Abstract
OBJECTIVE
In adults, the shape of the glucose response during an oral glucose tolerance test (OGTT) prospectively and independently predicts type 2 diabetes. However, no reports have described the utility of this indicator in younger populations. The purpose of this study was to compare type 2 diabetes risk factors in Latino adolescents characterized by either a monophasic or biphasic glucose response during an OGTT.
RESEARCH DESIGN AND METHODS
A total of 156 nondiabetic Latino adolescents completed a 2-h OGTT. Monophasic and biphasic groups were compared for the following type 2 diabetes risk factors: fasting and 2-h glucose, HbA1c, glucose area under the curve (AUC), insulin sensitivity (Matsuda index), insulin secretion (insulinogenic index), and β-cell function as measured by the disposition index (insulin sensitivity × insulin secretion).
RESULTS
Of the participants, 107 youth were categorized as monophasic and 49 were biphasic. Compared with the monophasic group, participants with a biphasic response exhibited lower HbA1c (5.4 ± 0.3 vs. 5.6 ± 0.3%, P < 0.01) and lower glucose AUC (14,205 ± 2,382 vs. 16,230 ± 2,537 mg ⋅ dL−1 ⋅ h−1, P < 0.001) with higher insulin sensitivity (5.4 ± 3.2 vs. 4.6 ± 3.4, P ≤ 0.05), higher insulin secretion (2.1 ± 1.3 vs. 1.8 ± 1.3, P = 0.05), and better β-cell function (10.3 ± 7.8 vs. 6.0 ± 3.6, P < 0.001). Differences persisted after adjusting for age, sex, and BMI.
CONCLUSIONS
These data suggest that the glycemic response to an OGTT may differentiate risk for type 2 diabetes in youth. This response may be an early marker of type 2 diabetes risk among high-risk youth.
In parallel with the current pediatric obesity epidemic, type 2 diabetes has emerged as a critical health concern among obese adolescents (1,2). Although type 1 diabetes is more prevalent in the pediatric population, data from the SEARCH for Diabetes in Youth Study highlight a disproportionate distribution of type 2 diabetes among certain subpopulations of adolescents (3). It is notable that for Hispanic females aged 15–19 years, the incidence of type 2 diabetes exceeds that of type 1 diabetes (4).
An important issue for the medical and research communities is to identify Latino youth at increased risk for premature type 2 diabetes so that appropriate prevention strategies may be initiated. In 1997, the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (5) introduced impaired fasting glucose and impaired glucose tolerance (IGT) as intermediate stages in the natural history of type 2 diabetes. In adults, prediabetes precedes frank type 2 diabetes by 5–10 years (6,7); however, similar data are limited in younger populations. Weiss et al. (8) have noted that obese youth with IGT decompensate to frank type 2 diabetes over a mean follow-up of 20 months. These data support the potential for a rapid progression to overt type 2 diabetes in youth, which may be exacerbated by pubertal insulin resistance (9,10). In contrast, Goran et al. (11) have shown that obese Latino youth may vacillate between normal glucose tolerance (NGT) and IGT over time. Therefore, in addition to prediabetes, other markers of type 2 diabetes risk may be necessary.
Several recent studies in adults use the shape of the glucose curve during an oral glucose tolerance test (OGTT) to identify metabolic dysregulation and the potential risk for future type 2 diabetes (12–17). Using a simple shape index, individuals with a monophasic response (inverted U shape) during an OGTT exhibit greater insulin resistance and decreased β-cell function compared with individuals with a biphasic response (a second rise of plasma glucose after first decline). A recent prospective study demonstrates that independent of fasting and/or postchallenge glucose concentrations, individuals with a monophasic response developed type 2 diabetes at a higher rate than those with a biphasic response (15). To our knowledge, whether the shape of the glucose response curve is associated with type 2 diabetes risk in younger populations has not been determined. Therefore, the purpose of this study was to compare diabetes risk factors in Latino youth characterized by either a monophasic or biphasic glucose response during a 2-h OGTT.
