Abstract
OBJECTIVE
To assess the association between serum adiponectin level and all-cause mortality in people with type 2 diabetes. Because of the insulin-sensitizing, anti-inflammatory, and antiatherogenic effects of adiponectin, we hypothesized that higher adiponectin level would be associated with lower all-cause mortality.
RESEARCH DESIGN AND METHODS
A total of 609 men and women aged 72 ± 6.3 years with type 2 diabetes and information on total and high molecular weight adiponectin were followed for a median of 5 years. The longitudinal association between adiponectin and all-cause mortality was analyzed with Cox proportional hazards models with time from adiponectin measurement to death as the time-to-event variable. Analyses were adjusted for demographic variables and significant diabetes parameters, significant cardiovascular parameters, and significant diabetes medications.
RESULTS
Total and high molecular weight adiponectin were highly correlated. The highest adiponectin quartile was strongly associated with higher all-cause mortality compared with the lowest quartile (hazard ratio = 4.0 [95% CI: 1.7–9.2]) in the fully adjusted model. These results did not change in analyses stratified by sex and thiazolidinedione use, after exclusion of people who died within one year of adiponectin measurement, or when change in weight before adiponectin measurement was considered.
CONCLUSIONS
Contrary to our hypothesis, higher adiponectin level was related to higher all-cause mortality. This association was not explained by confounding by other characteristics, including medications or preceding weight loss.
Nearly 24 million Americans are estimated to have type 2 diabetes, and more than half of those affected are >60 years of age (1). The prevalence of type 2 diabetes is expected to reach epidemic proportions by the year 2050 with a projected increase to 21–33% of the American population (2). Morbidity and mortality result from both microvascular and macrovascular complications, but the exact metabolic predictors of mortality are unknown. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial reported that tight glycemic control was associated with increased mortality (3), whereas tight lipid and blood pressure control had no association with mortality (4,5). These unexpected findings demonstrate the need to explore novel predictors of mortality risk in people with type 2 diabetes. Medications used to treat type 2 diabetes may affect insulin sensitivity, but this parameter is rarely used as an outcome predictor.
There is growing interest in adiponectin, a 244-amino acid protein that is secreted by adipocytes with receptors in liver and skeletal muscle, which has insulin-sensitizing, anti-inflammatory, and antiatherogenic effects (6,7). Adiponectin levels inversely correlate with adiposity, and higher adiponectin levels predict low risk of developing type 2 diabetes (8); thus, we might expect that higher adiponectin level would be associated with lower mortality, but the literature about the association between adiponectin and mortality is inconsistent. Although some authors describe no relationship between adiponectin and mortality (9), others describe an association of high adiponectin with increased mortality specifically in populations with prevalent coronary heart disease (CHD) (10), chronic kidney disease (11), chronic heart failure (12), healthy community dwellers (13), and in the elderly (14). To our knowledge, there are few data describing the relationship between adiponectin and all-cause mortality specifically in people with type 2 diabetes.
The objective of this study was to examine the association of adiponectin level with all-cause mortality in elderly people with type 2 diabetes. We hypothesized that higher adiponectin would be associated with lower all-cause mortality.
RESEARCH DESIGN AND METHODS
Design
This study is a cohort study of 609 elderly people with type 2 diabetes who were participants in the downstate sample of the Informatics in Diabetes Education and Telemedicine (IDEATel) Study. IDEATel was a randomized controlled trial with blinded outcome assessment. As described previously (15,16), subjects were enrolled through primary care practices in New York City, with the enrollment hub at Columbia University Medical Center, and in Upstate New York, where the enrollment hub was at State University of New York (SUNY) Upstate Medical University at Syracuse. The institutional review boards at each participating institution approved the study protocol. All participants provided informed consent. An independent Data and Safety Monitoring Board monitored the study to ensure participant safety and adherence to the protocol.
Sample
The original IDEATel sample consisted of 2,169 subjects residing in New York State, recruited and randomized between December 2000 and October 2005. Inclusion criteria were as follows: ≥55 years of age; current Medicare beneficiary; type 2 diabetes as defined by a physician’s diagnosis and treatment with diet, an oral hypoglycemic agent, or insulin; residence in a federally designated medically underserved area; and fluency in either English or Spanish. Exclusion criteria were as follows: moderate or severe cognitive impairment; severe visual, mobility, or motor coordination impairment; severe comorbid condition; severe expressive or receptive communication impairment; lack of electrical outlet for telemedicine unit; and more than 3 months a year spent at a location different from the person’s residence. Participants were recruited through their primary care provider practice and randomized to telemedicine case management or to usual care.
