Abstract
Germ cell development requires timely transition from primordial germ cell (PGC) self-renewal to meiotic differentiation. This is associated with widespread changes in gene expression, including downregulation of stem cell–associated genes, such as OCT4 and KIT, and upregulation of markers of germ cell differentiation and meiosis, such as VASA, STRA8, and SYCP3. The stem cell–expressed RNA-binding protein Lin28 has recently been demonstrated to be essential for PGC specification in mice, and LIN28 is expressed in human germ cell tumors with phenotypic similarities to human fetal germ cells. We have therefore examined the expression of LIN28 during normal germ cell development in the human fetal ovary, from the PGC stage, through meiosis to the initiation of follicle formation. LIN28 transcript levels were highest when the gonad contained only PGCs, and decreased significantly with increasing gestation, coincident with the onset of germ cell differentiation. Immunohistochemistry revealed LIN28 protein expression to be germ cell–specific at all stages examined. All PGCs expressed LIN28, but at later gestations expression was restricted to a subpopulation of germ cells, which we demonstrate to be primordial and premeiotic germ cells based on immunofluorescent colocalization of LIN28 and OCT4, and absence of overlap with the meiosis marker SYCP3. We also demonstrate the expression of the LIN28 target precursor pri-microRNA transcripts pri-LET7a/f/d and pri-LET-7g in the human fetal ovary, and that expression of these is highest at the PGC stage, mirroring that of LIN28. The spatial and temporal restriction of LIN28 expression and coincident peaks of expression of LIN28 and target pri-microRNAs suggest important roles for this protein in the maintenance of the germline stem cell state and the regulation of microRNA activity in the developing human ovary.
Introduction
The RNA-binding protein LIN28 was identified as a regulator of developmental timing in Caenorhabtidis elegans [1], and homologous genes exist in diverse species, including mammals [2]. LIN28 is expressed in pluripotent stem cells [3,4] and widely during mammalian embryogenesis [5,6], but displays restricted expression in adult tissues [5]. LIN28 inhibits the production of mature microRNAs (miRNAs) of the LET-7 (Mirlet7) family from precursor pri-miRNA transcripts [4,7]. LIN28 can also promote mRNA translation through direct association with target mRNAs [8–11], including that encoding the pluripotency-associated transcription factor OCT4 [12].
Lin28 is required for normal development of the primordial germ cell (PGC) population in vivo, and for derivation of PGC-like cells from embryonic stem (ES) cells in vitro [13]. In the absence of Lin28, Let-7-mediated translational repression inhibits the production of Blimp1, a key factor in PGC specification [14,15]. Lin28 expression is detectable in mouse PGCs from embryonic day (e) 7.5, with Lin28-negative germ cells first observed at e12.5, corresponding to the time at which sex determination occurs and immediately before entry into meiosis in the female [13]. In the postnatal mouse testis, Lin28 is repressed by retinoic acid (RA), resulting in increased Let-7 miRNA expression and possibly spermatogonial differentiation [16].
LIN28 is expressed by germ cells in the human fetal testis [17], and in some human gonadal tumors [13,17–19] characterized by retention of a fetal germ cell–like phenotype [20]. The expression of LIN28 in the developing human fetal ovary has not been previously reported. Here, we characterize the expression of LIN28 during human ovarian development, from the postmigratory PGC stage, through the onset of meiotic differentiation, to primordial follicle formation and meiotic arrest.
Results and Discussion
Expression of the LIN28 gene was investigated at 3 gestational ages representing key stages in human fetal ovarian germ cell differentiation. At 8–11 weeks of gestational age (wga), the fetal ovary contains only undifferentiated, proliferating PGCs. By 14–16 wga widespread germ cell differentiation has initiated, with germ cells forming syncytial clusters and some germ cells initiating meiosis. At 17–20 wga, germ cell clusters begin to break down, forming primordial follicles containing oocytes arrested at the diplotene stage of meiosis [21]. At later gestations (beyond 12–13 wga), germ cells at various stages of differentiation are seen in the same ovary, arising from considerable asynchrony in the progression of human fetal ovarian germ cell development [22,23]. Mitotic PGC-like cells are restricted to the periphery of the ovary, with progressively more differentiated germ cells found in a gradient toward the center [22–24].
