Abstract
OBJECTIVE
To determine how much physical activity (PA) is needed to protect against diabetes onset in older adults, whether protection is greater among overweight individuals, and whether taking up moderate activity in later life is beneficial.
RESEARCH DESIGN AND METHODS
Men (4,252) from a U.K. population-based cohort self-reported usual PA (regular walking and cycling, recreational activity, and sport) in 1996 and in 1998–2000, alongside other health behaviors and medical history. Fasting blood lipids were measured. Median follow-up was 7.1 years, during which 135 cases of type 2 diabetes (validated self-report) occurred.
RESULTS
Among 3,012 men free from cardiovascular disease and diabetes in 1998–2000, 9% reported no usual leisure-time PA, 23% occasional PA, and 15% vigorous PA. Compared with men reporting no activity, men reporting occasional, light, moderate, moderately vigorous, and vigorous PA had lower diabetes risks: hazard ratio (HR) 0.58 (95% CI 0.33–1.02), 0.39 (0.20–0.74), 0.38 (0.19–0.73), 0.39 (0.20–0.77), and 0.33 (0.16–0.70), respectively; P (trend) = 0.002, adjusted for age, social class, tobacco, alcohol, diet, and blood lipids. Adjustment for BMI, waist circumference, or fasting insulin attenuated HRs. HRs were stronger in men with BMI ≥28 vs. <28 kg/m2 (interaction P = 0.02). Compared with men reporting light activity or less in 1996 and 2000, men who became at least moderately active by 2000 or remained at least moderately active at both times had adjusted HRs of 0.62 (0.34–1.12) and 0.51 (0.31–0.82), respectively.
CONCLUSIONS
Even light PA markedly reduced diabetes risk in older men, especially among the overweight or obese. Taking up or maintaining at least moderate PA in older adulthood strongly protected against diabetes.
The prevalence of type 2 diabetes has increased markedly in high-income countries, resulting in high burdens of disease and related disability. The risks of type 2 diabetes are particularly high in older people, and as populations are aging (1), prevention of type 2 diabetes in older adults is a key priority.
In middle age, physical activity (PA) provides important protection against the onset of type 2 diabetes; a recent meta-analysis reported that even moderate-intensity leisure-time activity or brisk walking reduced diabetes incidence by ∼30% (2). However, the levels of PA required to protect against the onset of diabetes in older people (many of whom have low fitness levels or functional limitations) are much less established. The protection provided by PA may be stronger in older (>60 years) than younger adults; large intervention trials conducted in high-risk population groups, which compare medication with lifestyle changes, reported greater relative and absolute benefits from lifestyle changes in the older than in the younger age-groups (3,4). Yet, to date, few epidemiologic studies have examined associations between PA in later life and diabetes onset, but evidence suggests that even occasional activity in older adults compared with sedentariness is associated with ∼50% lower diabetes risks and also 28% lower total mortality risks (5,6). No epidemiologic studies have been performed on the influence of changes in PA in later life on diabetes risk in healthy populations, although increases in PA levels in later life are associated with reduced total and cardiovascular mortality risks (7). A further important and unresolved question is whether obese adults, who are at high risk of diabetes, gain greater protection against type 2 diabetes from PA than their leaner counterparts. Although some studies of middle-aged subjects suggest this (8–10), not all have yielded consistent findings (11,12). We have therefore investigated the prospective relations between PA in later life and risk of incident diabetes to clarify what levels of activity are protective in older adults. We investigated the underlying pathways linking PA to diabetes (including the roles of waist circumference, BMI, and insulin) and the possibility that adiposity modifies the influence of PA. Finally, we examined whether changes in PA in later life (particularly the influence of remaining or becoming at least moderately active in later life) reduce risks of diabetes in a prospective study.
RESEARCH DESIGN AND METHODS
In 1998–2000 (Q20), a total of 4,252 men (aged 60–79 years) from a single general practice (primary care center) in each of 24 British towns who were already participating in a prospective study of cardiovascular disease (CVD) attended for re-examination (77% response rate) (13). All relevant local research ethics committees provided ethical approval. All men provided informed written consent to the investigation, which was performed in accordance with the Declaration of Helsinki. Participants completed questionnaires including detailed information about tobacco use, medical history, and occupation. They completed a detailed, validated, 7-day recall food frequency questionnaire including questions about alcohol, coffee, and sugar-sweetened beverage consumption. Nutrient intakes were calculated using a validated program that multiplied the food frequency by the standard portion sizes for each food and by the nutrient composition of the foods obtained from the U.K. food composition tables, as reported elsewhere (14). Nurses made anthropometric measurements, recorded blood pressure (BP), and took fasting blood samples that were analyzed for lipids and insulin; methods have been published elsewhere (15).