From the total 2,169 subjects in IDEATel, 949 subjects were recruited in the New York City sample. Of these 949 New York City subjects, adiponectin levels were measured on 608 subjects who were also participants in an IDEATel substudy of aging and cognition that began data collection in 2004; these 608 subjects comprise the analytic sample for this study (Supplementary Fig. 1).
Adiponectin measurement
Blood samples were obtained fasting and frozen at −70°C. Total adiponectin and high molecular weight (HMW) adiponectin were measured at enrollment into the IDEATel cognition substudy (2004–2006) on frozen serum samples using radioimmunoassay (Linco Research, St. Charles, MO). The standard detection curve range was 0.78 to 100 ng/mL. The intra-assay coefficient of variation (CV) is from 1.78 to 6.21%, and the interassay CV was from 6.90 to 9.25%.
Covariates
Subjects were instructed to come to baseline and five yearly follow-up examinations fasting and having held their glycemic control medications. Assessments included resting blood pressure, HbA1c, total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, ultra-sensitive C-reactive protein (CRP), urine microalbumin-to-creatinine ratio, and anthropometric measurements as described in detail in previous publications (16). Demographic data and self-report of comorbid conditions were collected at each visit. For each subject, the covariates included in our analyses were from the same study visit during which the adiponectin sample was obtained.
The two original IDEATel study arms, the telemedicine intervention, and usual care (15,17) were also used as covariates. In the telemedicine intervention arm the target HbA1c was ≤7%, except for participants with significantly reduced life expectancy and/or severe hypoglycemic unawareness, for whom that target was ≤8%. Blood pressure and LDL goals are described in detail in previous publications (16). Patients in the usual care group received clinical care from their primary care providers, without other guidance or direction from study personnel.
Outcome
Vital status was determined through queries of the Center for Medicare and Medicaid Services database, which contains data updated weekly by automated cross-referencing with the Social Security Administration database. We last queried the Center for Medicare and Medicaid Services vital status database for this cohort on 30 November 2009.
Statistical analyses
The distributions of continuous variables were examined, and appropriate log transformations were performed for variables that were not normally distributed (total and HMW adiponectin, CRP and albumin-to-creatinine ratio [ACR]). Pearson correlation coefficients were calculated for continuous variables. Student t tests for continuous variables and Pearson’s χ2 for categorical variable were used to compare the associations between covariates (age, sex, race/ethnicity, BMI, HbA1c, systolic blood pressure (SBP), diastolic blood pressure (DBP), triglycerides, HDL, LDL, non-HDL cholesterol, ACR, CRP, history of myocardial infarction (MI), tobacco usage, medication usage, duration of diabetes, and randomization arm in IDEAtel study) with mortality. For categorical variables with ≥2 categories, pairwise comparisons were only examined if the overall χ2 was significant.
Because there is debate about its most active form and it is ∼15% higher in men, we chose to categorize adiponectin as follows: total adiponectin quartiles, female total adiponectin quartiles, male total adiponectin quartiles, HMW adiponectin quartiles, female HMW adiponectin quartiles, male HMW adiponectin quartiles, combined male and female quartiles of total adiponectin, and combined male and female quartiles of HMW adiponectin. ANOVA and χ2 were used to compare the distribution of covariates across adiponectin quartiles.