RESEARCH DESIGN AND METHODS
Data from 156 nondiabetic Latino adolescents (aged 12–21 years) who participated in a community-based diabetes registry were used in the present analysis. Participants arrived at the Arizona State University Clinical Research Unit after an overnight fast. Anthropometric measurements included height, weight and BMI, waist and hip circumference, and seated blood pressure. A blood sample (∼20 mL) was taken under fasting conditions to measure HbA1c and lipid profile, including total cholesterol, triglyceride, HDL, LDL, and VLDL. All laboratory tests were performed by a Clinical Laboratory Improvement Amendments–certified commercial laboratory (Sonora Quest Laboratories, Phoenix, AZ).
OGTT
Participants underwent a 2-h OGTT following a 10-h overnight fast. Subjects ingested a solution containing 75 g dextrose (1.75 g/kg), and venous blood samples were obtained at 0, 30, 60, 90, and 120 min for determination of plasma glucose and insulin concentrations. Plasma glucose was measured by the glucose oxidase method using a YSI 2300 STAT plus (YSI, Inc., Yellow Springs, OH), and insulin was measured in duplicate by ELISA (ALPCO Diagnostics, Windham, NH).
Classification of response curve
Glucose response phenotype (i.e., monophasic or biphasic) was classified according to previous studies (13–16), with a glucose threshold of 4.5 mg/dL as described by Tschritter et al. (13) to minimize fluctuations in glucose concentrations that may be caused by the method of glucose analysis rather than physiological reasons. A monophasic response was characterized by a gradual rise in plasma glucose concentrations until a peak was reached followed by a subsequent decrease until 120 min. A biphasic response was characterized by a gradual rise in glucose, followed by a ≥4.5 mg/dL fall, with a second rise of glucose of at least 4.5 mg/dL at a subsequent time point. Participants who exhibited a gradual increase in plasma glucose after glucose ingestion without a corresponding fall were deemed “unclassified” (n = 2) and were excluded for the present analysis (13).
Variables and calculation
Type 2 diabetes risk factors included fasting plasma glucose and insulin, 2-h plasma glucose and insulin, HbA1c, and glucose and insulin area under the curve (AUC). Total AUC for plasma glucose and insulin during the OGTT were calculated by the trapezoidal method using 30-min sampling time points (18). In addition to these indicators, insulin action was estimated by the homeostasis model assessment (HOMA) for insulin resistance (HOMA-IR) (19) and the whole-body insulin sensitivity index of Matsuda and DeFronzo (20), and insulin secretion was estimated by the insulinogenic index calculated using fasting and 30-min insulin and glucose concentrations (21). β-Cell function was estimated by the disposition index as the product of insulin action Matsuda index (20) and insulin secretion insulinogenic index (21).
Statistical analysis
Independent sample t tests and χ2 analyses were used to compare characteristics between glucose phenotypes. Two-way repeated-measures ANOVA was used to assess differences in the glucose and insulin levels at each time point during the OGTT. Analysis of covariance was used to compare phenotypes after adjusting for the potential confounding effects of age, sex, and BMI on type 2 diabetes risk factors. Data that did not meet the assumptions for normality (glucose values at 30 and 90 min and insulin values at each time point from the OGTT, HbA1c, and all indices for insulin sensitivity, secretion, and β-cell function) were log10 transformed; untransformed data are presented for ease of interpretation. Data were analyzed using PASW 18.0 statistical software package with significance set at P ≤ 0.05.