PA
In 1996 (Q96) and in 1998–2000 (Q20), men self-reported their usual pattern of PA under the headings of regular walking or cycling, recreational activity, and sporting (vigorous) activity. Regular walking and cycling related to weekday journeys that included travel to and from work. Recreational activity included gardening, pleasure walking, and do-it-yourself jobs. Sporting activity included running, golf, swimming, tennis, sailing, and digging. A PA score was derived for each man on the basis of frequency and type (intensity) of the PA. Scores were assigned for each type of activity and duration on the basis of the intensity and energy demands of the activities reported based on Minnesota intensity codes. The total score for each man is not a measure of total time spent in PA but is a relative measure of how much PA has been carried out. Men were categorized into six groups based on their total score: 1) inactive (score 0–2); 2) occasional (score 3–5; regular walking or recreational activity only); 3) light (score 6–8; more frequent recreational activities or sporting exercise less than once per week, or regular walking plus some recreational activity); 4) moderate (score 9–12; cycling or very frequent weekend recreational activities plus regular walking or sporting activity once per week); 5) moderately vigorous (score 13–20; sporting activity at least once per week, frequent cycling plus frequent recreational activities or walking, or frequent sporting activities only); and 6) vigorous (score >21; very frequent sporting exercise or frequent sporting exercise plus other recreational activities). The PA score has been validated in relation to heart rate and FEV1 (forced expiratory volume in 1 second) (16,17).
Type 2 diabetes
Incident type 2 diabetes cases after the Q20 examination were defined using 1) a record of diagnosis of diabetes in the two-yearly reviews of the patients’ notes (including details of patient encounters with primary care and all correspondence and diagnoses from secondary care) through to June 2006, or 2) self-reported data from one of the questionnaires completed after the Q20 examination comprising a doctor’s diagnosis of diabetes or new use of oral medications for diabetes (British National Formulary codes 06.01.02) (18). The medical-record review was compared with the patient's recall of doctor-diagnosed diabetes. In 97% of men who reported a doctor diagnosis of diabetes on the questionnaire, the diagnosis was confirmed by record review. Because of the high agreement, self-report of diabetes has been included. Participants (99%) were followed-up for incident type 2 diabetes from the Q20 examination to 1 June 2006 (median 7.1 years). Fatal cases were ascertained through the established tagging procedures provided by the National Health Service central registers (death certificates with ICD-9 code 250 or ICD-10 codes E10–E14 for diabetes).
Preexisting diabetes and CVD
Prevalent type 2 diabetes cases were defined using self-report of doctor diagnosis, medical record (see below), or fasting glucose ≥7 mmol/L (World Health Organization criteria) at the Q20 examination. Preexisting, physician-diagnosed coronary heart disease (CHD), stroke, or diabetes was identified from questionnaire data and general practitioner (GP) records as men who 1) reported that a doctor had ever told them that they had angina or myocardial infarction (MI) (heart attack or coronary thrombosis), stroke, “other heart trouble,” or diabetes at Q20, or 2) had a major, nonfatal MI or stroke event before then or had been diagnosed with diabetes, based on the regular surveillance of GP’s records. Men with preexisting, physician-diagnosed CHD, stroke, or diabetes were excluded from analysis, to reduce the risks of reverse causality.
Statistical methods
Means, medians, or proportions of behavioral and demographic factors selected a priori were calculated for men according to their activity level. Linear trends across activity categories were tested using linear regression analyses. Skewed variables (triglycerides) were natural log–transformed and adjusted for time of measurement if they showed significant diurnal variation. Systolic and diastolic BP, BMI, and waist circumference were also adjusted for interobserver variation.
Cox proportional hazards regression models were used to estimate associations between activity level and risk of diabetes in men without preexisting CHD and diabetes, with complete data on covariates. Survival times were censored at date of diabetes, death from any cause, or end of follow-up period, whichever occurred first. Date of entry into the study was used as the time origin. The hazard ratios (HRs) for each category of PA compared with the least-active group were estimated, and the overall association between PA and diabetes was adjusted for age (continuous variable) and region of residence. Models were then adjusted for covariates associated with both diabetes risk and PA, first established sociodemographic and behavioral confounders, and then diet and blood lipids. Further adjustments for BMI, waist circumference, and serum insulin were made separately, as these were expected to be on the causal pathway (further details in the Supplementary Methods). In a sensitivity analysis, regression models excluded the most active men (to see if the vigorous activity levels were driving any associations). We investigated interactions between PA and 1) BMI, categorized as <28 and ≥28 kg/m2, and 2) age (continuous variable). Interactions were evaluated using likelihood ratio (LR) tests. We investigated the changes in levels of PA between Q96 and Q20. In order to capture a true change in behavior, we compared the lowest three categories of PA against the highest three because this cut point required men to perform some kind of sporting exercise less than once per week in addition to the walking plus some recreational activity required in the bottom two categories.
RESULTS
Among 4,097 participants with complete PA data (96% of those attending the Q20 rescreen), 1,140 participants with a history (self-report of physician diagnosis or medical record) of MI, stroke, or diabetes were excluded, leaving 3,012 men for inclusion in the main analysis. Their mean age at the start of follow-up (Q20) in 1998–2000 was 68.3 years. During a median 7.1 years of follow-up (20,023 person-years), 135 new cases of diabetes occurred (6.7 per 1,000 person-years of follow-up).