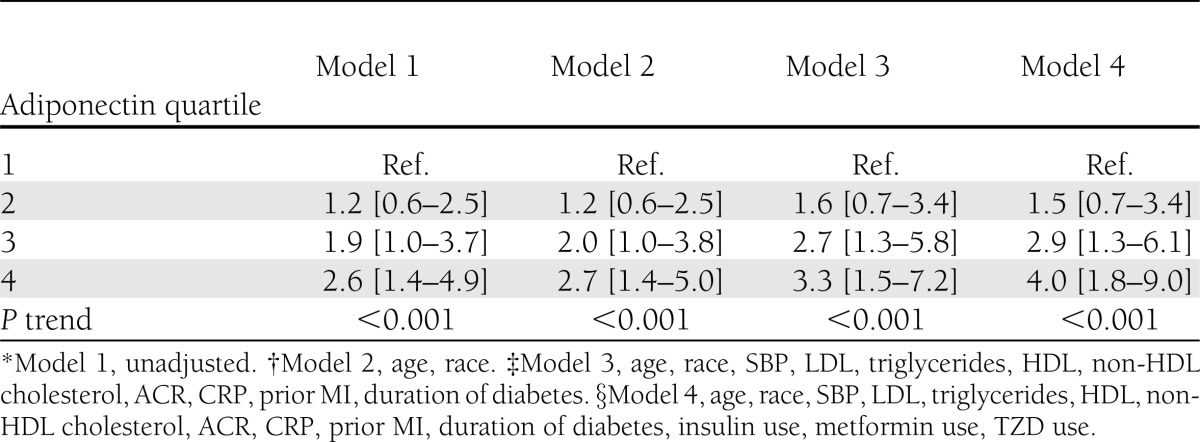
The association between adiponectin and mortality was investigated using multivariable Cox proportional hazards regression with time from adiponectin measurement to death as the outcome variable. The proportional hazards assumption was verified by modeling an interaction term of time to death by the main exposure. Adiponectin was examined as a log-transformed continuous variable and as quartiles described above. Four separate models were evaluated and only covariates that were significantly related to the outcome or to adiponectin were included. The first model was an unadjusted analysis; the second model was adjusted for demographics (age and race); the third model was adjusted for significant cardiovascular and diabetes parameters (SBP, LDL, triglycerides, HDL, non-HDL cholesterol, ACR, CRP, prior MI, and duration of diabetes); and the fourth model was adjusted for demographics, significant cardiovascular and diabetes parameters, and significant medications (insulin, metformin, and thiazolidinediones [TZDs]). A test for trend across the adiponectin quartiles was performed for each model using the P value for the adiponectin quartile variable in a model with the covariates but without the adiponectin quartile indicator variables. Forty subjects were missing CRP values, and sensitivity analyses excluding CRP demonstrated similar results; thus data are presented with CRP included as a covariate.
We examined effect modification by sex, TZD use, and history of previous MI in stratified analyses by these variables and by examining interactions terms of these variables with the adiponectin quartile variable. A sensitivity analysis was conducted excluding those subjects who died within 1 year of adiponectin measurement to rule out the effect of subclinical disease processes.