RESULTS
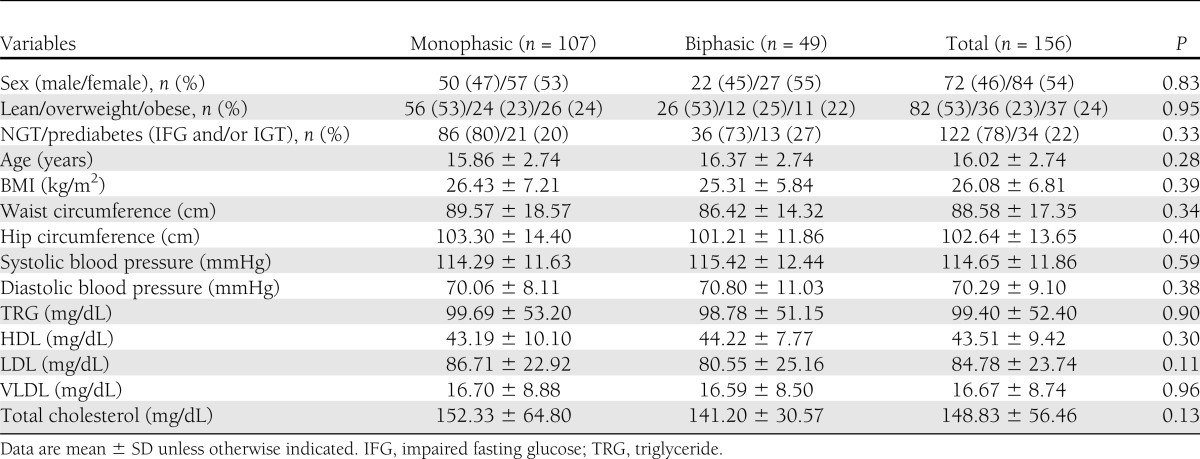
Descriptive characteristics of participants are presented in Table 1. No differences in sex, BMI categories (lean vs. overweight vs. obese), or glycemic status (NGT vs. prediabetes) were noted between glucose phenotypes. In addition, no significant differences were noted for age, anthropometrics (BMI and waist and hip circumference), lipids, or blood pressure.
Table 1.
Descriptive characteristics of participants by phenotype
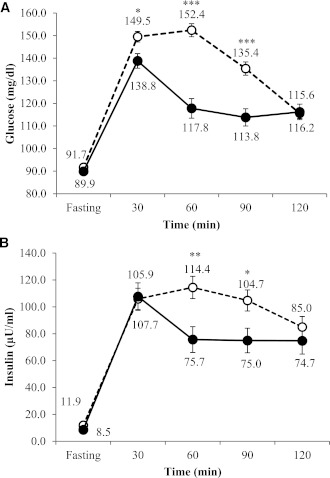
Two-way repeated-measures ANOVA for plasma glucose concentrations during the OGTT demonstrated significant effects for group and time as well as group × time interaction, indicating differences between groups over the course of the OGTT (all P < 0.0001). Glucose and insulin concentrations for each OGTT time point are presented in Fig. 1. Within each glucose phenotype, all time points across the OGTT (i.e., 0, 30, 60, 90, and 120) were significantly different from each other (P < 0.0001). The monophasic group exhibited significantly higher blood glucose levels at 30, 60, and 90 min compared with the biphasic group, while no differences were noted for either fasting or 2-h glucose concentrations between groups. In terms of insulin response during the OGTT, there were significant effects for time (P < 0.0001) but not for group. The monophasic group had significantly higher insulin values at 60 and 90 min compared with the biphasic group.
Figure 1.
Glucose (A) and insulin (B) response curves during OGTT in monophasic (white circle and dashed line) and biphasic (black circle and solid line) groups. *P < 0.05, **P < 0.01, ***P < 0.001.
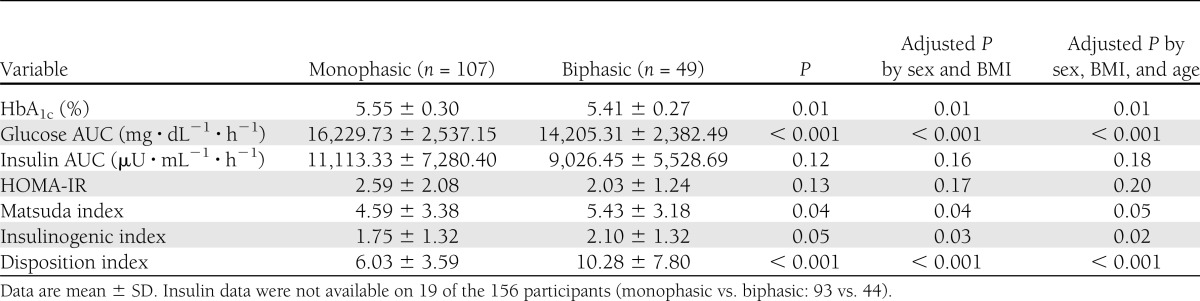
Measures of glycemia are presented in Table 2. Participants with a monophasic response exhibited slightly but significantly higher HbA1c than biphasic participants, and these differences remained significant after adjusting for sex, BMI, and age. Glucose AUC in the monophasic group was 14.3% higher than in the biphasic group, and these differences were independent of sex, age, or BMI (Table 2).