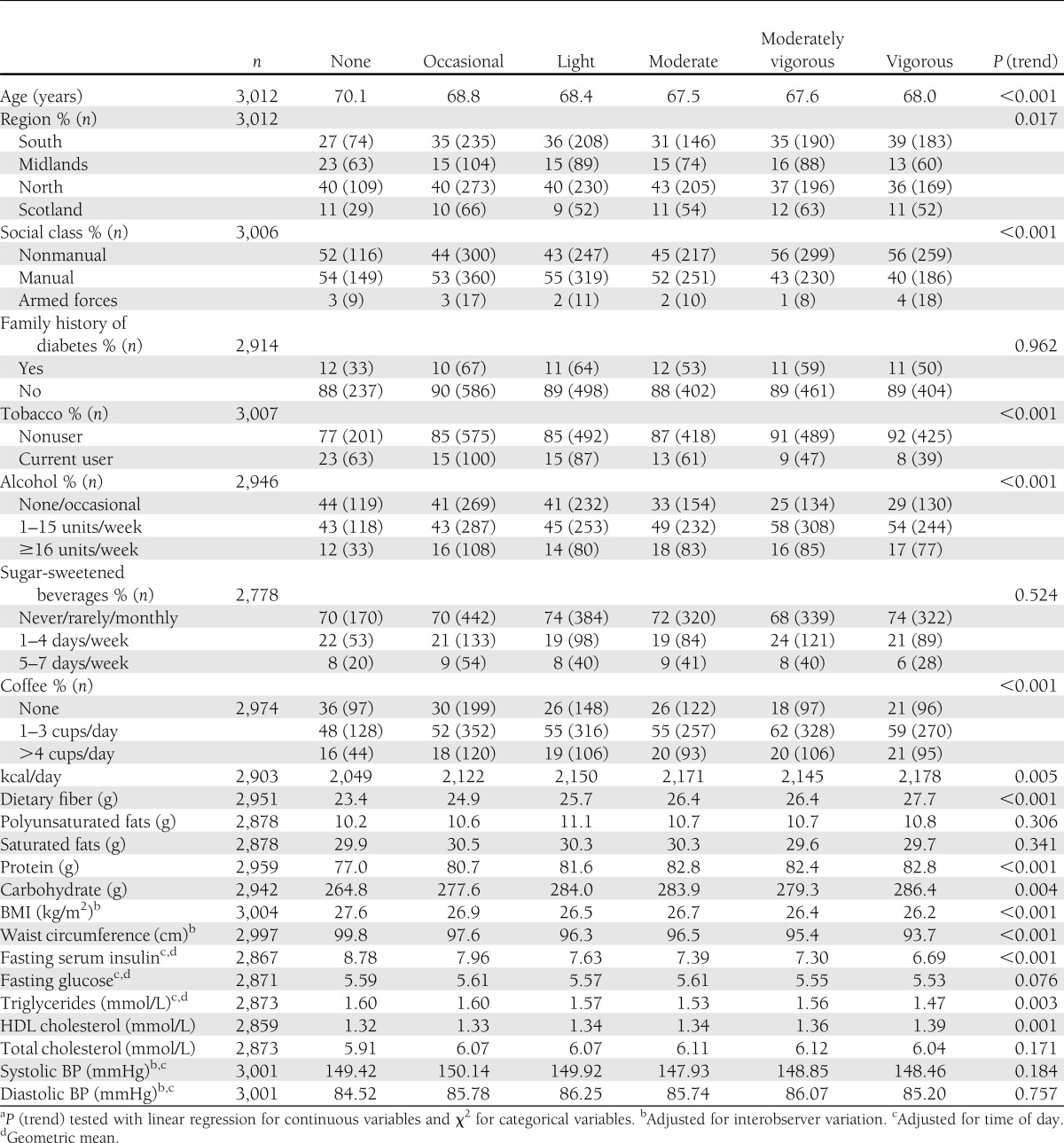
Table 1 shows the characteristics of men according to their usual PA level: 9% (n = 275) were classified as performing no usual leisure-time PA, 23% (n = 678) were classified as performing occasional activity, which was the most common group, 19% (n = 579) were in the light activity group, 16% (n = 479) were in the moderate activity group, 18% (n = 537) were in the moderately vigorous activity group, and 15% (n = 464) were in the highest vigorous activity group. Overall, 49% of men in the analysis sample were classified as performing moderate activity or more. Men who were more active were more likely to be from nonmanual social classes, to smoke, to be non/occasional or heavier alcohol drinkers, and to drink coffee. More active men consumed more kilocalories, fiber, protein, and carbohydrates per day, had lower mean BMI, waist circumference, fasting triglycerides, serum insulin and glucose, and higher HDL cholesterol. There was no evidence that usual PA level was associated with systolic or diastolic BP, family history of diabetes, or consumption of fats or sugar-sweetened beverages.
Table 1.
