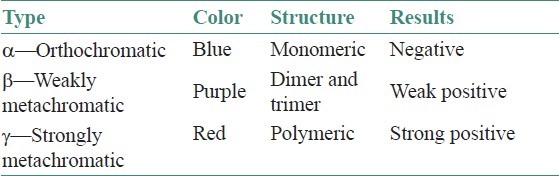
The color shift is always from a blue or violet dye to yellow or red stain meaning that the color absorption shifts to shorter wavelengths, leaving only the longest wavelengths to be seen. This is believed to represent polymerization of the dye. The greater the degree of polymerization, the stronger is the metachromasia. Metachromasia requires water between dye molecules to form the polymer and does not survive dehydration and clearing.[10] The absorption spectrum of TB with an orthochromatic tissue is maximum at about 630 nm and staining result is blue. With a metachromatic substance the absorption spectrum is maximum at 480–540 nm and the staining is red in color.[4] Van der Waals attraction between TB and polyanions contribute to affinity when binding to DNA or RNA as does hydrophobic bonding. However, flexible, high charge density glycosaminoglycans (GAG) permit charge neutralization of dye aggregates or stacks formed due to dye-to-dye van der Waals attractions and hydrophobic bonding.[9]
Mast cells were first recognized by virtue of metachromasia in 1877 by Paul Ehrlich. The compound responsible for metachromasia was identified as heparin, a heteroglycan rich in half-sulfate esters. TB is a small weakly hydrophilic cationic dye. Attached to DNA or RNA, in chromatin or Nissl substance, this dye has a blue color. Attached to glycosaminoglycans, in mast cell granules or cartilage matrix, the dye displays a purple metachromatic color.[9] TB is typically applied from weakly acidic aqueous solutions. DNA, RNA, and GAG are then polyanionic, whereas most proteins are protonated and so polycationic. Thus basic dye cations exchange with mobile tissue cations associated with the various polyanions, a process termed basic dyeing. This ion exchange is entropy driven as it increases the randomness of the system. Because polycationic proteins are not associated with mobile cations, ion exchange cannot occur minimizing background staining.[9]
APPLICATION OF TOLUIDINE BLUE
Connective tissue mucins, especially acid mucins. The tissue stains purple to red, while the background is stained blue.
Mast cell granules stain purple in color due to the presence of heparin and histamine.
Amyloid stain blue but under polarized light they give a bright red birefringence.
Endocrine cell granules are stained purple to red. The concentration of stain used here is 0.01%.
Sulfatides stain red brown or yellow. Only lipids that are sufficiently acidic to induce a metachromatic shift are stained.
Corneybacterium diphtheria contains granules with polymerized inorganic polyphosphate, which stains red violet color.
Helicobacter stains dark blue against a variably blue background. The concentration of TB used is 1%.[11]
TB can also be used to stain frozen section because of the rapidity of the staining procedure (10–20 s) and better clarity of the cells.
VITAL STAINING
The use of TB as a vital stain was first proposed by Richart to disclose dysplasia and carcinoma in situ of the uterine cervix. Neibel and Chomet and Shedd and co-workers were the first to report vital application of TB for the detection of premalignant and malignant lesions of the oral cavity. They confirmed the property of TB to verify clinically suspicious lesions as neoplastic, to delineate margins of premalignant and malignant growth, and to detect unnoticed or satellite tumors.[12]
TB is used based on the fact that dysplastic and neoplastic cells may contain quantitatively more nucleic acids than normal tissues. Also, malignant epithelium may contain intracellular canals that are wider than normal epithelium, which may facilitate penetration of the dye.[1] The other proposals about the uptake of TB in dysplastic and carcinomas include the high density of nuclear material, loss of cell cohesion, and increased mitosis.[3]
The use of TB as an adjuvant diagnostic aid in the identification of malignant lesions has led to publishing of numerous studies, however, with diverse results. The diversity could be due to variations in methodology, the population studies, and lesions analyzed. In addition, in some of the studies, biopsies of the lesions that did not retain TB were not performed. These factors may interfere with the evaluation of staining, thus limiting the value of the results obtained.[13]
TB is generally prepared in 1% concentration for oral application. A 100 mL of 1% TB consists of 1 gm TB powder, 10 mL of 1% acetic acid, 4.19 mL absolute alcohol, and 86 mL distilled water to make up 100 mL. The pH is usually regulated to 4.5.[14] The technique of application usually involves rinsing of the mouth twice with water for 20 s to remove debris. And 1% acetic acid is then applied for 20 s to remove ropey saliva. This is followed by 1% TB application for 20 s either with cotton swab when a mucosal lesion was seen or given as rinse when no obvious lesion was detected. Again, 2 rinses with 1% acetic acid were performed to reduce the extent of mechanically retained stain. Finally the mouth is rinsed with water.[14] The interpretation is based on the color; a dark blue (royal or navy) stain is considered positive, light blue staining is doubtful and when no color is observed, it is interpreted as negative stain. Under normal conditions, nucleated scales covering the papillae on the dorsum of the tongue as well as the pores of seromucinous glands in hard palate are frequently stained with TB.[15]
Several studies have been performed over the years to determine the sensitivity and specificity of in vivo TB staining. Earlier studies have found sensitivity in the range of 86–100% and specificity in the range of 44–100%.[14,16–18] Lingen et al,[7] in their review, mentioned the sensitivity and specificity of TB in the detection of oral cancer to be in the range of 78–100% and 31–100%, respectively. The evaluation of TB staining for detection of oral premalignant lesions and carcinoma in animal models showed high false-negative results in premalignancies (95.2%) seeding doubt on whether TB is sensitive enough for the detection of premalignant lesions. In contrast, it was found that in vivo staining with TB was highly sensitive in detecting carcinoma.[12]
In 1989, meta-analysis of available data assessing the effectiveness of TB application in identification of oral squamous cell carcinoma determined sensitivity ranging from 93.5% to 97.8% and specificity ranging from 73.3% to 92.9%.[19] Epstein et al[1] showed sensitivity and specificity of 92.5% and 63.2%, respectively, in their results.
