Abstract
OBJECTIVE
To assess the prevalence of distal sensorimotor polyneuropathy (DSPN) in an older population and to examine its relationship with prediabetes.
RESEARCH DESIGN AND METHODS
Glucose tolerance status was determined in 61- to 82-year-old participants (n = 1,100) of the population-based Cooperative Health Research in the Region of Augsburg (KORA) F4 Survey (2006–2008). Clinical DSPN was defined as bilaterally impaired foot-vibration perception and/or foot-pressure sensation.
RESULTS
Prevalence of clinical DSPN was similar in subjects with known diabetes (22.0%) and subjects with combined impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) (23.9%). Among prediabetic subgroups, IFG-IGT, but not isolated-IFG and -IGT, was associated with a higher risk of clinical DSPN, compared with normal glucose tolerance. A J-shaped association was observed between clinical DSPN and quartiles of 2-h postchallenge glucose, but not with fasting glucose and HbA1c levels.
CONCLUSIONS
Subjects with IFG-IGT and known diabetes had a similar prevalence of clinical DSPN. Elevated 2-h postload glucose levels appeared important for disease risk.
Distal sensorimotor polyneuropathy (DSPN) is an important complication of type 2 diabetes. It is still unclear whether nerve disorders are already manifest in subjects with prediabetes (1).
The aim of the current study was to evaluate the prevalence of DSPN in a representative sample of the older population and to study the association between prediabetes and DSPN. In addition, we examined the relationship between glucose (fasting and 2-h postload) and HbA1c levels and the disorder.
RESEARCH DESIGN AND METHODS
The current study is performed using the follow-up data of the Cooperative Health Research in the Region of Augsburg (KORA) S4 Survey (1999–2001): the KORA F4 Survey (2006–2008) (2). Of the 1,209 participants, 923 participants subsequently completed an oral glucose tolerance test (OGTT) (according to World Health Organization criteria) (3), and self-reported diabetes was validated for 177 participants. Clinical DSPN was defined as bilaterally impaired foot-vibration perception (tuning fork) and/or bilaterally impaired foot-pressure sensation (10-g monofilament). Details on a validation study of the DSPN definition are provided in the Supplementary Data online.
Age- and sex-adjusted differences in characteristics were evaluated for the different categories of glucose tolerance using ANOVA. Multivariate logistic regression models were fitted to study associations between diabetes, prediabetes (combined impaired fasting glucose [IFG] and impaired glucose tolerance [IGT], isolated IFG [i-IFG], and isolated IGT [i-IGT]), and the presence of clinical DSPN. In addition, relationships between glucose concentrations, HbA1c, and clinical DSPN were studied. Analyses were performed with STATA (version 11; StataCorp, College Station, TX).
RESULTS
The results are based on 1,100 participants with complete information on glucose tolerance status, clinical DSPN, and other covariables. The study population included 577 subjects with normal glucose tolerance (NGT), 55 with i-IFG, 183 with i-IGT, 46 with IFG-IGT, 62 with undiagnosed diabetes, and 177 with known diabetes. Participants with known and undiagnosed diabetes were physically less active and had a larger waist circumference, a higher prevalence of hypertension, and prior cardiovascular events compared with subjects with NGT.
