Abstract
OBJECTIVE
Diabetic peripheral neuropathy (DPN) alters walking. Yet, the compensatory role of central locomotor circuits remains unclear. We hypothesized that walking outcomes would be more closely related to regional gray matter volumes in older adults with DPN as compared with nonneuropathic diabetic patients and nondiabetic control subjects.
RESEARCH DESIGN AND METHODS
Clinically important outcomes of walking (i.e., speed, stride duration variability, and double support time) were measured in 29 patients with DPN (type 2 diabetes with foot-sole somatosensory impairment), 68 diabetic (DM) patients (type 2 diabetes with intact foot-sole sensation), and 89 control subjects. Global and regional gray matter volumes were calculated from 3 Tesla magnetic resonance imaging.
RESULTS
DPN subjects walked more slowly (P = 0.005) with greater stride duration variability (P < 0.001) and longer double support (P < 0.001) as compared with DM and control subjects. Diabetes was associated with less cerebellar gray matter volume (P < 0.001), but global gray matter volume was similar between groups. DPN subjects with lower gray matter volume globally (P < 0.004) and regionally (i.e., cerebellum, right-hemisphere dorsolateral prefrontal cortex, basal ganglia, P < 0.005) walked more slowly with greater stride duration variability and/or longer double support. Each relationship was stronger in DPN than DM subjects. In control subjects, brain volumes did not relate to walking patterns.
CONCLUSIONS
Strong relationships between brain volumes and walking outcomes were observed in the DPN group and to a lesser extent the DM group, but not in control subjects. Individuals with DPN may be more dependent upon supraspinal elements of the motor control system to regulate several walking outcomes linked to poor health in elderly adults.
Diabetic peripheral neuropathy (DPN) is a debilitating condition caused by peripheral nerve deterioration that affects 30–50% of people with type 2 diabetes (1,2). DPN-related foot-sole somatosensory impairment leads to diminished walking speed and increased stride-to-stride movement variability (3,4)—important patient outcomes independently linked to survival (5) and fall-risk (6) in older adults. Still, despite often severe somatosensory impairments, some DPN patients maintain walking patterns that are similar to those observed in age-matched healthy people (4). Identification of factors underlying this intersubject variability in functional reserve may provide new targets for preventative and rehabilitative programs for older adults with DPN.
Walking is governed by a complex motor control system comprising somatosensory, visual, and vestibular elements that interact with spinal, supraspinal, and peripheral motor circuitry. In the presence of chronic impairment to one or more of its elements, this system may compensate and maintain functionality by placing increased dependence upon other, intact elements (7–10). In those older adults who perform poorly on a foot-sole somatosensory test, standing balance is more disrupted by the performance of concurrent cognitive tasks (11). We therefore contend that DPN-related foot-sole somatosensory impairment is associated with increased dependence upon supraspinal elements of the motor control system to regulate walking.
Population-based studies indicate that community-dwelling older adults with less regional gray matter (GM) volume walk more slowly with increased movement variability (12,13). We hypothesized that the relationship between GM volumes within sensorimotor and cognitive brain regions and walking outcomes would be significantly stronger in older adults with DPN as compared with diabetic patients without DPN and control subjects.
RESEARCH DESIGN AND METHODS
Subjects
This study examined the relationships between brain volumes and walking in individuals with and without DPN. Community-dwelling older adults with and without type 2 diabetes were consecutively recruited using advertisements and screened by medical history and physical, neurologic, and laboratory examinations. We screened 229 individuals aged 50–85 years, of which 186 were eligible: 97 with type 2 diabetes and 89 control subjects. These cohorts were matched for age and sex on a frequency distribution.
Inclusion criteria for type 2 diabetes (52 men, 45 women) were physician diagnosis and treatment for ≥5 years. Inclusion criteria for control subjects (43 men, 46 women) were normal fasting glucose and no history of metabolic disorder. For the current analysis, subjects with type 2 diabetes were divided into two groups. The DPN group (n = 29) consisted of subjects with type 2 diabetes and foot-sole somatosensory impairment as defined by an inability to perceive 10 g of pressure on the plantar aspect of either hallux. The diabetic (DM) group (n = 68) consisted of subjects with type 2 diabetes yet normal foot-sole sensation.