Table 2.
Measures of insulin and glucose homeostasis and β-cell function
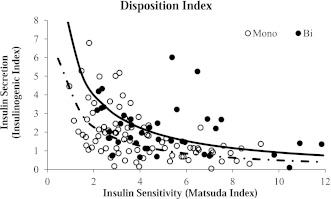
Insulin measures are presented in Table 2. No significant differences between groups were noted for insulin AUC or HOMA-IR. However, insulin sensitivity as measured by the Matsuda index and insulin secretion as measured by the insulinogenic index were both significantly higher in youth exhibiting the biphasic phenotype. β-Cell function as measured by the disposition index was 42% higher in the biphasic group, and this difference remained significant after adjusting for covariates. Figure 2 displays the hyperbolic relationship between insulin sensitivity and insulin secretion for each group using the product of the Matsuda index and insulinogenic index. The best-fit line derived from the individual points of the monophasic group is shifted toward the origin (down and to the left) compared with the biphasic group (Fig. 2).
Figure 2.
Hyperbolic relationship between insulin sensitivity and insulin secretion in monophasic (white circle and dashed line) and biphasic (black circle and solid line) groups.
CONCLUSIONS
In the current study, we demonstrated that the shape of the plasma glucose response during an OGTT differentiates diabetes risk factors in Latino adolescents. Participants with a biphasic response exhibited lower glucose AUC and HbA1c, higher whole-body insulin sensitivity (Matsuda index) and insulin secretion, and better β-cell function compared with individuals with a monophasic response. These data extend previous studies in adults and suggest that the glucose response curve may be an early indicator of type 2 diabetes risk in adolescents.
Studies in adults have established that the shape of the glucose curve is related to both type 2 diabetes risk factors (12–14,16,17) and the development of type 2 diabetes (15). Tschritter et al. (13) studied glucose curves from 551 nondiabetic Caucasian adults and found that the biphasic response was associated with lower BMI, younger age, and higher insulin sensitivity and disposition index. The authors also reported that females and individuals with NGT were more likely to be characterized by a biphasic response. These findings were confirmed and expanded upon by Tura et al. (17) who used glucose excursions during 3-h OGTTs from nearly 600 Austrian women screened for gestational diabetes. The authors noted that a 3-h OGTT captured even greater variations in glucose response, with some individuals exhibiting up to five phases. Greater complexity of the glucose curve (i.e., increasing number of phases) was associated with a healthier metabolic profile as indicated by higher insulin sensitivity and β-cell function as well as a lower prevalence of prediabetes and type 2 diabetes. These cross-sectional studies were confirmed prospectively where prediabetic adults with a monophasic glucose response during an OGTT exhibited nearly double the risk of developing type 2 diabetes during a 7- to 8-year follow-up compared with prediabetic subjects with a biphasic response (15). These studies suggest that the biphasic phenotype is associated with lower risk of type 2 diabetes potentially as a result of higher insulin sensitivity and better β-cell function.
In adults, insulin resistance and insulin secretory dysfunction are independently and interactively related to type 2 diabetes risk (22–24). Specifically, the inability of the β-cell to compensate for insulin resistance is a primary determinant of type 2 diabetes (22,25). Compared with what is known in adults, the natural history of type 2 diabetes in youth is less well understood. Recent studies support β-cell dysfunction as a key feature of type 2 diabetes in adolescents (26,27). Not only does β-cell dysfunction contribute to type 2 diabetes in adolescents but it also was recently noted that Latino children and adolescents with prediabetes exhibit significantly lower β-cell function compared with their normoglycemic peers (28,29). These data suggest that β-cell dysfunction contributes to prediabetes and type 2 diabetes in children and adolescents.