Finally, a secondary analysis was conducted to consider change in weight before adiponectin sampling. We were unable to calculate a change in weight for those subjects whose adiponectin was drawn at the first study visit, and the resulting sample size was n = 446. Change in weight was operationalized with indicator variables comparing both those who lost more than 10% and those who gained more than 10% of their body weight to those with no change in weight. Cox regression was performed with change in weight variables added to the fully adjusted model described previously.
All tests for significance were two sided, with P < 0.05 considered statistically significant. All statistical analyses were conducted using SAS software, version 9.2 for Windows (copyright 2002–2008; SAS Institute, Cary, NC).
RESULTS
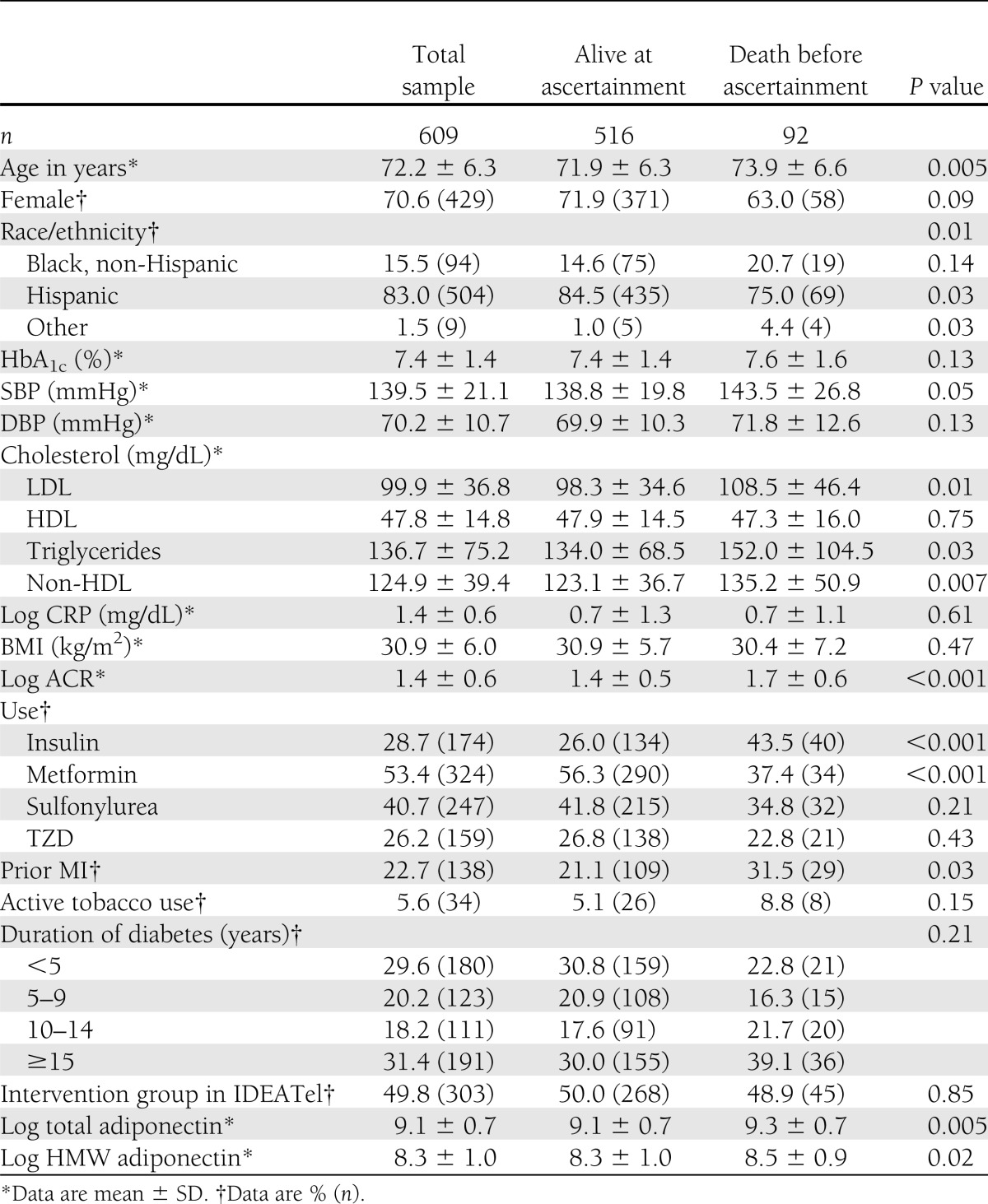
Characteristics of the study participants are shown in Table 1. Among the study participants, the average age was 72 ± 6.3 years, most were female, and the overwhelming majority was Hispanic or African American. The average HbA1c was 7.4%, and other diabetes control parameters were reasonably well controlled. As expected, total adiponectin was higher in women compared with men (13263.9 ± 11,265.0 vs. 9,110.4 ± 7,418.7 ng/mL; P < 0.001). The same was true for HMW adiponectin. Compared with the excluded downstate sample, our analytic sample was slightly younger, had more Hispanic but fewer Black non-Hispanic participants, had less education, and had lower mortality (Supplementary Table 1).
Table 1.
Characteristics of total sample, those alive at ascertainment, and those who died before ascertainment (30 November 2009)
Total and HMW adiponectin were highly correlated (r = 0.88663, P < 0.001). Positive correlations were also present between adiponectin and HDL, LDL, total cholesterol, and ACR, whereas negative correlations were seen for CRP, triglycerides, DBP, and HbA1c (Supplementary Table 2).
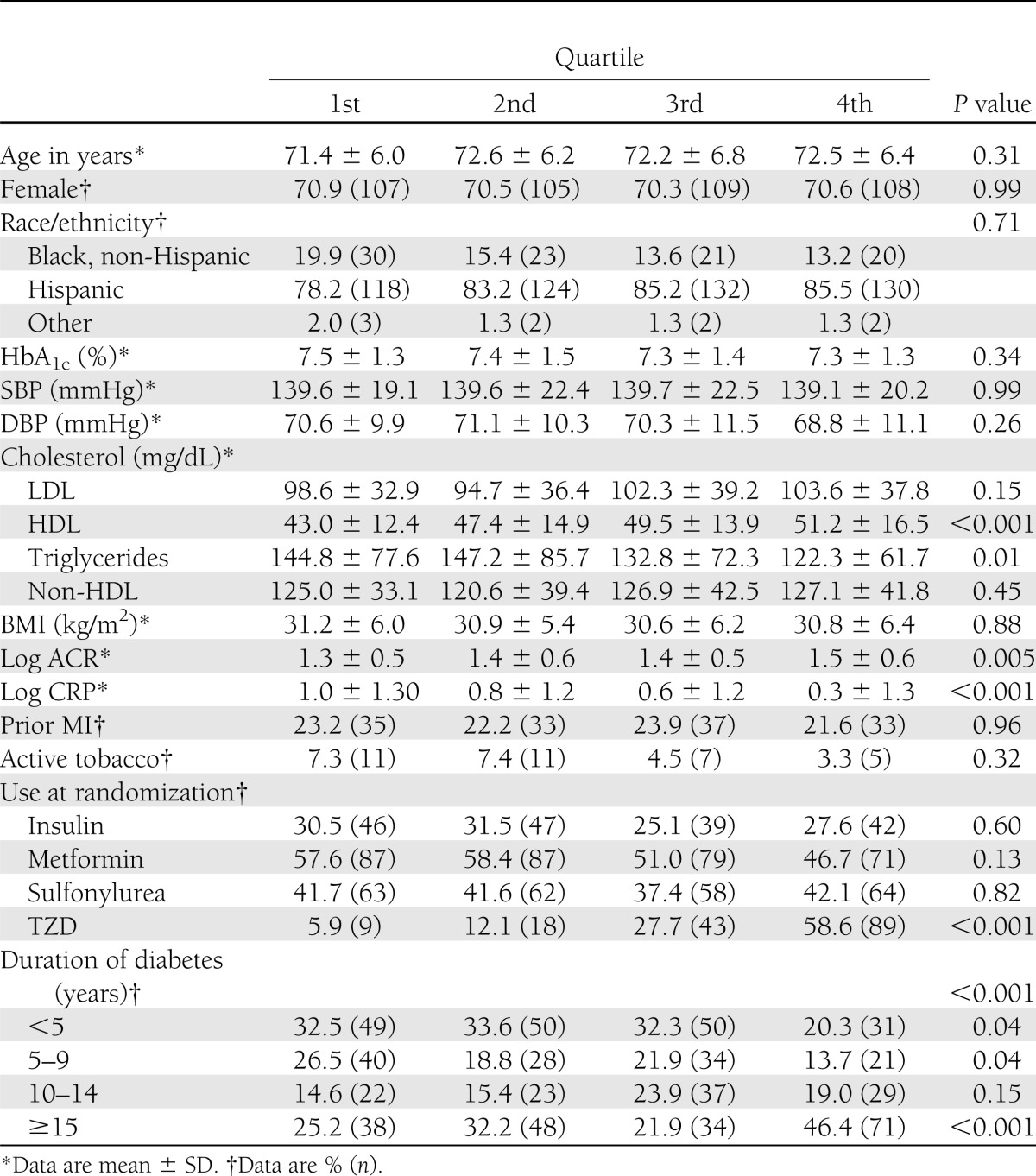
Those participants who died (n = 92) were older; had higher SBP, LDL, triglycerides, and ACR; were more likely to have had an MI; were more likely to use insulin and less likely to use metformin; and had higher total and HMW adiponectin, compared with those participants that did not die (n = 516) (Table 1). When compared with participants