The analysis of TB in vivo stain in 145 oral mucosal lesions among 102 enrolled patients revealed 100% sensitivity and 62% specificity. It was suggested that TB staining is an invaluable option in the surveillance of high-risk subjects and in addition it has a remarkable sensitivity in the detection of invasive carcinoma. The study also found that lesions with limited dysplasia or atypia do not stain consistently with TB. Oral premalignant lesions showed false-negative rate of 20.5%, which could be due to the lack of objective criteria for evaluation of stain uptake.[6]
The application of TB in 81 lesions of which 48 lesions were considered clinically suspicious and 33 were clinically benign showed that 28 cases had no stain, 20 had equivocal stain and 33 had positive stain. On biopsy of these lesions 54 were nonmalignant and 27 were carcinoma. The study found 100% sensitivity and 52% specificity.[2]
Onofre et al[13] evaluated the TB staining in premalignancies, and superficial oral ulceration suggesting malignancy. The study showed 100% sensitivity in the detection of in situ and invasive carcinoma and no false-negative results occurred. The lesions that were diagnosed as dysplasia did not retain stain, and thus gave false-negative results. The reasons could be that the exact mechanisms by which the dye differentially stains malignant or dysplastic tissues remain unknown. Staining specificity was 65% because the inflammatory lesions were eliminated for the first time and re-stained after 14 days. In lesions without epithelial dysplasia or atypical cells, false positivity was 35%.
Hegde et al[15] found a sensitivity of 97.29% and specificity of 62.5%. False positivity of 7.69% and false negativity of 16.67% was noticed. The authors suggested that specificity was reduced because of retention of the dye in some benign lesions. Gupta et al[20] demonstrated sensitivity of 96.9% and specificity of 86% for detection of malignancy. The false-positive rate was 14%, which could be due to ulcerated, inflammatory, or traumatic lesions. The study also showed 64% sensitivity and 86% specificity for premalignant lesions.
False-positive retention in nondysplastic conditions has been reported with varying frequency. Mashberg[14] reported a false-positive rate of 8.5%. They suggested that false positivity could be reduced by a second examination typically 14 days later that assisted in excluding lesions where staining was associated with inflammation.
Selective staining of malignant and dysplastic cells might be explained by the fact that these cells contain quantitatively more nucleic acid than normal tissue. In vivo TB staining might be the result of immediate binding of sulfated mucopolysaccharides, which are found in higher quantity in tissues that are actively growing, such as tumors and tissues that are healing which may explain false-positive results.[17] A study by Martin et al[21] showed 42% false negative for in situ carcinoma and 58% false negativity in epithelial dysplasia. This could be due to the sample analyzed and the fact that these authors considered only lesions with stippled or dark blue staining as positive.