Taken together, our results extend findings on glucose response patterns in adults and type 2 diabetes pathophysiology in adults and youth to suggest that a monophasic glucose response may be associated with an increased risk for type 2 diabetes. This seemingly increased risk is evidenced by a lower disposition index that is due to significantly lower insulin sensitivity and secretion. When the disposition index of each group is plotted on the same graph (Fig. 2), the best-fit line representing the disposition index for the monophasic group is shifted closer to the origin (i.e., down and to the left) compared with that for the biphasic group. This shift is a hallmark feature of type 2 diabetes and is considered one of the earliest indicators of β-cell dysfunction (25). It is important to note that the lower disposition index among the monophasic group was independent of BMI and, therefore, may confer additional type 2 diabetes risk beyond that of obesity. Furthermore, despite the lower disposition index observed in the monophasic group, neither the levels of fasting and 2-h glucose nor the percentage of prediabetic subjects were significantly different between groups. When the dataset was restricted to only those participants with NGT, the disposition index in the monophasic group remained significantly lower than that of the biphasic group (P ≤ 0.05). Collectively, these findings suggest that the shape of the glucose response curve may be a very early marker of glucose dysregulation and type 2 diabetes risk and is detectable even before traditional indicators of hyperglycemia (30). Whether the shape of the glucose curve is similarly predictive of the development of type 2 diabetes as traditional diabetes risk factors is an important question that should be addressed in future studies.
The physiological mechanisms responsible for the various glucose response curves are poorly understood. Although we found lower β-cell function (lower insulin sensitivity and secretion) in the monophasic group, we do not know whether this represents a cause or an effect of the phenotype or whether there are common biologic or genetic pathways linking these phenotypic characteristics. It may be that higher insulin sensitivity and secretion contribute to the biphasic response through more efficient and faster glucose clearance compared with the monophasic response. It is also possible that the timing of the insulin response may contribute to differences in the shape of the glucose response curve. Therefore, we divided individuals into either early (30-min) or late (≥60-min) responders based on the timing of peak insulin concentrations. The biphasic group exhibited a higher percentage of “early responders” compared with the monophasic group (57 vs. 32%; P < 0.01); however, including insulin timing as a covariate in the final models did not change the results (data not shown). In addition, it is possible that an early return of plasma glucose concentrations toward baseline may stimulate a subtle counterregulatory response (14,16), leading to a second rise in plasma glucose at 60 or 90 min. Another possible explanation may be prolonged or delayed gastric emptying among monophasic individuals. Previous studies suggest that delayed gastric emptying is more common among adults with type 2 diabetes compared with control subjects, and prolonged gastric emptying is positively correlated with plasma glucose concentration (31). Among nondiabetic individuals, the incretin response following an oral glucose challenge is directly related to the rate of gastric emptying, which is inversely associated with postchallenge glucose and insulin concentrations (32). Taken together, it is possible that differences in gastric emptying as well as alterations in the incretin response may be associated with a monophasic glucose response and, ultimately, increases in type 2 diabetes risk with this phenotype.
To our knowledge, this is the first study to examine the shape of the glucose response curve in relation to type 2 diabetes risk among the pediatric population. We focused on Latino adolescents because this group represents a vulnerable population at increased risk for developing type 2 diabetes. We used defined glucose thresholds based on objective criteria to identify when and if more than one glucose peak was achieved. Very few researchers have published specific glucose thresholds for identifying differences between time points, and we believe using rigid criteria (i.e., a minimum of 4.5 mg/dL glucose excursion between a peak and a subsequent trough) rather than simply characterizing glucose curves through observation will minimize misclassification. When we analyzed our data using a previously published relative threshold of ≥2% difference between consecutive glucose time points (17), the overall results and interpretations were not affected. Despite these strengths, we acknowledge potential limitations in our data that should be considered.