Exclusion criteria were type 1 diabetes, history of stroke, myocardial infarction within six months, other clinically important cardiac diseases, arrhythmias, significant nephropathy, kidney or liver transplant, renal or congestive heart failure, carotid artery stenosis (over 50% by medical history and magnetic resonance angiography), and any neurologic or systemic disorders (aside from peripheral neuropathy). Magnetic resonance imaging (MRI) exclusion criteria were incompatible metal implants, pacemakers, arterial stents, and claustrophobia.
This study was approved by the Committee on Clinical Investigations at the Beth Israel Deaconess Medical Center, and written consent was obtained from all subjects.
Protocol
Studies were conducted in the Syncope and Falls in the Elderly (SAFE) Laboratory, the Magnetic Resonance Imaging Center, and the Clinical Research Center at the Beth Israel Deaconess Medical Center.
Diabetic peripheral neuropathy assessment.
The criterion for inclusion of diabetic patients into the DPN group was foot-sole somatosensory impairment defined by the inability to perceive 10 g of pressure on the plantar aspect of either hallux as determined using a 5.07-gauge Semmes-Weinstein monofilament (North Coast Medical) and standard testing procedures (14). This test has low incidence of false-positive results when compared with electrodiagnostics (15) and is thus a conservative test for the presence of peripheral neuropathy. The clinical signs and symptoms of DPN were further assessed with the Toronto Clinical Neuropathy Score, which is derived from a combination of neurologic assessment of symptoms (i.e., foot pain, numbness, tingling, weakness, ataxia, upper-limb symptoms) and sensory tests (i.e., pinprick, temperature, light touch, vibration, position sense) (16).
Walking test.
A 12-min walk was completed along a 75-m course on an 80 × 4 m indoor hallway. Subjects wore standard walking shoes instrumented with heel and toe footswitches (Mega Electronics, Kuopio, Finland) to record the timing of each heel and toe ground contact for both feet. Subjects were instructed to walk at their preferred speed. The time taken to complete each length and total distance were recorded. The relatively long trial duration was originally chosen to enable study of the effects of fatigue on walking. Because this was not the focus of the present analysis, we only examined data from the first 75 m of the trial (i.e., one hallway length, zero turns).
MRI.
All studies were performed on a 3T GE HDx MRI scanner (GE Medical Systems, Milwaukee, WI) using three-dimensional T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) sequences (TR/TE/TI = 6.5/2.8/1100 ms, 3.0 mm slice thickness, 52 slices, bandwidth = 122 Hz per pixel, flip angle = 15°, 24 cm × 24 cm FOV, 256 × 192 matrix size). Images were acquired continuously throughout the brain with 0 mm skip between slices to allow for calculation of regional volumes of the whole brain (see image analysis).
Data analysis
Walking analysis.
Walking outcomes were computed from the first 75 m of walking, which did not include turns. Average walking speed (in meters per second) was determined by dividing distance by time. Stride duration variability and double support time were calculated using foot switch data to create time-series of every “heel-strike” and “toe-off” event for each foot. Stride duration variability (in percentage) was determined by calculating the coefficient of variation about the average stride duration (i.e., heel-strike to heel-strike of the right foot) and multiplying by 100. Double support (in percentage) was calculated as the average time of each stride spent with both feet on the ground (i.e., right heel-strike to left toe-off plus left heel-strike to right toe-off), dividing by average stride time and multiplying by 100.
Image analysis.
MP-RAGE images were analyzed using Interactive Data Language (IDL; Research Systems, Boulder, CO) and MATLAB (MathWorks, Natick, MA) software. MP-RAGE images were skull stripped and coregistered to the MNI152 standard template within the statistical parametric mapping software package (SPM; University College, London, UK). Generated maps of GM, white matter, and cerebrospinal fluid were segmented based upon the LONI Probabilistic Brain Atlas (17) and used to calculate regional volumes. Global GM volume and nine specific regions linked to the control of walking and/or sensorimotor function (12,18,19) were selected for analysis. Regions-of-interest included the right and left precentral gyri, the right and left basal ganglia, and cerebellum (i.e., motor function); the right and left postcentral gyri (i.e., sensory function); and the right and left dorsolateral prefrontal cortex (i.e., executive function/attention). All volumes were normalized to percent intracranial volume.