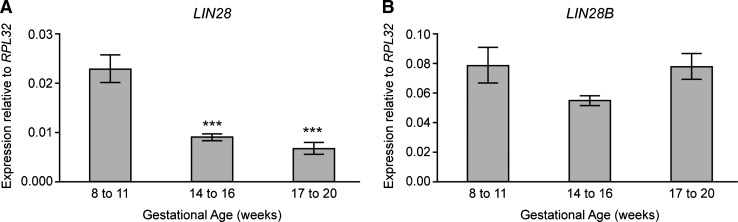
LIN28 gene expression decreased significantly between 8–11 wga and 14–16 wga (Fig. 1A) coincident with the onset of meiotic germ cell differentiation, and remained low at 17–20 wga. This pattern of expression is strikingly similar to that we have reported previously for POU5F1 (encoding OCT4) [22], and is the opposite to that observed for markers of more mature germ cells such as DAZL and VASA [22,23], and genes encoding proteins involved in meiosis, such as STRA8, SYCP3, and SPO11, that are upregulated from 11 to 12 wga [25–27]. This suggests that LIN28 expression is associated with PGCs in the human fetal ovary and downregulated at the onset of germ cell differentiation. The paralogous gene LIN28B has been implicated in the timing of human puberty and menopause [28], but its expression was unchanged (Fig. 1B).
FIG. 1.
Expression of LIN28 and LIN28B during human fetal ovarian development. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis reveals expression of LIN28 (A) to be highest at 8–11 wga, and significantly lower at 14–16 wga and 17–20 wga, coincident with the onset of germ cell differentiation [***P<0.001 vs. 8–11 wga (ANOVA with Neumann-Keuls post hoc testing), n=6–8 ovaries per gestational group]. LIN28B expression (B) remained unchanged across gestation. Values denote mean±SEM. ANOVA, analysis of variance; SEM, standard error of the mean.
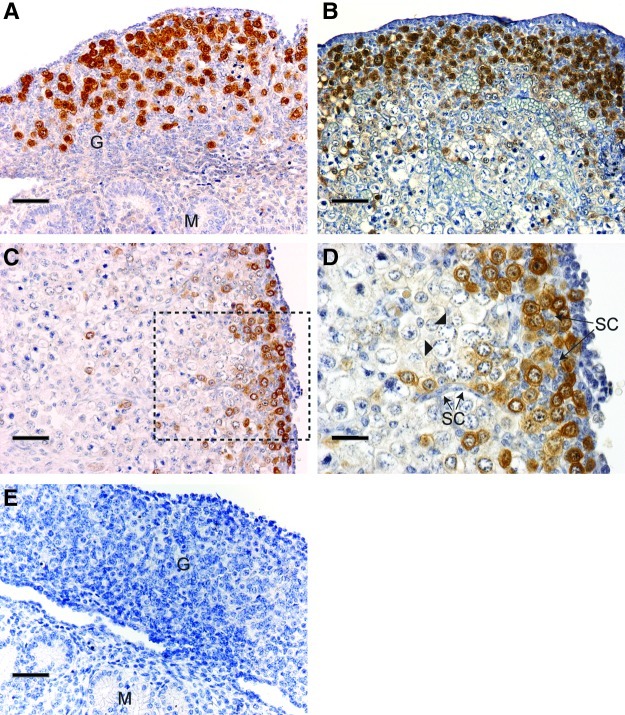
To establish whether the pattern of LIN28 gene expression was conserved at the protein level, we performed immunohistochemistry for LIN28 on sections of human fetal ovaries. At 9 wga, intense LIN28 immunostaining was detected exclusively in PGCs, in both the nucleus and the cytoplasm (Fig. 2A), and was present in all germ cells at this stage. By 14 wga expression was restricted to a population of germ cells at the periphery of the fetal ovary, with some immunopositive germ cells detected deeper in the ovarian cortex (Fig. 2B). By 18 wga, almost all immunopositive germ cells were restricted to a thin margin at the periphery of the ovary (Fig. 2C, D). Large germ cells with cartwheel chromatin indicative of the leptotene stage of meiosis [29] displayed no staining (Fig. 2D), supporting the hypothesis that LIN28 expression is downregulated as germ cells differentiate. Additionally, we noted a possible change in the predominant subcellular localization of the LIN28 protein with gestation; at 9 wga all germ cells displayed both nuclear and cytoplasmic staining, while most LIN28-positive germ cells at 18 wga appeared to have immunonegative nuclei (compare Fig. 2A and Fig. C, D). Ovarian (Fig. 2D) and mesonephric (Fig. 2A) somatic cells were immunonegative at all stages examined, as was the ovarian surface epithelium, despite recent reports of the isolation of LIN28-positive germ cell–like cells from the adult ovarian surface epithelium [30], and the expression of LIN28 by a subpopulation of epithelial ovarian cancer cells [31]. Negative controls also displayed no staining (Fig. 2E).