First, we based our phenotype on the response to a single OGTT, which may have limited reproducibility in youth. Libman et al. (33) demonstrated poor reproducibility of the OGTT in overweight youth in terms of identifying hyperglycemia. Whether the shape of the glucose response is an inherent and, hence, reproducible biological process warrants further examination before using this assessment in longitudinal studies. In addition, our classification of glucose phenotype was derived from the 2-h OGTT. By using a longer OGTT (i.e., 3-h OGTT) and/or more frequent sampling intervals (i.e., every 10 min) it is possible to capture more sophisticated curve types that will provide greater information on type 2 diabetes risk (16,17). Second, family history of diabetes, exposure to gestational diabetes in utero, and pubertal stage were not available for our analysis. It is well established that family history of diabetes is a strong risk factor for type 2 diabetes in both adults and youth (34–36), and exposure to gestational diabetes in utero is a hypothesized risk factor for type 2 diabetes in youth (37). In addition, cross-sectional and longitudinal studies show that puberty is associated with insulin resistance, which may further contribute to type 2 diabetes risk (10,38,39). Although we did not assess pubertal status in the current study, we did attempt to minimize the confounding effects by adjusting for age. Nonetheless, age is not an ideal surrogate for pubertal stage, and we further acknowledge the relatively wide spectrum of age in our heterogeneous sample that should be addressed in future studies. Third, the utility of HbA1c as a potential type 2 diabetes risk factor in youth has not been well established (40). It is also not clear whether this result is of clinical significance because differences were subtle. Lastly, the cross-sectional nature of our study precludes the ability to draw causal inferences about the shape of the glucose curve and type 2 diabetes risk. Given that type 2 diabetes is a progressive, chronic disease and typically presents in adulthood, examining markers that may identify risk in younger cohorts can offer temporal insight into the pathophysiological mechanisms of diabetes. It is interesting to note that previous studies suggest that adults with a biphasic response are characterized by younger age compared with those with a monophasic response (12,13). In our cohort, >31% of the participants exhibited a biphasic response, which is higher than the percentages typically observed in adult cohorts (12–17).
In summary, the pattern of plasma glucose response during an OGTT may provide an early marker of type 2 diabetes risk in youth. We have demonstrated that participants with a biphasic response have significantly better β-cell function secondary to higher insulin sensitivity and secretion as well as lower glucose AUC and HbA1c. Moreover, our data suggest that the shape of the glucose curve may differentiate type 2 diabetes risk independent of obesity and before dysregulation of fasting or 2-h glucose. Longitudinal studies to investigate whether glucose response phenotypes prospectively predict the development of type 2 diabetes in younger populations are warranted.
Acknowledgments
This work was supported by Health Research Alliance Arizona and the Center for Metabolic Biology at Arizona State University. Data management support was provided by Grant UL1-RR-024150 from the Mayo Clinic to use Research Electronic Data Capture (REDCap).
No potential conflicts of interest relevant to this article were reported.
J.Y.K. researched and analyzed data and wrote the manuscript. D.K.C. and L.J.M. researched data and reviewed and edited the manuscript. G.Q.S. researched data and wrote, reviewed, and edited the manuscript. G.Q.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors are grateful to the children and their families who participated in this study. The authors thank Veronica Vital (Brookline College, Phoenix, AZ) and the staff of the Clinical Research Unit for their help with enrolling and testing participants.
References
- 1.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA 2002;288:1728–1732 [DOI] [PubMed] [Google Scholar]
- 2.Fagot-Campagna A. Emergence of type 2 diabetes mellitus in children: epidemiological evidence. J Pediatr Endocrinol Metab 2000;13(Suppl. 6):1395–1402 [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Bell RA, D’Agostino RB, Jr, et al. Writing Group for the SEARCH for Diabetes in Youth Study Group Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 4.Lawrence JM, Mayer-Davis EJ, Reynolds K, et al. SEARCH for Diabetes in Youth Study Group Diabetes in Hispanic American youth: prevalence, incidence, demographics, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care 2009;32(Suppl. 2):S123–S132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 6.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med 1988;319:1500–1506 [DOI] [PubMed] [Google Scholar]
- 8.Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care 2005;28:902–909 [DOI] [PubMed] [Google Scholar]