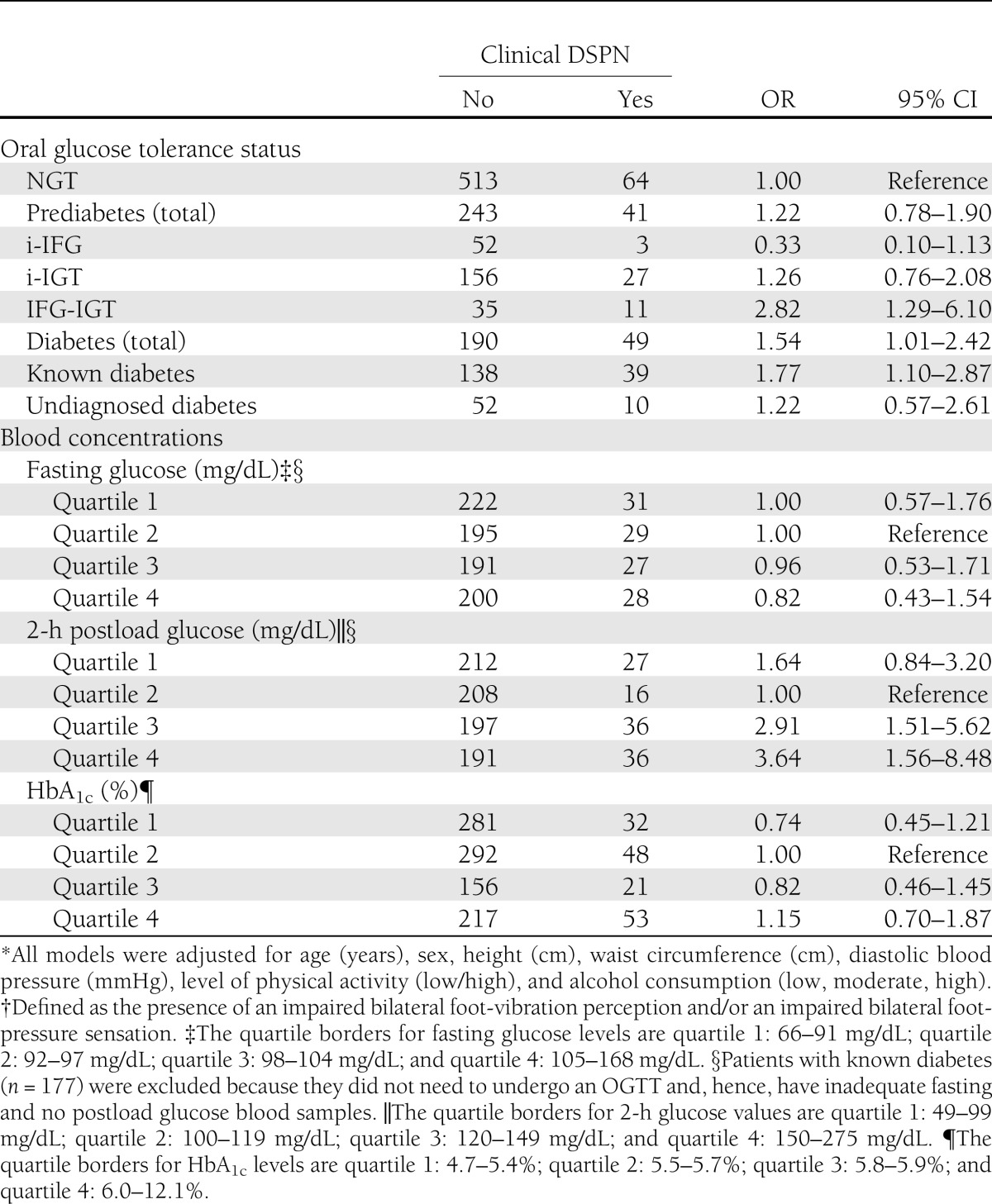
The prevalence of clinical DSPN was similar among participants with IFG-IGT (23.9% [95% CI 12.6–38.8]) and those with known diabetes (22.0 [16.2–28.9]). Prevalence in subjects with NGT, i-IFG, i-IGT, and undiagnosed diabetes was 11.1 (8.6–13.9), 5.5 (1.1–15.1), 14.8 (10.0–20.7), and 16.1 (8.0–27.7), respectively. Subjects with clinical DSPN were slightly older, taller, and physically less active than subjects without DSPN and had a larger waist circumference and higher HbA1c levels.
The total prediabetic group did not show statistically significantly increased odds of having clinical DSPN compared with the NGT group (Table 1). When the subgroups were examined separately, a positive association was observed between IFG-IGT and clinical DSPN (odds ratio [OR] 2.82 [95% CI 1.29–6.10]). Compared with NGT subjects, participants with diabetes (undiagnosed and diagnosed) had increased odds of having clinical DSPN (1.54 [1.01–2.42]). This elevated risk was largely attributable to subjects with known diabetes, given that no association was observed for subjects with undiagnosed diabetes.
Table 1.
Adjusted ORs* and 95% CIs for clinical DSPN† according to oral glucose tolerance status and blood concentrations: KORA F4 (2006–2008)
A J-shaped relationship was observed between quartiles of 2-h postload blood glucose and the presence of clinical DSPN (Table 1). There was no relationship between quartiles of fasting blood glucose and quartiles of HbA1c levels and the disorder. In addition, there was no association between (components of) metabolic syndrome and clinical DSPN (data not shown).
CONCLUSIONS
The present population-based study showed a similar prevalence of clinical DSPN in participants with IFG-IGT and those with known diabetes. Among the prediabetic subgroups, only IFG-IGT was positively associated with having clinical DSPN, while the total prediabetic group was not related to having the disorder. A J-shaped relationship was observed between quartiles of 2-h postload glucose and the presence of clinical DSPN. No associations were seen with fasting glucose and HbA1c levels.
In the current literature, the prevalence of DSPN varies between 18 and 42% for populations with known diabetes (4,5) and between 4 and 19% for populations with prediabetes (6–10). The prevalence found in the current study for both diabetic groups is in concordance with the literature. Published prevalences for subjects with prediabetes, or IGT only, ranged from 5 to 16% (4,7,11,12). The proportion of clinical DSPN that we found in subjects with IGT is in line with these observations. Occurrence of DSPN in IFG was reported less frequently (11–13) yet was within a range of 5.7–11.3% comparable with our study estimate. Prevalence of clinical DSPN in individuals suffering from IFG-IGT has not been published before. Our findings indicate that participants with IFG-IGT were similar to participants with diabetes regarding characteristics and frequency of clinical DSPN. Consequently, subjects with IFG-IGT represent a high-risk group for DSPN.