Characteristics of men according to PA score (n = 3,012 men without preexisting CHD, stroke, or diabetes)
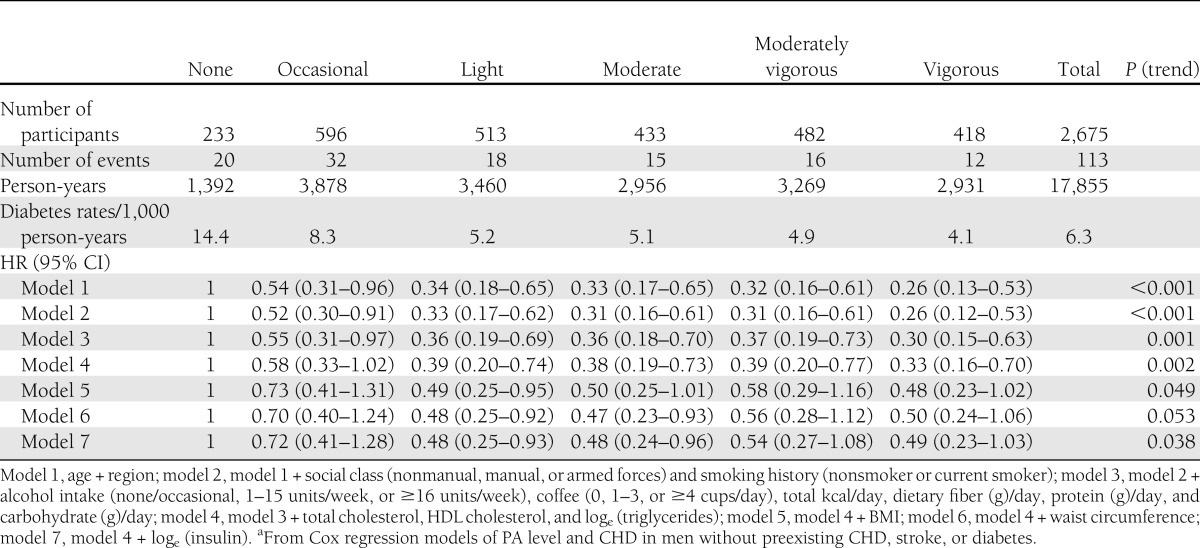
Table 2 shows the rates and HRs for type 2 diabetes by PA level; compared with men reporting no usual leisure-time PA, men reporting higher activity levels had significantly reduced HRs for diabetes, with evidence for a dose-response association, and there was a significant linear trend across all categories (model 1, P < 0.001). Adjustment for alcohol and tobacco use, social class, dietary factors, BP, and lipids did little to change the HRs or trends; P (trend) = 0.002. Further separate adjustments for BMI, waist circumference, or serum insulin attenuated the estimates, although a clear protective effect of increasing PA levels was still observed. Additional adjustment for serum insulin to the model with BMI (model 4 + insulin) or waist circumference (model 5 + insulin) attenuated the estimates further. In separate sensitivity analyses, conclusions were not altered when 1) the “vigorous activity” group was excluded or 2) the first two years of follow-up were excluded.
Table 2.
HRs for risk of type 2 diabetes in men by PA scorea
Association between PA and diabetes: influence of BMI?
The modifying effect of adiposity on the association between PA and diabetes was tested using a cut point of BMI ≥28 kg/m2. The prevalence of BMI ≥28 kg/m2 increased from 20% among very vigorously active men up to 46% of men reporting no usual PA; P (trend) < 0.001. There was evidence for an interaction between PA at Q20 low active (light, occasional, or no habitual PA) versus moderate or more active and BMI (cut point BMI of 28); P (LR test) = 0.02 (model 1). Among men with BMI <28 kg/m2 (n = 1,851; 48 cases of diabetes), compared with low-active men, those reporting moderate, moderately vigorous, or vigorous activity had an adjusted (model 4) HR for diabetes of 1.12 (95% CI 0.62–1.99). The equivalent estimate for men with BMI ≥28 kg/m2 (n = 817; 65 cases of diabetes) was 0.43 (0.24–0.75). The absolute rates (95% CI) per 1,000 person-years for men with BMI <28 kg/m2 were 3.64 (2.44–5.43) for low active (light, occasional, or no habitual PA) and 3.72 (2.55–5.42) for moderate or more active. Equivalent rates for men with BMI ≥28 kg/m2 were 18.31 (14.21–25.58) and 8.45 (5.66–12.60).
Within the 60- to 79-year age-group, we did not find evidence for interactions between PA and age; P (LR test) = 0.7 for model 1.