Statistical analysis
Statistical analyses were performed using JMP software (SAS Institute, Cary, NC). Descriptive statistics (means ± SD) were used to summarize all numeric variables. Potential group differences in demographics, DM duration since diagnosis, blood glucose, glycated hemoglobin (HbA1c) levels, and hypertension were examined with one-way ANOVAs or logistic regression.
The effect of group (i.e., DPN, DM, and control subjects) on global and regional GM volumes was examined with ANCOVAs. Models were adjusted for age and sex. Because each volumetric (i.e., global plus nine regional GM volumes) was analyzed with a separate model, a Bonferroni adjustment of P < 0.005 was used to determine significance. Tukey’s post hoc testing was used to analyze group differences within significant models. The effects of variables related to DM and DPN severity (i.e., DM duration, fasting blood glucose, HbA1c, Toronto Clinical Neuropathy Score) on GM volumes were also explored.
Linear regression analyses were used to assess the effects of GM volume, group, and their interactions on walking outcomes (i.e., walking speed, stride duration variability, double support). Models were adjusted for age, sex, and body mass. Because separate models were used to examine each GM volumetric, a Bonferroni adjustment of P < 0.005 was again used to determine significance. For those models with a significant group effect, Tukey’s post hoc testing was used to analyze the group difference in the walking outcome. For those models with a significant group by volumetric interaction, contrast tests were used to examine group differences in the relationship between the GM volumetric and walking outcome. The relationships between walking outcomes and variables related to DM and DPN severity (i.e., DM duration, fasting blood glucose, HbA1c, Toronto Clinical Neuropathy Score) and symptoms (i.e., pain) were also examined using linear models.