FIG. 2.
LIN28 expression is germ cell specific and becomes restricted to a subpopulation of germ cells with increasing gestation. At 9 wga (A), all germ cells in the gonad (G) express LIN28 (brown staining) in both the nucleus and cytoplasm. Somatic cells in the gonad (G) and mesonephros (M) display no staining. By 14 wga (B), LIN28-positive cells are concentrated at the periphery of the ovary, although some LIN28-positive germ cells are distributed toward the center of the ovary. At 18 wga (C), LIN28-positive germ cells occupy a thin margin at the edge of the ovary; no staining is detected in more mature germ cells toward the center of the ovary. (D) Magnified image of area identified in (C); meiotic germ cells (identified by their characteristic chromatin arrangement) are LIN28 negative (arrowheads). Somatic cells (SC) in streams and interspersed between LIN28-positive germ cells are also immunonegative. (E) No staining is detected in negative controls. Scale bars in A, B, C, and E: 50 μm; D: 20 μm.
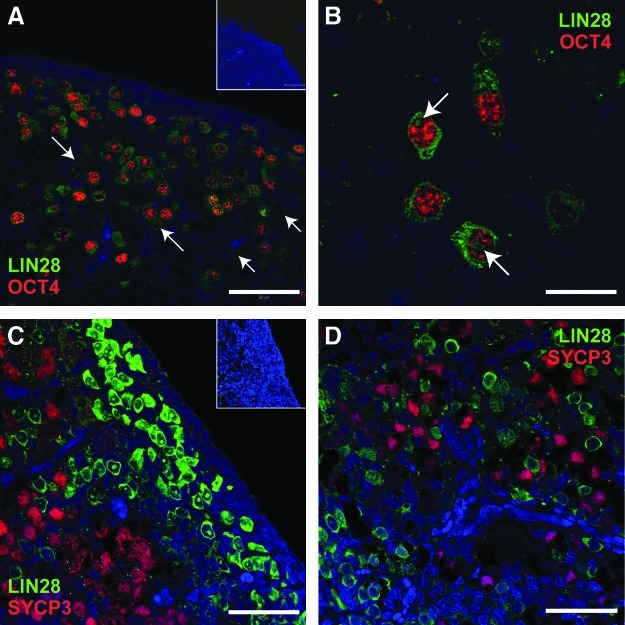
As human germ cell development is highly asynchronous, we performed double immunofluorescence to compare the distribution of LIN28 with stage-specific markers of germ cell maturation, namely, the PGC marker OCT4 [22], and the meiosis-associated synaptonemal complex protein SYCP3 [32]. We detected extensive overlap between the expression of LIN28 and OCT4 at 14 wga (Fig. 3A). All OCT4-positive germ cells appeared to express LIN28, however, OCT4-negative/LIN28-positive germ cells could also be detected, revealing persistence of LIN28 expression beyond the stage at which germ cells downregulate OCT4. High-magnification confocal images revealed that in germ cells in which LIN28 localized to the nucleus, expression was restricted to a distinct subnuclear compartment from which OCT4 appeared to be excluded (Fig. 3B), a finding consistent with previous reports of nucleolar localization of LIN28 [8]. No coexpression of LIN28 and SYCP3 was detected at 15 wga at either the periphery (Fig. 3C) or deeper within the cortex (Fig. 3D) of the fetal ovary, confirming that LIN28 is downregulated before extensive germ cell meiotic differentiation. Negative controls also displayed no staining (insets, Fig. 3A, C). It appears therefore that in the human fetal ovary LIN28 marks OCT4-positive PGC-like germ cells and, additionally, a population of germ cells at a slightly later yet still premeiotic stage of differentiation.