- 9.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care 2005;28:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes 2001;50:2444–2450 [DOI] [PubMed] [Google Scholar]
- 11.Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor beta-cell function, and increasing visceral fat. Diabetes 2008;57:3007–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchigami M, Nakano H, Oba K, Metori S. [Oral glucose tolerance test using a continuous blood sampling technique for analysis of the blood glucose curve]. Nihon Ronen Igakkai Zasshi 1994;31:518–524 [in Japanese] [DOI] [PubMed] [Google Scholar]
- 13.Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003;26:1026–1033 [DOI] [PubMed] [Google Scholar]
- 14.Kanauchi M, Kimura K, Kanauchi K, Saito Y. Beta-cell function and insulin sensitivity contribute to the shape of plasma glucose curve during an oral glucose tolerance test in non-diabetic individuals. Int J Clin Pract 2005;59:427–432 [DOI] [PubMed] [Google Scholar]
- 15.Abdul-Ghani MA, Lyssenko V, Tuomi T, Defronzo RA, Groop L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev 2010;26:280–286 [DOI] [PubMed] [Google Scholar]
- 16.Trujillo-Arriaga HM, Román-Ramos R. Fitting and evaluating the glucose curve during a quasi continuous sampled oral glucose tolerance test. Comput Biol Med 2008;38:185–195 [DOI] [PubMed] [Google Scholar]
- 17.Tura A, Morbiducci U, Sbrignadello S, Winhofer Y, Pacini G, Kautzky-Willer A. Shape of glucose, insulin, C-peptide curves during a 3-h oral glucose tolerance test: any relationship with the degree of glucose tolerance? Am J Physiol Regul Integr Comp Physiol 2011;300:R941–R948 [DOI] [PubMed] [Google Scholar]
- 18.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 21.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 1994;11:286–292 [DOI] [PubMed] [Google Scholar]
- 22.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin secretion and increased insulin resistance are independently related to the 7-year risk of NIDDM in Mexican-Americans. Diabetes 1995;44:1386–1391 [DOI] [PubMed] [Google Scholar]
- 24.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993;329:1988–1992 [DOI] [PubMed] [Google Scholar]
- 25.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 2002;51(Suppl. 1):S212–S220 [DOI] [PubMed] [Google Scholar]
- 26.Arslanian S. Type 2 diabetes in children: clinical aspects and risk factors. Horm Res 2002;57(Suppl. 1):19–28 [DOI] [PubMed] [Google Scholar]
- 27.Weiss R, Gillis D. Patho-physiology and dynamics of altered glucose metabolism in obese children and adolescents. Int J Pediatr Obes 2008;3(Suppl. 1):15–20 [DOI] [PubMed] [Google Scholar]
- 28.Goran MI, Bergman RN, Avila Q, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab 2004;89:207–212 [DOI] [PubMed] [Google Scholar]
- 29.Weigensberg MJ, Ball GD, Shaibi GQ, Cruz ML, Goran MI. Decreased beta-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care 2005;28:2519–2524 [DOI] [PubMed] [Google Scholar]
- 30.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 31.Horowitz M, Harding PE, Maddox AF, et al. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1989;32:151–159 [DOI] [PubMed] [Google Scholar]
- 32.Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993;36:857–862 [DOI] [PubMed] [Google Scholar]
- 33.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 2008;93:4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arslanian SA, Bacha F, Saad R, Gungor N. Family history of type 2 diabetes is associated with decreased insulin sensitivity and an impaired balance between insulin sensitivity and insulin secretion in white youth. Diabetes Care 2005;28:115–119 [DOI] [PubMed] [Google Scholar]
- 35.Kelly LA, Lane CJ, Weigensberg MJ, et al. Parental history and risk of type 2 diabetes in overweight Latino adolescents: a longitudinal analysis. Diabetes Care 2007;30:2700–2705 [DOI] [PubMed] [Google Scholar]
- 36.Kuo CK, Lin LY, Yu YH, Chang CH, Kuo HK. A family history of diabetes mellitus is associated with poor glycemic control and increased metabolic risks among people with diabetes: data from the National Health and Nutrition Examination Survey 1999-2004. Intern Med 2010;49:549–555 [DOI] [PubMed] [Google Scholar]
- 37.Fetita LS, Sobngwi E, Serradas P, Calvo F, Gautier JF. Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab 2006;91:3718–3724 [DOI] [PubMed] [Google Scholar]
- 38.Reinehr T, Wabitsch M, Kleber M, de Sousa G, Denzer C, Toschke AM. Parental diabetes, pubertal stage, and extreme obesity are the main risk factors for prediabetes in children and adolescents: a simple risk score to identify children at risk for prediabetes. Pediatr Diabetes 2009;10:395–400 [DOI] [PubMed] [Google Scholar]
- 39.Ball GDC, Huang TTK, Gower BA, et al. Longitudinal changes in insulin sensitivity, insulin secretion, and beta-cell function during puberty. J Pediatr 2006;148:16–22 [DOI] [PubMed] [Google Scholar]
- 40.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1C: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952 [DOI] [PMC free article] [PubMed]