Change in PA level
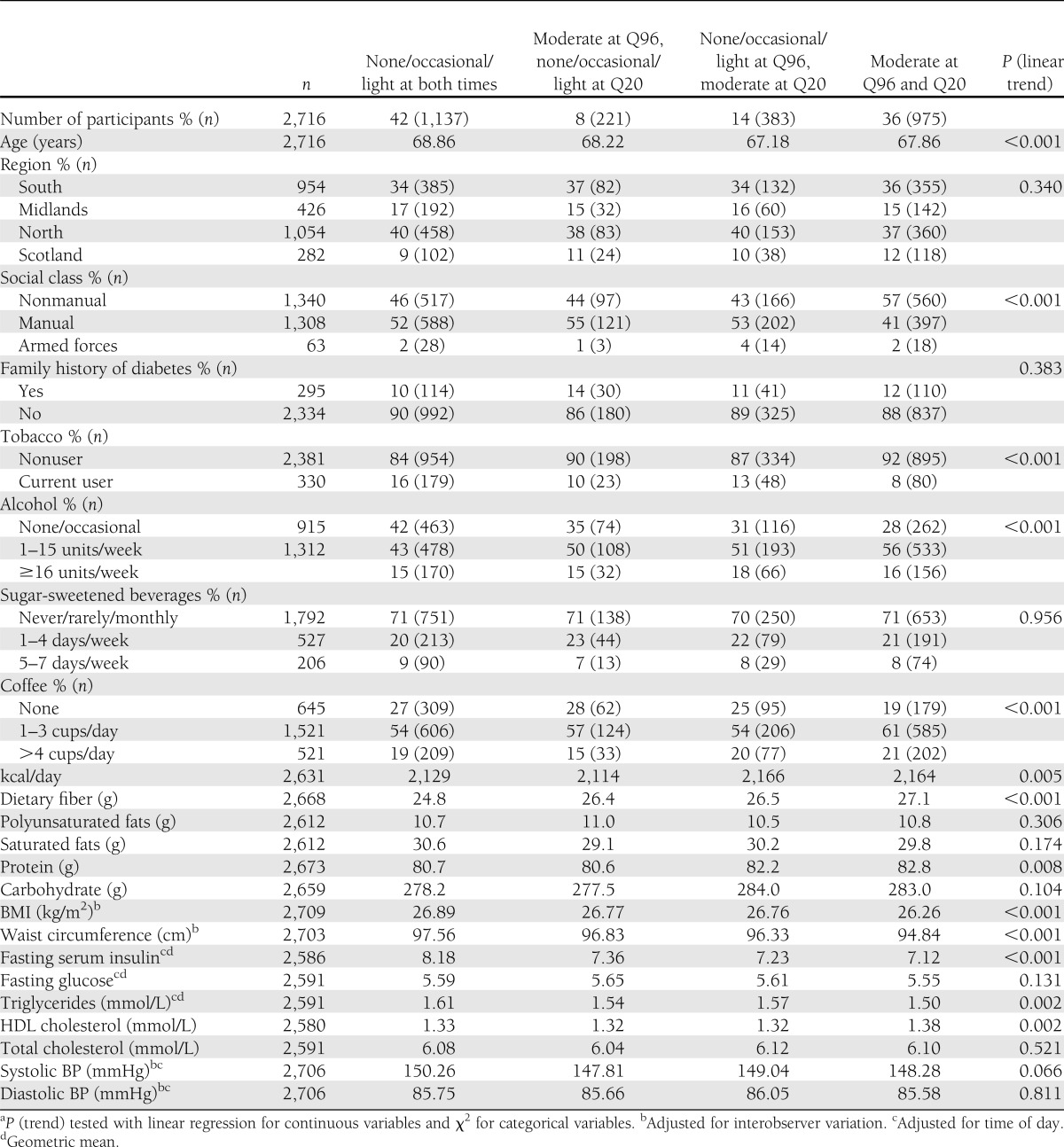
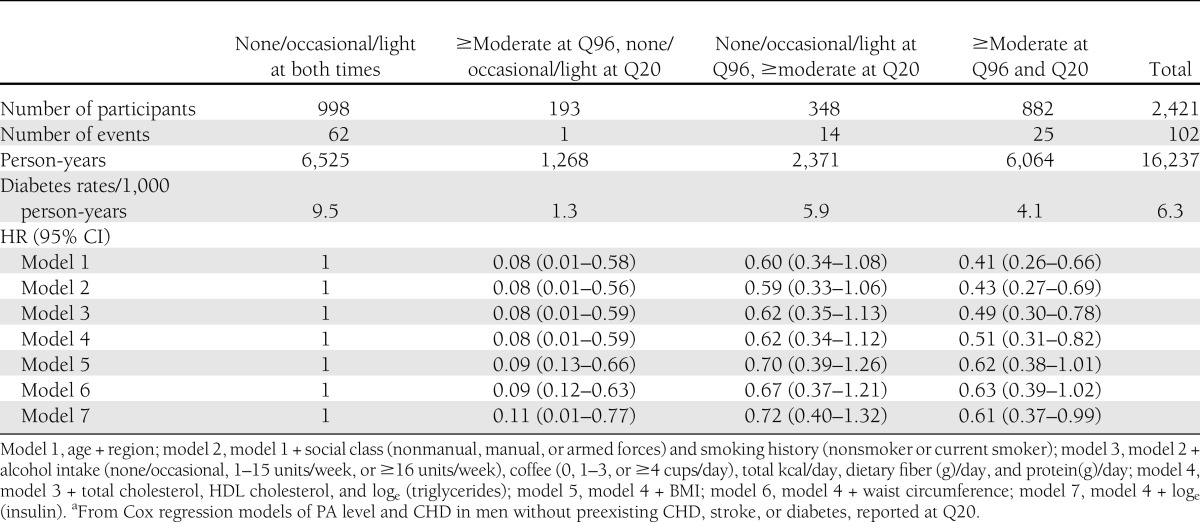
Forty-two percent of men were low active (light, occasional, or no habitual PA) at both Q96 and Q20, 14% took up at least moderate activity (moderate or more vigorous usual PA level), and 36% maintained at least moderate activity at both times (Table 3). Compared with the men who remained low active, the men who took up or maintained moderate activity were younger and more likely to be from the south of England or Scotland, from the nonmanual class, and regular drinkers and nonsmokers. The moderately active men had lower BMI, waist circumference, serum insulin, and triglycerides and higher HDL cholesterol. Men who were low active at Q96 but became at least moderately active at Q20 had lower risk of diabetes than men who were low active at both times; the adjusted HR (model 4) was 0.62 (95% CI 0.34–1.12), which was did not significantly change on further adjustment for BMI, waist circumference, and insulin level (Table 4). There was only one case of diabetes among men who became less active at Q20; thus, effect estimates have wide CIs. However, the adjusted HR (model 4) was 0.51 (0.31–0.82) for men reporting at least moderate PA at both Q96 and Q20 compared with men reporting light activity or less at both times. Additional adjustment for BMI, waist circumference, and serum insulin attenuated estimates.
Table 3.
Associations between PA score and demographic variables in men without preexisting CHD, stroke, or diabetes (n = 2,716)
Table 4.
HRs for risk of type 2 diabetes in men by change in PA score between 1996 and 2000a
CONCLUSIONS
Among older men, even low levels of habitual PA more than halved the risk of new-onset diabetes, and the protective effects were greater at higher PA levels. Established risk factors (blood lipids, socioeconomic position, diet, and alcohol and tobacco use) did not account for much of the associations but, as expected, adiposity and insulin levels were important mediators. Novel findings among these older men were, firstly, that the protective effects of PA were most pronounced among the men at higher risk of diabetes from being overweight or obese (with BMI >28). Secondly, taking up at least moderate habitual activity levels in later life or remaining consistently active compared with remaining sedentary or doing only light activities over a 4-year period each offered an ∼40% reduction in risk of diabetes. Although intervention studies of behavioral changes have focused on high-risk groups, to our knowledge, this is the first population-based, epidemiologic study including healthy lower-risk adults to study changes in activity levels in older adults in relation to diabetes onset.