Problems with studies of TB include that no studies were carried out in a primary care environment, data from studies in secondary care are not necessarily applicable to general population, no randomized controlled trials, some studies only include carcinoma or dysplasias, and some include both (no uniformity), histologic diagnosis is rarely used as the “gold standard,” no single standard staining method used, confusion over inclusion of equivocal (pale) staining as positive or negative.[7]
TB application is an important adjunct to the clinical examination because it may not only increase the clinical suspicion of the examination but also assist in identifying sites in need of biopsy and delineating margins of the lesions, which may lead to a more timely diagnosis allowing benefits of earlier treatment and in directing surgical management.[22] Although the specificity of the technique is less which could be attributed to the inability to biopsy normal tissues which does not take up TB, it can still be used as an important adjuvant as a diagnostic aid because in a clinical setting, false-positive results are of less concern than false-negative results and any positive findings should be confirmed on biopsy for the presence of dysplasia or carcinoma. In addition, any lesion that is not clinically suspicious but shows TB uptake (due to irritating and inflammatory factors) should be re-evaluated after 10–14 days after the regression of the condition. If TB positivity still persists, the lesion should be considered suspicious and biopsy should be performed to rule out carcinoma. A uniform staining methods and interpretation (e.g., doubts regarding inclusion of equivocal staining as positive or negative) would further enhance the utility of TB staining. As evident from the previous literature, most of the studies were done using insufficient parameters; further studies comprising all the relevant parameters (normal, inflammatory, preneoplasia, and neoplasia) would validate the routine use of TB as a vital stain.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Epstein JB, Scully C, Spinelli J. Toluidine blue and Lugol's iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160–3. doi: 10.1111/j.1600-0714.1992.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JB, Oakley C, Millner A, Emerton S, van der Meij E, Le N. The utility of toluidine blue application as a diagnostic aid in patients previously treated for upper oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:537–47. doi: 10.1016/s1079-2104(97)90117-7. [DOI] [PubMed] [Google Scholar]
- 3.Gandalfo S, Pentenero M, Broccoletti R, Pagano M, Carrozzo M, Scully C. Toluidine blue uptake in potentially malignant lesions in vivo: Clinical and histological assessment. Oral Oncol. 2006;42:89–95. doi: 10.1016/j.oraloncology.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Culling CF, Allison TR. 4th ed. London: Butterworths; 1985. Cellular Pathology Technique. [Google Scholar]
- 5.Siddiqui IA, Farooq MU, Siddiqyi RA, Rafe SM. Role of toluidine blue in early detection of oral cancer. Pak J Med Sci Q. 2006;22:184–7. [Google Scholar]
- 6.Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of OraScanÒ toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25:97–103. doi: 10.1111/j.1600-0714.1996.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 7.Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drupy RA, Wallington EA. 5th ed. Oxford University Press; 1980. Carleton's Histological Technique. [Google Scholar]
- 9.Kumar GL, Kiernan JA. 2nd ed. Dako North America, California: 2010. Education guide: Special stains and H & E. [Google Scholar]
- 10.Staining theory. Cell Path. E-book. [Last accessed on 2011 Nov 11]. pp. 67–104. Available from: http://www.scionpublishing.com .
- 11.Bancroft J, Gamble . 5th ed. Philadelphia: Churchill Livingstone; 2005. Theory and Practice of Histological Techniques. [Google Scholar]
- 12.Miller RL, Simms BW, Gould AR. Toluidine blue staining for detection of oral premalignant lesions and carcinomas. J Oral Pathol Med. 1988;17:73–8. doi: 10.1111/j.1600-0714.1988.tb01510.x. [DOI] [PubMed] [Google Scholar]
- 13.Onofre MA, Sposto MR, Navarro CM. Reliability of toluidine blue application in the detection of oral epithelial dysplasia and in situ and invasive squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:535–40. doi: 10.1067/moe.2001.112949. [DOI] [PubMed] [Google Scholar]
- 14.Mashberg A. Reevaluation of toluidine blue application as A diagnostic adjunct in the detection of asymptomatic oral squamous cell carcinoma: a continuing prospective study of oral cancer III. Cancer. 1980;46:758–63. doi: 10.1002/1097-0142(19800815)46:4<758::aid-cncr2820460420>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Hegde CM, Kamath PN, Sreedharan S, Dannana NK, Raju RM. Supravital staining: It's role in detecting early malignancies. Indian J Otolaryngol Head Neck Surg. 2006;58:31–4. doi: 10.1007/BF02907735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy CR, Ramulu C, Sundareshwar B, Raju MV, Gopal R, Sarma R. Toluidine blue staining of oral cancer and precancerous lesions. Indian J Med Res. 1973;61:1161–4. [PubMed] [Google Scholar]
- 17.Silverman S, Jr, Migliorati C, Barbosa J. Toluidine blue staining in the detection of oral precancerous and malignant lesions. Oral Surg Oral Med Oral Pathol. 1984;57:379–82. doi: 10.1016/0030-4220(84)90154-3. [DOI] [PubMed] [Google Scholar]
- 18.Onofre MA, Sposto MR, Navarro CM, Scully C. Assessment of blue toluidine stain in oral lesions with suspicions of malignancy. J Dent Res. 1995;74:782. [Google Scholar]
- 19.Rosenberg D, Cretin S. Use of meta-analysis to evaluate tolonium chloride in oral cancer screening. Oral Surg Oral Med Oral Pathol. 1989;67:621–7. doi: 10.1016/0030-4220(89)90286-7. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A, Singh M, Ibrahim R, Mehrotra R. Utility of toluidine blue staining and brush biopsy in precancerous and cancerous oral lesions. Acta Cytol. 2007;51:788–94. doi: 10.1159/000325843. [DOI] [PubMed] [Google Scholar]
- 21.Martin IC, Kerawala CJ, Reed M. The application of toluidine blue as a diagnostic adjunct in the detection of epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:444–6. doi: 10.1016/s1079-2104(98)90071-3. [DOI] [PubMed] [Google Scholar]
- 22.Portugal LG, Wilson KM, Biddinger PW, Gluckman JL. The role of toluidine blue in assessing margin status after resection of squamous cell carcinoma of upper aerodigestive tract. Arch Otolaryngol Head Neck Surg. 1996;122:517–9. doi: 10.1001/archotol.1996.01890170051010. [DOI] [PubMed] [Google Scholar]