RESULTS
Participants
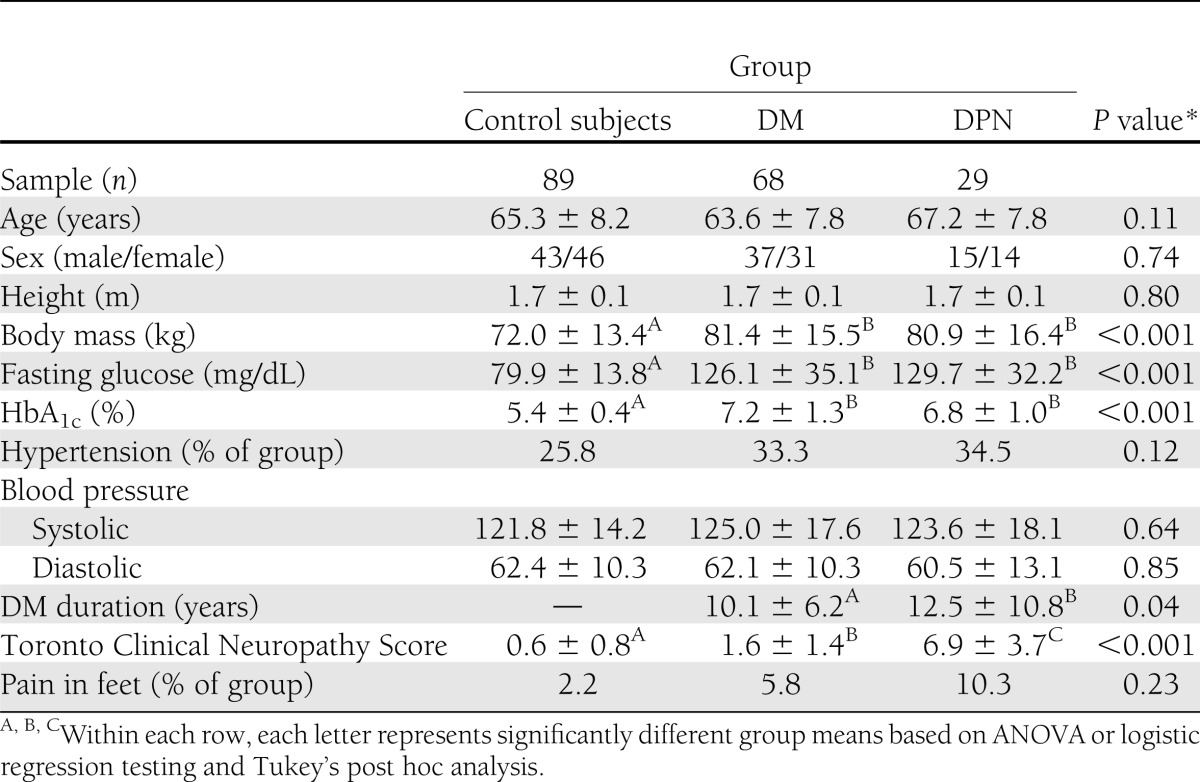
Subject demographics were similar among groups (Table 1). The DPN and DM groups had greater body mass, blood glucose, and HbA1c as compared with control subjects, but did not differ from each other. Diabetes duration was longer in the DPN group as compared with the DM group (12.5 ± 10.8 vs. 10.1 ± 6.2 years; ANOVA: P = 0.04). Based on our classification criterion for peripheral neuropathy, all DPN subjects were unable to detect the 5.07-gauge monofilament on the plantar surface of at least one hallux, whereas all DM and control subjects were able to detect the monofilament on both halluces. The Toronto Clinical Neuropathy Score was greatest in the DPN group, lower in the DM group, and lowest in control subjects (ANOVA: P < 0.001). The percentage of subjects with self-reported foot pain did not differ between groups.
Table 1.
Group demographics and health characteristics (mean ± SD)
GM volumetrics
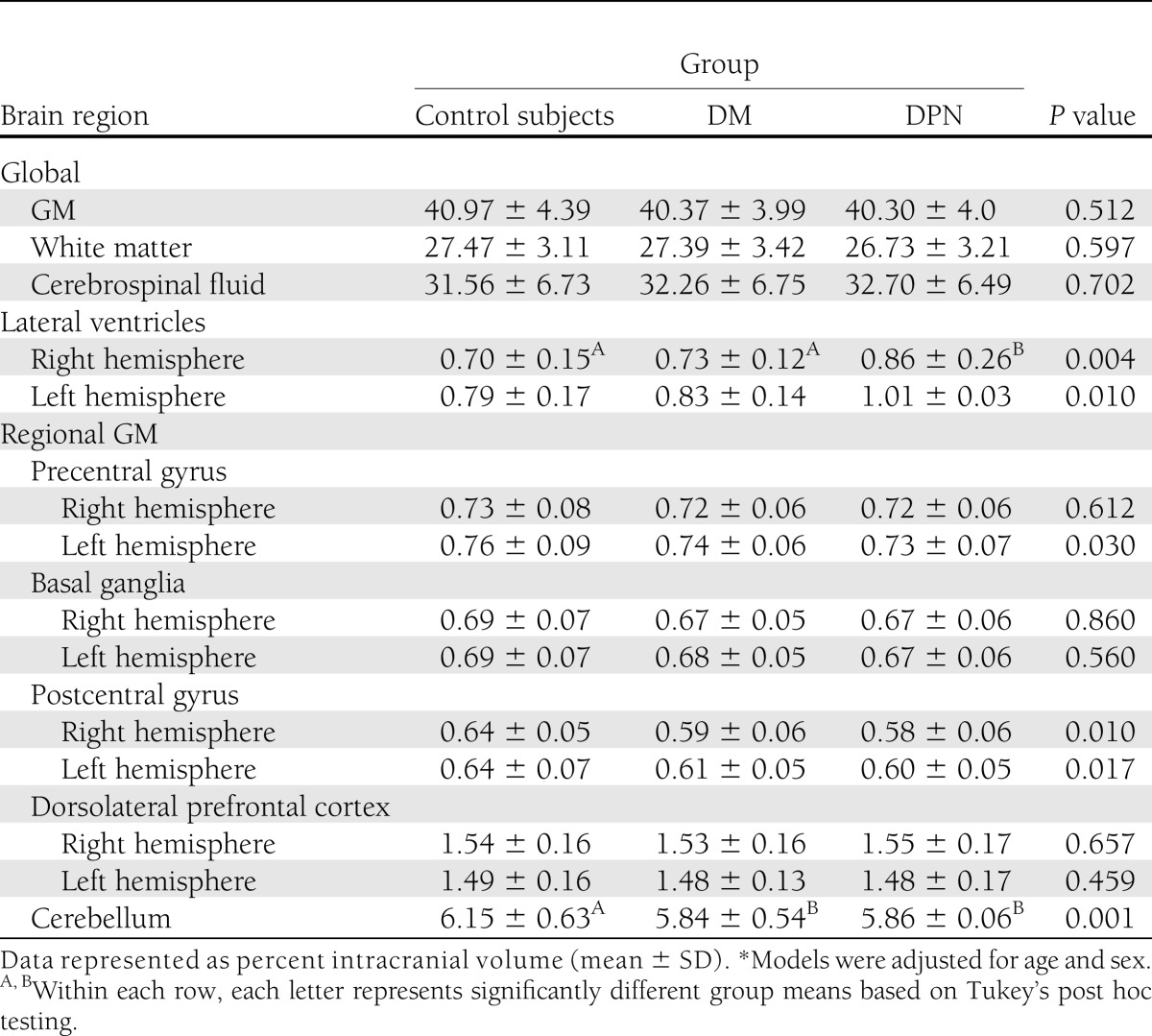
Global GM volume did not differ across the groups (Table 2). As compared with control subjects, the DPN and DM groups had lower GM volume within the cerebellum (F = 4.2, P = 0.001). GM volume within each hemisphere of the postcentral gyrus was also reduced in the DPN and DM groups (P = 0.01), but this difference did not achieve significance. Neither global nor regional GM volumes were significantly associated with DM duration, fasting blood glucose, HbA1c, or the Toronto Clinical Neuropathy Score.
Table 2.
Brain volumetrics
Global white matter and cerebrospinal fluid volumes were similar between the groups. The DPN group had larger lateral ventricle volumes as compared with the control group (F = 5.3, P = 0.005) (Table 2).
Effects of DPN on walking
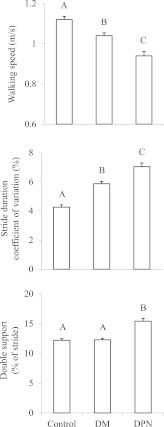