Strengths and weaknesses
This cohort study benefits from prospective data with a PA score validated against other measures (e.g., FEV1 and heart rate) (16,17). Diabetes cases were validated by GP notes, medication use, and self-report, but as oral glucose tolerance tests were not available at baseline and follow-up, it is possible that we missed some cases of asymptomatic diabetes. The repeated data on PA in later life enabled us to examine the importance of changes in PA on the incidence of diabetes. Importantly, we were able to include information about a wide range of social and behavioral confounders, and we studied the role of biological factors that may be on the causal pathway between PA and diabetes risk. To reduce the risks of reverse causality, men with preexisting CHD, stroke, or diabetes were excluded because they have increased mortality risks and may be limited in their physical activities due to their health. Our study is limited in examining only men, so we cannot generalize results to older women. Future studies could investigate sedentary behavior and distinguish between aerobic and resistance training, which we could not.
Effect size and shape of the dose-response curve
We found evidence for a dose-response association with higher usual PA levels associated with increased protection against the onset of type 2 diabetes, in line with expectations from limited data on older adults and a larger body of evidence from middle-aged adults (8,10,19,20). The incremental benefits were greatest at the transition from occasional activity (risk reduction of 45%) to light activity (risk reduction of 65%), and more intense activity was associated with even greater benefits. Adjustment for BMI attenuated the risk reduction associated with light or moderate usual PA levels from nearly two-thirds to about one-half of the risk compared with inactive adults. These estimates suggest greater protective effects than were estimated for moderate activity in a meta-analysis reporting reductions in risk of ∼30%, reducing to 20% on adjustment for BMI (2). However most of the participants included in the meta-analysis were in middle rather than older age. Two other studies with data on older adults, reported 50 and ∼40% reductions in risk of onset of type 2 diabetes (5,21). Data from large trials comparing medication to PA and other lifestyle changes, including diet and weight loss, reported a greater protective effect of PA in individuals >60 years of age than those at younger ages (3,4).