FIG. 3.
LIN28 is expressed in undifferentiated germ cells, but not in those undergoing meiosis. Double immunofluorescence reveals the extensive overlap between the expression domains of LIN28 (green) and OCT4 (red) (A). All OCT4-positive cells express LIN28, although some germ cells are LIN28-positive only (arrows). High-magnification images of the human fetal ovary at 14 wga (B) reveal that LIN28 occupies a distinct subnuclear compartment (arrows) from which OCT4 protein is excluded. No colocalization of LIN28 (green) with the meiosis marker SYCP3 (red) is detected at 15 wga at the periphery (C) or deeper within the cortex (D). No staining is detected on negative controls (insets, A and C). Scale bars in A, C, and D: 50 μm; B: 10 μm, blue staining denotes cell nuclei.
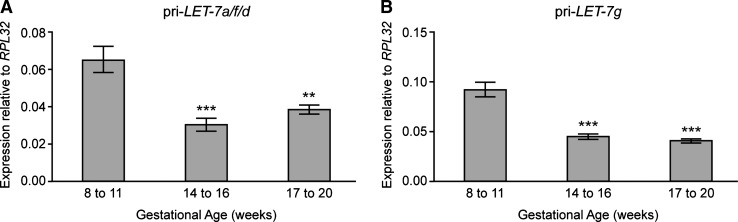
As LIN28 inhibits the production of mature LET-7 miRNAs from precursor pri-miRNA transcripts, we examined the expression of the LIN28-target pri-LET-7a/f/d and pri-LET-7g miRNAs during human fetal ovarian development. We found significant decreases in the expression of pri-LET-7a/f/d and pri-LET-7g (Fig. 4A, B) between 8–11 wga and 14–16 wga, mirroring the changes in LIN28 expression. These data suggest that LET-7 miRNAs may be important during early human germ cell development. Furthermore, the coincident peak expression of both LIN28 and its target pri-miRNAs suggests that in early human germ cells LIN28 maybe required to inhibit the high levels of pri-LET-7 transcripts from being processed into mature miRNAs. In mouse ES cells, pri-Let-7g expression is directly regulated by Oct4 [33], and the pattern of pri-LET-7 expression reported here, which reflects that of OCT4, [22] may indirectly suggest that this regulatory relationship is preserved in human fetal germ cells.
FIG. 4.
Expression of precursor LET-7 pri-microRNA transcripts during human fetal ovarian development. qRT-PCR analysis reveals developmentally regulated expression of (A) pri-LET-7a/f/d and (B) pri-LET-7g during human fetal ovary development. Expression of both transcripts is highest at 8–11 wga, and significantly downregulated at 14–16 and 17–20 wga. ***P<0.001 versus 8–11 wga, **P<0.01 versus 8–11 wga (ANOVA, with Neumann-Keuls post hoc testing n=6–8 ovaries per gestational group). Values denote mean±SEM.
Conclusions
In this report we have demonstrated the expression of the RNA-binding protein LIN28 by immature germ cells during the development of the human fetal ovary. LIN28 is downregulated before germ cells have undergone extensive meiotic differentiation, a finding consistent with the loss of Lin28 expression in fetal mouse gonads from e12.5 [13], just before the initiation of meiosis. In mice [34,35] and possibly humans [25,27] meiotic entry is thought to be promoted by the action of RA, which has recently been reported to repress Lin28 expression in the postnatal mouse testis [16]. It will be important to establish therefore whether downregulation of LIN28 in the human fetal ovary results, in part, from RA-mediated repression, thus facilitating germ cell entry into meiosis.