The DPN group walked more slowly with greater stride duration variability than the DM group, which in turn walked more slowly with greater stride duration variability than the control group (walking speed: F = 5.3, P = 0.005; stride duration variability: F = 25.6, P < 0.001) (Fig. 1). The DPN group spent more time in double support as compared with both the DM and control groups (F = 17.0, P < 0.0001).
Figure 1.
The effects of DPN on walking outcomes (means ± SE). For each metric, bars with different A, B, and C symbols reflect significantly different group means based on Tukey’s post hoc testing of adjusted models. The DPN group walked more slowly, demonstrated greater stride duration variability, and spent more time in double support as compared with the DM and/or control groups.
Across all subjects, those with a greater Toronto Clinical Neuropathy Score walked more slowly (r2 = 0.21, P = 0.003) with longer double support (r2 = 0.18, P = 0.01). Walking outcomes were not related to pain, DM duration, fasting glucose, or HbA1c. Those who walked more slowly tended to spend more time in double support (r2 = 0.12, P = 0.04).
Relationship between GM volume and walking outcomes
Global GM volume.
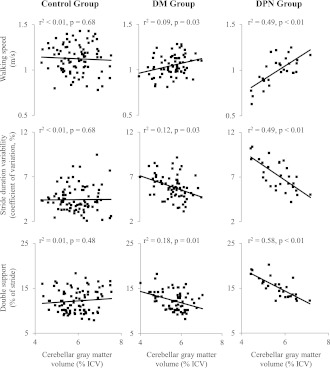
Less global GM volume was related to worse walking outcomes in DM patients, and especially in those with DPN. Specifically, linear models adjusted for age, sex, and body mass revealed group differences in the relationship between global GM volume and each walking outcome, i.e., groups by GM volume interactions were observed for walking speed (F = 14.3, P < 0.001), stride duration variability (F = 5.7, P = 0.004), and double support (F = 7.2, P = 0.001) (Fig. 2). Diabetic subjects with smaller global GM volume walked more slowly, demonstrated greater stride duration variability, and spent more time in double support. These relationships were most prominent in the DPN group (P = 0.008). In contrast, global GM volume was not related to any of the walking outcomes in the control group.