Mediators
As expected, we found that adiposity mediated the effects of PA on type 2 diabetes risk; separate adjustments for BMI and waist circumference somewhat attenuated associations, but the trends with increasing levels of activity remained. This fits with data from other studies (2), suggesting that PA may affect diabetes risk separately from just its effect on weight loss and reduction in adipose tissue. We also observed that PA and change in PA levels were associated with components of the metabolic syndrome. Lower PA levels at Q20 were associated with insulin resistance, for which insulin levels have been used as a marker. Men who were consistently inactive at Q96 and Q20 also had much higher insulin levels than men who became or remained at least moderately active. Insulin was a dominant mediator of the effects of PA on type 2 diabetes risk, as previously demonstrated in middle age (20). In line with data from intervention studies (22), we hypothesized that part of the effect of activity on diabetes would operate through reductions in LDL cholesterol and triglyceride levels and increases in HDL cholesterol. We observed clear associations between PA and these lipids, as was observed in middle age (20). Although we cannot entirely exclude the possibility of residual confounding in our results, we report results effect estimates adjusted for a wide range of important social and behavioral factors; few other studies adjust associations for social position.
Effect modifiers
Overweight or obese men (with BMI >28) were strongly protected against the onset of diabetes by higher PA levels (indicated by the HRs), whereas there was little evidence of a protective effect among leaner men. However, the absolute rates of diabetes incidence were much higher in the active men with BMI >28 than in the active or inactive leaner men, highlighting the importance of high BMI in the onset of diabetes. Our findings fit with other studies that find an enhanced protective effect of PA in high-risk individuals (8–10), although not all studies have found this to be the case (11,12,21). In line with another study, we did not find any evidence that the association between PA and risk of diabetes varied by age within the range of 60–79 years (21).
Change in activity levels
Large-scale intervention studies have found that multiple lifestyle changes (weight loss, exercise, and dietary changes) are highly effective in preventing diabetes in high-risk adults (23–25). Post hoc analyses reported clear benefits of increases in low-, moderate-, and high-intensity leisure-time activity on risk of diabetes onset (26). Epidemiologic studies of general populations have reported protective effects of increases in PA levels over time on total mortality or on CHD events in older adults (7,27), including in this cohort when participants were middle aged (28). Associations between changes in PA patterns in older adults and diabetes onset have not been thoroughly studied, so our study importantly extends current literature. It has been reported that PA in middle age is associated with metabolic syndrome and diabetes nearly three decades later (29), but changes in PA during the intervening period were not accounted for, so conclusions cannot be drawn about changes in activity in later life. In observational studies of changes in PA in younger adults, persistently active adults are reported to have lower risks of metabolic syndrome than their inactive counterparts (30). We found that compared with older men remaining at low or light activity levels on both occasions 4 years apart, men who were more active on both occasions or who took up at least moderate PA levels had about half the risk of onset of diabetes. In order to be classified as at least moderately active on our score, men had to report walking, recreational activities, and some kind of additional sporting activity (e.g., heavy gardening or do-it-yourself jobs) regularly, but less than weekly. Hence, men able to do some kind of moderate activity on a regular basis had substantially reduced risks of diabetes. This fits with a previous report about changes in activity levels in this cohort; the prevalence of metabolic syndrome at Q20 was much lower in the men who took up or maintained activity compared with men who remained sedentary (31). The results also fit with data suggesting that the protective effects of PA in terms of physical adaptation to exercise are quickly lost when activity levels are reduced (32). However we had insufficient data to draw firm conclusions about the effects of becoming less active.
Among contemporary older men free from existing diabetes and CVD, higher PA levels were associated with reductions in risk of diabetes, with greatest benefits occurring above moderate activity levels. However, even low or modest levels of PA, which are attainable by older adults, were associated with reductions in diabetes risks. Obese or overweight adults who are at raised risk of diabetes may benefit most from PA, even at low levels; thus, prevention may be especially important and effective in this group. Given that sustained levels of moderate or more intense activity was strongly protective and that taking up moderate activity also conferred substantially reduced risks of onset, adults who are free from existing disease but at high risk of diabetes should be encouraged to maintain their PA levels and, if appropriate, slowly increase their activity levels to moderate levels.
Acknowledgments
A.G. Shaper established the British Regional Heart Study, which is a British Heart Foundation Research Group (program grant RG/08/013/25942). B.J.J. is supported by a National Institute for Health Research postdoctoral fellowship. This study contains independent research arising from a postdoctoral fellowship.
The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health Research, the Department of Health, or the British Heart Foundation.
No potential conflicts of interest relevant to this article were reported.
B.J.J. researched data, wrote the manuscript, and contributed to discussion. P.H.W. contributed to discussion and reviewed and edited the manuscript. L.L. researched data. S.G.W. researched data, contributed to discussion, and reviewed and edited the manuscript. B.J.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2280/-/DC1.