The expression of the LET-7 family precursor pri-miRNAs, a target of LIN28, also declines with increasing germ cell differentiation, consistent with a conserved functional interaction between the two. Elucidation of the LIN28–LET-7 relationship in human germ cells will enhance our understanding of how human PGCs switch from the stem cell state to meiotic differentiation, and provide insight into manipulating these processes for therapeutic intervention.
Materials and Methods
Collection of human fetal ovaries
Ovaries (8–20 weeks of gestation, calculated from the date of the last menstrual period) were obtained from morphologically normal human fetuses after elective termination of pregnancy. Informed consent was obtained, and the study was approved by the Lothian Research Ethics Committee (study code: LREC 08/S1101/1). Gestational age was determined by ultrasonography, and further confirmed (for 14–20-wga specimens) by measurement of foot length. The sex of 8–11-wga gonads was determined by polymerase chain reaction (PCR) for the SRY gene as described previously [36], using the following primers: SRY-Fwd, 5′-ACAGTAAAGGCAACGTCCAG-3′ and SRY-Rev, 5′-ATCTGCGGGAAGCAAACTGC-3′ [37]. Gonads were dissected into Dulbecco's phosphate balanced salt solution (Life Technologies) and either snap-frozen and stored at −80°C for extraction of total RNA for quantitative reverse transcription (qRT)-PCR analysis or fixed in Bouin's solution and processed into wax by standard methods for immunohistochemistry. All extragonadal tissue, including mesonephroi, was dissected away from the gonad before freezing.
RNA extraction and qRT-PCR
Total RNA was extracted from fetal ovaries using the RNeasy Micro and RNeasy Mini kits (QIAGEN) with on-column DNaseI digestion as per the manufacturer's instructions. First-strand cDNA was prepared using the Superscript VILO Mastermix kit (Life Technologies). Gene expression was assessed by qRT-PCR using SYBR green technology on an ABI7900HT Fast thermal cycler (Life Technologies) as described previously [36]. The expression level of each gene of interest was normalized to that of the housekeeping gene RPL32 within the same sample. Negative (cDNA prepared in the absence of reverse transcriptase) and no-template (in which dH2O was substituted for template cDNA) controls were included to exclude contamination or amplification of residual genomic DNA. Primer sequences used in qRT-PCR can be found in Table 1.
Table 1.
Oligonucleotide Primer Sequences Used in SYBR Green Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis
Immunohistochemistry
Immunohistochemistry was performed on fixed sections of fetal ovary as described previously [36]. Briefly, 5-μm-thick sections of Bouin's-fixed, paraffin-embedded tissues were mounted on electrostatically charged glass slides; dewaxed; and rehydrated through xylene (2×5 min) and graded alcohols [100% (2×30 s), 95% (30 s), and 70% (30 s)]. Antigen retrieval was performed by pressure cooking in 0.01 M sodium citrate buffer (pH 6.0) for 5 min, followed by incubation in 3% (v/v) hydrogen peroxide in methanol for 30 min to block endogenous peroxidase activity. Slides were subsequently blocked using the avidin/biotin blocking kit (Vector Laboratories, Inc.) and incubation in Tris Buffered Saline (TBS), supplemented with 5% bovine serum albumin (BSA) and 20% normal serum (NS). Polyclonal rabbit anti-LIN28 primary antibody (AB46020; Abcam) was diluted 1:500 in 5% BSA/NS/TBS and applied to the sections, and slides were incubated at 4°C overnight in a humidified chamber. Primary antibodies were detected using a goat anti-rabbit secondary antibody (DAKO; 30 min, diluted 1:500 in BSA/TBS/NS) and incubation with avidin-biotin-HRP complex (Vector Laboratories). Bound antibodies were visualized using 3,3-diaminobenzidine tetrahydrochloride (DAB; DAKO). Negative controls in which the primary antibody was omitted were included in each experiment. Slides were counterstained with hematoxylin, dehydrated through graded alcohols and xylene, and permanently mounted using PerTex before being photographed on a Provis (Carl Zeiss Ltd.) microscope with cold-capture camera (Olympus). A minimum of 4 sections per ovary were examined.