Figure 2.
The effects of DPN and DM on the relationship between walking outcomes and global GM volume. Pairwise r2 and P values are listed above each scatter plot. GM volumes were normalized to intracranial volumes (ICV). Linear regression models adjusted for age, sex, and body mass revealed that the relationships between global GM volume and each walking outcome were significantly stronger within the DPN group as compared with the DM group, and within the DM group as compared with control subjects.
Regional GM volumes.
The relationships between cerebellar GM volume and walking outcomes were also strongest within the DPN group. Similar to global GM volume, linear models adjusted for age, sex, and body mass revealed group differences in the relationship between cerebellum GM volume and each walking outcome, i.e., groups by GM volume interactions were observed for walking speed (F = 8.5, P = 0.002), stride duration variability (F = 6.8, P = 0.003), and double support (F = 6.5, P = 0.004). In both the DPN and DM groups, smaller cerebellum volume was associated with slower walking speed, greater stride duration variability, and longer double support. Each relationship was stronger in the DPN than the DM group (P = 0.01). Within the control group, GM volume within this region was not related to any walking outcome.
In the DPN group, GM volume within the dorsolateral prefrontal cortex and basal ganglia was also associated with walking outcomes. Less GM volume within the right dorsolateral prefrontal cortex associated with slower walking speed (F = 6.7, P = 0.005), and less GM volume within the right basal ganglia associated with increased stride duration variability (F = 6.7, P = 0.005). In contrast, GM volumes in these regions were not related to any walking outcome in either the DM or control groups.
GM volumes within the other analyzed regions (i.e., the left dorsolateral prefrontal cortex, the right or left pre/postcentral gyri, the left basal ganglia) were not related to walking outcomes in any group.
CONCLUSIONS
Subjects with DPN walked more slowly, demonstrated greater variability in stride duration, and spent longer time in double support as compared with both age-matched control subjects and DM patients without foot-sole somatosensory impairment. Within the DPN group, abnormalities in walking patterns were strongly associated with GM volume. Those with smaller global GM volume walked more slowly with greater stride duration variability and longer double support. Less GM volume within the cerebellum, as well as within the right hemisphere of the dorsolateral prefrontal cortex and basal ganglia, was also associated with worse performance in one or more walking outcome within the DPN group. In control subjects, on the other hand, neither global nor regional GM volumes were associated with any of the measured walking outcomes.
Older adults with slower, more variable walking patterns are at greater risk of future functional decline (20) and falls (6). In DPN patients, such alterations have been attributed to peripheral nerve deterioration and related sensorimotor complications (3,21–26). In the current study, diabetes was associated with less GM volume only within the cerebellum. Walking outcomes, however, were strongly related to cortical GM volumes both globally and across multiple sensorimotor regions within this population. In fact, in those DPN subjects with relatively large GM volumes, walking outcomes were comparable with many of their healthy age-matched counterparts (e.g., Fig. 2). This observation suggests that intersubject differences in cortical GM volume are in part responsible for the high interindividual variance in walking outcomes often observed in the DPN population. Although further longitudinal studies are needed, the current study highlights the notion that DPN-related walking disturbances may not arise solely as a result of peripheral nerve impairment, but rather stem from the interplay between the peripheral and central sensorimotor systems.
In contrast with the strong relationships observed between GM volumes and walking outcomes in the DPN group, and to a lesser extent the DM group, neither global nor regional GM volumes related to any walking outcome within the group of age-matched control subjects. Together, these observations suggest that in the presence of impairment to one or more elements of the motor control system, balance may become more dependent upon cortical GM volume. This observation is supported by our previous report of poststroke standing postural sway, i.e., tissue volumes within unaffected brain regions were closely related to the magnitude of postural sway in patients with chronic cerebral infarction, but not in age-matched control subjects (9). Still, because both that study and the current study were based on relationships among brain structure and behavioral outcomes, research using functional neuroimaging during standing and walking is warranted to determine whether the relationships between the characteristics of cortical activity and behavioral outcomes also differ between healthy and diseased older adults.