Considering glycemia, 2-h postload glucose concentrations appeared more important for DSPN risk than fasting glucose levels. Risk of clinical DSPN was increased at postload glucose concentrations that were still within the normal range of the glycemic metabolism according to the World Health Organization (3). Previous studies show similar curvilinear relationships between 2-h postload glucose concentrations and risk of (cardiovascular) mortality (14,15). Altogether, these results suggest an important role of postprandial glucose levels, including those below the diagnostic threshold, in the development of diabetes complications.
The current study has some limitations. Since there is no uniform consensus on a definition of DSPN for use in epidemiological studies, we may have under- or overestimated the true prevalence of DSPN. However, a validation study of our clinical DSPN definition showed excellent diagnostic performance (see Supplementary Data online). Additional adjustment of our analyses for other risk factors of DSPN (smoking and diseases causing neurologic damage) did not change the results. Finally, stratified analyses resulted in some small subgroups.
An important methodological strength of the current study, as opposed to other previous population-based studies, is the use of the OGTT. This enables studying the entire spectrum of glucose disorders through identification of subjects with undiagnosed diabetes and prediabetes. Another strength of the study is the use of different neurologic bedside tests, facilitating a relatively accurate definition of clinical DSPN.
Replication of the present findings in large, well-defined, population-based studies may shed light on the developmental mechanism of DSPN and on identifying high-risk individuals.
Acknowledgments
The current study was funded by a grant from the German Research Foundation (RA-45913/3-1). The German Diabetes Center is funded by the German Federal Ministry of Health and the Ministry of Innovation, Science, Research, and Technology of the State of North Rhine-Westphalia.
No potential conflicts of interest relevant to this article were reported.
B.W.C.B. performed data analyses and wrote the manuscript. W.R. planned the study, contributed to data analyses, and wrote the manuscript. B.K., C.H., D.S., and C.M. reviewed and edited the manuscript. D.Z. planned the study, contributed to data analyses and discussion, and reviewed and edited the manuscript. W.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to Dr. Margit Heier for performing the neurologic examinations and interviews in all participants of the KORA F4 Survey.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2028/-/DC1.
References
- 1.Papanas N, Vinik Al, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol; 2011;7:682–690. [DOI] [PubMed] [Google Scholar]
- 2.Rathmann W, Haastert B, Icks A, et al. High prevalence of undiagnosed diabetes mellitus in southern Germany: target populations for efficient screening. The KORA survey 2000. Diabetologia 2003;46:182–189 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Report of a WHO Consultation: Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Geneva, World Health Organization, 1999 [Google Scholar]
- 4.Shaw JE, Zimmet PZ, Gries FA, Ziegler D. Epidemiology of diabetic neuropathy. In Textbook of Diabetic Neuropathy. Gries FA, Cameron NE, Low PA, Ziegler D, Eds. Stuttgart, Germany, Thieme, 2003, p. 64–82 [Google Scholar]
- 5.Herder C, Lankisch M, Ziegler D, et al. Subclinical inflammation and diabetic polyneuropathy: MONICA/KORA Survey F3 (Augsburg, Germany). Diabetes Care 2009;32:680–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rith-Najarian SJ, Stolusky T, Gohdes DM. Identifying diabetic patients at high risk for lower-extremity amputation in a primary health care setting. A prospective evaluation of simple screening criteria. Diabetes Care 1992;15:1386–1389 [DOI] [PubMed] [Google Scholar]
- 7.Herman WH, Aubert RE, Engelgau MM, et al. Diabetes mellitus in Egypt: glycaemic control and microvascular and neuropathic complications. Diabet Med 1998;15:1045–1051 [DOI] [PubMed] [Google Scholar]
- 8.Shaw JE, Hodge AM, de Courten M, et al. Diabetic neuropathy in Mauritius: prevalence and risk factors. Diabetes Res Clin Pract 1998;42:131–139 [DOI] [PubMed] [Google Scholar]
- 9.Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1995;333:89–94 [DOI] [PubMed] [Google Scholar]
- 10.Dyck PJ, Clark VM, Overland CJ, et al. Impaired glycemia and diabetic polyneuropathy: the OC IG Survey. Diabetes Care 2012;35:584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, KORA Study Group Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469 [DOI] [PubMed] [Google Scholar]
- 12.Tapp RJ, Shaw JE, de Courten MP, Dunstan DW, Welborn TA, Zimmet PZ, AusDiab Study Group Foot complications in type 2 diabetes: an Australian population-based study. Diabet Med 2003;20:105–113 [DOI] [PubMed] [Google Scholar]
- 13.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, KORA Study Group Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009;10:393–400 [DOI] [PubMed] [Google Scholar]
- 14.Seshasai SR, Kaptoge S, Thompson A, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowall B, Rathmann W, Heier M, et al. Categories of glucose tolerance and continuous glycemic measures and mortality. Eur J Epidemiol 2011;26:637–645 [DOI] [PubMed] [Google Scholar]