Immunofluorescence
Double immunofluorescence was performed as described previously [38] using either rabbit polyclonal anti-SYCP3 (AB15093; Abcam; diluted 1:50,000) or mouse monoclonal anti-OCT4 (SC-5279, which recognizes only the pluripotency-associated OCT4A isoform [39]; Santa Cruz Biotechnology; diluted 1:250) on day 1 of the procedure, and rabbit polyclonal anti-LIN28 (AB46020; Abcam; diluted 1:500) primary antibody on day 2. Anti-SYCP3/anti-OCT4 antibodies were detected using a goat anti-mouse or a goat anti-rabbit peroxidase secondary antibody (Vector Laboratories Ltd.; diluted 1:200), and then incubated with Tyramide Fluorescein Green (TSA Plus Cyanine 5 System; diluted 1:50) for 10 min at room temperature. Anti-LIN28 primary antibody was detected using a goat anti-rabbit biotinylated secondary antibody (Vector Laboratories Ltd.; diluted 1:500), followed by incubation with Streptavidin-488 (Molecular Probes, Invitrogen Life Technologies; diluted 1:200) for 1 h at room temperature. Slides were counterstained with DAPI, and mounted using PermaFluor Aqueous Mounting Medium (Beckman Coulter). Fluorescent images were captured using an LSM510 Meta confocal microscope (Carl Zeiss Ltd.). A minimum of 4 sections per ovary were examined.
Statistical analyses
Gestational range qRT-PCR data (n=6–8 ovaries from individual fetuses per gestational group) were analyzed by one-way analysis of variance with Neumann-Keuls post hoc testing in Graphpad Prism Software. P values of <0.05 were considered statistically significant.
Acknowledgments
The authors are very grateful to Anne Saunderson and the staff of the Bruntsfield Suite of the Royal Infirmary of Edinburgh for patient recruitment and provision of samples for these studies, to members of the Anderson lab for assistance with sample collection and preparation, and to Ronnie Grant for assistance with figures. This work was supported by Medical Research Scotland (research grant 354 FRG to A.J.C.) and the Medical Research Council (program grant G1100357 to R.A.A.).
Disclosure Statement
The authors declare that they have no competing interests to disclose.
Note Added in Proof
While this article was in peer review and production, El-Khairi et al. [40] also reported increasing expression of LIN28 in the human fetal ovary between 6 and 9 weeks post conception (approximately equivalent to 8–11 weeks gestational age) and co-localization of LIN28 with OCT4 in germ cells at this stage.
References
- 1.Moss EG. Lee RC. Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 2.Moss EG. Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol. 2003;258:432–442. doi: 10.1016/s0012-1606(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 3.Richards M. Tan SP. Tan JH. Chan WK. Bongso A. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2004;22:51–64. doi: 10.1634/stemcells.22-1-51. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan SR. Daley GQ. Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang DH. Moss EG. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr Patterns. 2003;3:719–726. doi: 10.1016/s1567-133x(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama S. Hashimoto M. Shimizu H. Ueno-Kudoh H. Uchibe K. Kimura I. Asahara H. Dynamic gene expression of Lin-28 during embryonic development in mouse and chicken. Gene Expr Patterns. 2008;8:155–160. doi: 10.1016/j.gep.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bussing I. Slack FJ. Grosshans H. Let-7 microRNAs in development, stem cells and cancer. Trends Mol Med. 2008;14:400–409. doi: 10.1016/j.molmed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Polesskaya A. Cuvellier S. Naguibneva I. Duquet A. Moss EG. Harel-Bellan A. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 2007;21:1125–1138. doi: 10.1101/gad.415007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu B. Huang Y. Histone H2a mRNA interacts with Lin28 and contains a Lin28-dependent posttranscriptional regulatory element. Nucleic Acids Res. 2009;37:4256–4263. doi: 10.1093/nar/gkp372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu B. Zhang K. Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15:357–361. doi: 10.1261/rna.1368009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng S. Chen LL. Lei XX. Yang L. Lin H. Carmichael GG. Huang Y. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells. 2011;29:496–504. doi: 10.1002/stem.591. [DOI] [PubMed] [Google Scholar]