In the DPN group, GM volumes within regions linked to motor control (i.e., the cerebellum and right basal ganglia) as well as cognitive processing and executive function (i.e., the right dorsolateral prefrontal cortex) were related to one or more walking outcome. First, less GM within the cerebellum was associated with worse performance in each walking outcome, and less GM within the right basal ganglia was specifically associated with increased stride duration variability. These results are supported by a large body of evidence implicating both regions in the control of motor control and balance (12,13,27–30) and the basal ganglia in particular with the timing of motor output (12,31). On the other hand, Rosano et al. (19) reported that GM volume within these regions did not predict step length or double support time in a large sample of older adults. That study, however, was population based and did not examine relationships separately in those individuals with and without somatosensory impairment. Second, within the DPN group only, less GM volume within the right dorsolateral prefrontal cortex related to slower walking speed. This observation is supported by Rosano et al. (19), who reported a positive relationship between GM volume in this region and several outcomes related to walking speed in relatively healthy older adults. Because the dorsolateral prefrontal cortex supports executive function and attentional processes (32), the link between this region and walking may indicate that DPN patients require greater higher-order cognitive processing to control walking speed. This premise is supported by the observation that performing a concurrent cognitive task induces greater walking disturbances in DPN patients as compared with their healthy counterparts (33).
DPN subjects were diagnosed with type 2 diabetes more than two years longer, on average, than DM subjects. Type 2 diabetes severity (i.e., HbA1c and fasting glucose) was similar between groups, however, and measures of diabetes duration and severity were not related to any walking outcome. The group difference in diabetes duration therefore did not likely influence relationships between brain volumes and walking outcomes. Although foot pain affects balance (34), relatively few subjects reported this symptom and it did not relate to walking outcomes in this cohort. Regions-of-interest were selected based on known contributions to sensory, motor, and/or cognitive function. GM volume within other regions may also relate to walking outcomes in older adults with or without DPN and should be explored. Finally, studies examining cortical activity during walking in older adults with and without DPN will provide additional insight into the changing role of supraspinal elements in the control of walking in older adults with DPN and related foot-sole somatosensory impairment.
Acknowledgments
This work was conducted with the support of a KL2 Medical Research Investigator Training (MeRIT) award (1KL2RR025757-04) from Harvard Catalyst, the National Institutes of Health (R01-NS045745, AG023480), the American Diabetes Association (1-06-CR-25), and The Harvard Clinical and Translational Science Center (UL 1RR025757).
No potential conflicts of interest relevant to this article were reported.
B.M. collected data, performed statistical analyses, and wrote the manuscript. E.N. oversaw statistical analyses and contributed to manuscript preparation. A.A. analyzed imaging data and contributed to manuscript preparation. V.N. designed the study and oversaw all aspects of the study conduct and manuscript preparation. B.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 17th Annual Meeting of the Organization for Human Brain Mapping, Québec City, Canada, 26–30 June 2011.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
References
- 1.Candrilli SD, Davis KL, Kan HJ, Lucero MA, Rousculp MD. Prevalence and the associated burden of illness of symptoms of diabetic peripheral neuropathy and diabetic retinopathy. J Diabetes Complications 2007;21:306–314 [DOI] [PubMed] [Google Scholar]
- 2.Gregg EW, Sorlie P, Paulose-Ram R, et al. 1999-2000 national health and nutrition examination survey Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care 2004;27:1591–1597 [DOI] [PubMed] [Google Scholar]
- 3.Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil 2004;85:245–252 [DOI] [PubMed] [Google Scholar]
- 4.Manor B, Wolenski P, Li L. Faster walking speeds increase local instability among people with peripheral neuropathy. J Biomech 2008;41:2787–2792 [DOI] [PubMed] [Google Scholar]
- 5.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011;305:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009;13:881–889 [DOI] [PubMed] [Google Scholar]