References
- 1.Office for National Statistics. Population estimates for UK, England and Wales, Scotland and Northern Ireland - current datasets. Mid 2007 UK Wales Scotland and Northern Ireland [Internet], 2008. Available from http://www.statistics.gov.uk/statbase/Product.asp?vlnk=15106 Accessed 11 December 2008
- 2.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007;30:744–752 [DOI] [PubMed] [Google Scholar]
- 3.Lindström J, Peltonen M, Eriksson JG, et al. Finnish Diabetes Prevention Study (DPS) Group Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care 2008;31:857–862 [DOI] [PubMed] [Google Scholar]
- 4.Crandall J, Schade D, Ma Y, et al. Diabetes Prevention Program Research Group The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci 2006;61:1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demakakos P, Hamer M, Stamatakis E, Steptoe A. Low-intensity physical activity is associated with reduced risk of incident type 2 diabetes in older adults: evidence from the English Longitudinal Study of Ageing. Diabetologia 2010;53:1877–1885 [DOI] [PubMed] [Google Scholar]
- 6.Sundquist K, Qvist J, Sundquist J, Johansson SE. Frequent and occasional physical activity in the elderly: a 12-year follow-up study of mortality. Am J Prev Med 2004;27:22–27 [DOI] [PubMed] [Google Scholar]
- 7.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med 1993;328:538–545 [DOI] [PubMed] [Google Scholar]
- 8.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA 1992;268:63–67 [PubMed] [Google Scholar]
- 9.Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS., Jr Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 1991;325:147–152 [DOI] [PubMed] [Google Scholar]
- 10.Hu G, Lindström J, Valle TT, et al. Physical activity, body mass index, and risk of type 2 diabetes in patients with normal or impaired glucose regulation. Arch Intern Med 2004;164:892–896 [DOI] [PubMed] [Google Scholar]
- 11.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 12.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 13.Walker M, Shaper AG, Lennon L, Whincup PH. Twenty year follow-up of a cohort based in general practices in 24 British towns. J Public Health Med 2000;22:479–485 [DOI] [PubMed] [Google Scholar]
- 14.Wannamethee SG, Whincup PH, Thomas MC, Sattar N. Associations between dietary fiber and inflammation, hepatic function, and risk of type 2 diabetes in older men: potential mechanisms for the benefits of fiber on diabetes risk. Diabetes Care 2009;32:1823–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emberson JR, Whincup PH, Morris RW, Walker M, Lowe GD, Rumley A. Extent of regression dilution for established and novel coronary risk factors: results from the British Regional Heart Study. Eur J Cardiovasc Prev Rehabil 2004;11:125–134 [DOI] [PubMed] [Google Scholar]
- 16.Shaper AG, Wannamethee G, Weatherall R. Physical activity and ischaemic heart disease in middle-aged British men. Br Heart J 1991;66:384–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wannamethee SG, Lowe GD, Whincup PH, Rumley A, Walker M, Lennon L. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation 2002;105:1785–1790 [DOI] [PubMed] [Google Scholar]
- 18.Joint Formulary Committee British National Formulary. London, British Medical Association and Royal Pharmaceutical Society of Great Britain, 1999 [Google Scholar]
- 19.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol 2005;99:1193–1204 [DOI] [PubMed] [Google Scholar]
- 20.Wannamethee SG, Shaper AG, Alberti KG. Physical activity, metabolic factors, and the incidence of coronary heart disease and type 2 diabetes. Arch Intern Med 2000;160:2108–2116 [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the Cardiovascular Health Study. Arch Intern Med 2009;169:798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindström J, Louheranta A, Mannelin M, et al. Finnish Diabetes Prevention Study Group The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–3236 [DOI] [PubMed] [Google Scholar]
- 23.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 24.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 26.Laaksonen DE, Lindström J, Lakka TA, et al. Finnish diabetes prevention study Physical activity in the prevention of type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes 2005;54:158–165 [DOI] [PubMed] [Google Scholar]
- 27.Talbot LA, Morrell CH, Fleg JL, Metter EJ. Changes in leisure time physical activity and risk of all-cause mortality in men and women: the Baltimore Longitudinal Study of Aging. Prev Med 2007;45:169–176 [DOI] [PubMed] [Google Scholar]
- 28.Wannamethee SG, Shaper AG, Walker M. Changes in physical activity, mortality, and incidence of coronary heart disease in older men. Lancet 1998;351:1603–1608 [DOI] [PubMed] [Google Scholar]
- 29.Holme I, Tonstad S, Sogaard AJ, Larsen PG, Haheim LL. Leisure time physical activity in middle age predicts the metabolic syndrome in old age: results of a 28-year follow-up of men in the Oslo study. BMC Public Health 2007;7:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Telama R, Hirvensalo M, Mattsson N, Viikari JS, Raitakari OT. The longitudinal effects of physical activity history on metabolic syndrome. Med Sci Sports Exerc 2008;40:1424–1431 [DOI] [PubMed] [Google Scholar]
- 31.Wannamethee SG, Shaper AG, Whincup PH. Modifiable lifestyle factors and the metabolic syndrome in older men: effects of lifestyle changes. J Am Geriatr Soc 2006;54:1909–1914 [DOI] [PubMed] [Google Scholar]
- 32.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009;41:1510–1530 [DOI] [PubMed] [Google Scholar]