- 12.Qiu C. Ma Y. Wang J. Peng S. Huang Y. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res. 2010;38:1240–1248. doi: 10.1093/nar/gkp1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West JA. Viswanathan SR. Yabuuchi A. Cunniff K. Takeuchi A. Park IH. Sero JE. Zhu H. Perez-Atayde A, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. 2009;460:909–913. doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohinata Y. Payer B. O'Carroll D. Ancelin K. Ono Y. Sano M. Barton SC. Obukhanych T. Nussenzweig M, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- 15.Vincent SD. Dunn NR. Sciammas R. Shapiro-Shalef M. Davis MM. Calame K. Bikoff EK. Robertson EJ. The zinc finger transcriptional repressor Blimp1/Prdm1 is dispensable for early axis formation but is required for specification of primordial germ cells in the mouse. Development. 2005;132:1315–1325. doi: 10.1242/dev.01711. [DOI] [PubMed] [Google Scholar]
- 16.Tong MH. Mitchell D. Evanoff R. Griswold MD. Expression of Mirlet7 family microRNAs in response to retinoic acid-induced spermatogonial differentiation in mice. Biol Reprod. 2011;85:189–197. doi: 10.1095/biolreprod.110.089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillis AJ. Stoop H. Biermann K. van Gurp RJ. Swartzman E. Cribbes S. Ferlinz A. Shannon M. Oosterhuis JW. Looijenga LH. Expression and interdependencies of pluripotency factors LIN28, OCT3/4, NANOG and SOX2 in human testicular germ cells and tumours of the testis. Int J Androl. 2011;34:e160–e174. doi: 10.1111/j.1365-2605.2011.01148.x. [DOI] [PubMed] [Google Scholar]
- 18.Cao D. Allan RW. Cheng L. Peng Y. Guo CC. Dahiya N. Akhi S. Li J. RNA-binding protein LIN28 is a marker for testicular germ cell tumors. Hum Pathol. 2011;42:710–718. doi: 10.1016/j.humpath.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Xue D. Peng Y. Wang F. Allan RW. Cao D. RNA-binding protein LIN28 is a sensitive marker of ovarian primitive germ cell tumours. Histopathology. 2011;59:452–459. doi: 10.1111/j.1365-2559.2011.03949.x. [DOI] [PubMed] [Google Scholar]
- 20.Rajpert-De Meyts E. Bartkova J. Samson M. Hoei-Hansen CE. Frydelund-Larsen L. Bartek J. Skakkebaek NE. The emerging phenotype of the testicular carcinoma in situ germ cell. Apmis. 2003;111:267–278. doi: 10.1034/j.1600-0463.2003.11101301.x. discussion 278–279. [DOI] [PubMed] [Google Scholar]
- 21.Maheshwari A. Fowler PA. Primordial follicular assembly in humans—revisited. Zygote. 2008;16:285–296. doi: 10.1017/S0967199408004802. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RA. Fulton N. Cowan G. Coutts S. Saunders PT. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol. 2007;7:136. doi: 10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoop H. Honecker F. Cools M. de Krijger R. Bokemeyer C. Looijenga LH. Differentiation and development of human female germ cells during prenatal gonadogenesis: an immunohistochemical study. Hum Reprod. 2005;20:1466–1476. doi: 10.1093/humrep/deh800. [DOI] [PubMed] [Google Scholar]
- 24.Fulton N. Martins da Silva SJ. Bayne RA. Anderson RA. Germ cell proliferation and apoptosis in the developing human ovary. J Clin Endocrinol Metab. 2005;90:4664–4670. doi: 10.1210/jc.2005-0219. [DOI] [PubMed] [Google Scholar]