- 7.Black FO, Paloski WH, Doxey-Gasway DD, Reschke MF. Vestibular plasticity following orbital spaceflight: recovery from postflight postural instability. Acta Otolaryngol Suppl 1995;520:450–454 [DOI] [PubMed] [Google Scholar]
- 8.Kloter E, Wirz M, Dietz V. Locomotion in stroke subjects: interactions between unaffected and affected sides. Brain 2011;134:721–731 [DOI] [PubMed] [Google Scholar]
- 9.Manor B, Hu K, Zhao P, et al. Altered control of postural sway following cerebral infarction: a cross-sectional analysis. Neurology 2010;74:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez NI, Rama JI, Martinez Vila E. Vision preference in dynamic posturography analysed according to vestibular impairment and handicap. Rev Laryngol Otol Rhinol (Bord) 2004;125:215–221 [PubMed] [Google Scholar]
- 11.Manor B, Costa MD, Hu K, et al. Physiological complexity and system adaptability: evidence from postural control dynamics of older adults. J Appl Physiol 2010;109:1786–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2007;62:1048–1055 [DOI] [PubMed] [Google Scholar]
- 13.Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology 2007;29:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Schlösser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg 2009;50:675–682 [DOI] [PubMed] [Google Scholar]
- 15.Kamei N, Yamane K, Nakanishi S, et al. Effectiveness of Semmes-Weinstein monofilament examination for diabetic peripheral neuropathy screening. J Diabetes Complications 2005;19:47–53 [DOI] [PubMed] [Google Scholar]
- 16.Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care 2002;25:2048–2052 [DOI] [PubMed] [Google Scholar]
- 17.Shattuck DW, Mirza M, Adisetiyo V, et al. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage 2008;39:1064–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson RR, Guttmann CR, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology 2002;58:48–55 [DOI] [PubMed] [Google Scholar]
- 19.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci 2008;63:1380–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc 2011;59:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: the Health, Aging, and Body Composition (Health ABC) study. Diabetes Care 2008;31:1767–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giacomozzi C, Caselli A, Macellari V, Giurato L, Lardieri L, Uccioli L. Walking strategy in diabetic patients with peripheral neuropathy. Diabetes Care 2002;25:1451–1457 [DOI] [PubMed] [Google Scholar]
- 23.Manor B, Li L. Characteristics of functional gait among people with and without peripheral neuropathy. Gait Posture 2009;30:253–256 [DOI] [PubMed] [Google Scholar]
- 24.Richardson JK, Ashton-Miller JA. Peripheral neuropathy: an often-overlooked cause of falls in the elderly. Postgrad Med 1996;99:161–172 [PubMed] [Google Scholar]
- 25.Richardson JK, Thies SB, DeMott TK, Ashton-Miller JA. A comparison of gait characteristics between older women with and without peripheral neuropathy in standard and challenging environments. J Am Geriatr Soc 2004;52:1532–1537 [DOI] [PubMed] [Google Scholar]
- 26.Volpato S, Blaum C, Resnick H, Ferrucci L, Fried LP, Guralnik JM, Women’s Health and Aging Study Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women’s Health and Aging Study. Diabetes Care 2002;25:678–683 [DOI] [PubMed] [Google Scholar]
- 27.Chastan N, Westby GW, Yelnik J, et al. Effects of nigral stimulation on locomotion and postural stability in patients with Parkinson’s disease. Brain 2009;132:172–184 [DOI] [PubMed] [Google Scholar]
- 28.Ilg W, Giese MA, Gizewski ER, Schoch B, Timmann D. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain 2008;131:2913–2927 [DOI] [PubMed] [Google Scholar]
- 29.Takezawa N, Mizuno T, Seo K, Kondo M, Nakagawa M. Gait disturbances related to dysfunction of the cerebral cortex and basal ganglia. Brain Nerve 2010;62:1193–1202 [in Japanese] [PubMed] [Google Scholar]
- 30.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord 2008;23:329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crenna P, Carpinella I, Lopiano L, et al. Influence of basal ganglia on upper limb locomotor synergies. Evidence from deep brain stimulation and L-DOPA treatment in Parkinson’s disease. Brain 2008;131:3410–3420 [DOI] [PubMed] [Google Scholar]
- 32.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev 2002;9:637–671 [DOI] [PubMed] [Google Scholar]
- 33.Paul L, Ellis BM, Leese GP, McFadyen AK, McMurray B. The effect of a cognitive or motor task on gait parameters of diabetic patients, with and without neuropathy. Diabet Med 2009;26:234–239 [DOI] [PubMed] [Google Scholar]
- 34.Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA 2009;302:2214–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]