- 25.Childs AJ. Cowan G. Kinnell HL. Anderson RA. Saunders PT. Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS One. 2011;6:e20249. doi: 10.1371/journal.pone.0020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houmard B. Small C. Yang L. Naluai-Cecchini T. Cheng E. Hassold T. Griswold M. Global gene expression in the human fetal testis and ovary. Biol Reprod. 2009;81:438–443. doi: 10.1095/biolreprod.108.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bouffant R. Guerquin MJ. Duquenne C. Frydman N. Coffigny H. Rouiller-Fabre V. Frydman R. Habert R. Livera G. Meiosis initiation in the human ovary requires intrinsic retinoic acid synthesis. Hum Reprod. 2010;25:2579–2590. doi: 10.1093/humrep/deq195. [DOI] [PubMed] [Google Scholar]
- 28.Perry JR. Stolk L. Franceschini N. Lunetta KL. Zhai G. McArdle PF. Smith AV. Aspelund T. Bandinelli S, et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat Genet. 2009;41:648–650. doi: 10.1038/ng.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bendsen E. Byskov AG. Andersen CY. Westergaard LG. Number of germ cells and somatic cells in human fetal ovaries during the first weeks after sex differentiation. Hum Reprod. 2006;21:30–35. doi: 10.1093/humrep/dei280. [DOI] [PubMed] [Google Scholar]
- 30.Virant-Klun I. Skutella T. Stimpfel M. Sinkovec J. Ovarian surface epithelium in patients with severe ovarian infertility: a potential source of cells expressing markers of pluripotent/multipotent stem cells. J Biomed Biotechnol. 2011;2011:381928. doi: 10.1155/2011/381928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng S. Maihle NJ. Huang Y. Pluripotency factors Lin28 and Oct4 identify a sub-population of stem cell-like cells in ovarian cancer. Oncogene. 2010;29:2153–2159. doi: 10.1038/onc.2009.500. [DOI] [PubMed] [Google Scholar]
- 32.Yuan L. Liu JG. Hoja MR. Wilbertz J. Nordqvist K. Hoog C. Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science. 2002;296:1115–1118. doi: 10.1126/science.1070594. [DOI] [PubMed] [Google Scholar]
- 33.Marson A. Levine SS. Cole MF. Frampton GM. Brambrink T. Johnstone S. Guenther MG. Johnston WK. Wernig M, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koubova J. Menke DB. Zhou Q. Capel B. Griswold MD. Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowles J. Knight D. Smith C. Wilhelm D. Richman J. Mamiya S. Yashiro K. Chawengsaksophak K. Wilson MJ. Rossant J. Hamada H. Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 36.Childs AJ. Kinnell HL. Collins CS. Hogg K. Bayne RA. Green SJ. McNeilly AS. Anderson RA. BMP signaling in the human fetal ovary is developmentally regulated and promotes primordial germ cell apoptosis. Stem Cells. 2010;28:1368–1378. doi: 10.1002/stem.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friel A. Houghton JA. Glennon M. Lavery R. Smith T. Nolan A. Maher M. A preliminary report on the implication of RT-PCR detection of DAZ, RBMY1, USP9Y and Protamine-2 mRNA in testicular biopsy samples from azoospermic men. Int J Androl. 2002;25:59–64. doi: 10.1046/j.1365-2605.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 38.Duffin K. Bayne RA. Childs AJ. Collins C. Anderson RA. The forkhead transcription factor FOXL2 is expressed in somatic cells of the human ovary prior to follicle formation. Mol Hum Reprod. 2009;15:771–777. doi: 10.1093/molehr/gap065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atlasi Y. Mowla SJ. Ziaee SA. Gokhale PJ. Andrews PW. OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells. 2008;26:3068–3074. doi: 10.1634/stemcells.2008-0530. [DOI] [PubMed] [Google Scholar]
- 40.El-Khairi R. Parnaik R. Duncan AJ. Lin L. Gerrelli D. Dattani MT. Conway GS. Achermann JC. Analysis of LIN28A in early human ovary development and as a candidate for primary ovarian insufficiency. Mol Cell Endocrinol. 2012;351:264–268. doi: 10.1016/j.mce.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]