in the first quartile (with the lowest adiponectin), those in the fourth quartile (with the highest adiponectin) had higher HDL and ACR, and lower triglycerides and CRP. Subjects in the fourth quartile were also more likely to be prescribed a TZD and to have had type 2 diabetes for >15 years (Table 2). Sensitivity analyses were performed with adiponectin quartiles as described in research design and methods, and the results were similar for each analysis (Supplementary Tables 3–5).
Table 2.
Comparison of characteristics across combined sex-specific quartiles of total adiponectin
Participants were followed for a median of 5.0 years (IQR 5.7–5.3 years), and the Cox proportional hazards assumption was satisfied (P = 0.06). Cox regression analysis using adiponectin as a log-transformed continuous variable demonstrated a positive association between adiponectin and all-cause mortality (unadjusted hazard ratio [HR] = 1.5 [95% CI: 1.2–2.0], P = 0.003 and fully adjusted HR = 1.8 [1.2–2.6], P = 0.004) (Supplementary Table 6). Because the relationship between adiponectin and mortality was not linear (P = 0.05 for adiponectin squared term) and to remain consistent with the literature, we present results of Cox hazards regression analysis with quartiles of adiponectin, specifically combined sex quartiles of total adiponectin; however, the results for other operationalizations of adiponectin quartiles are similar and available in Supplementary Tables 7–9. Compared with those with the lowest adiponectin, those with the highest adiponectin had an increased hazard of death (crude HR = 2.6 [1.4–4.9], P for trend <0.001) (Table 3). Adjusting for demographic factors, medication use, and diabetes and cardiovascular control parameters actually strengthens this association (fully adjusted HR = 4.0 [1.8–9.0], P for trend <0.001). The association between adiponectin level and mortality did not differ by age as evidenced by a nonsignificant interaction term (P = 0.80). There was also no effect modification by history of prior MI (P for interaction term = 0.41).
Table 3.
Relation of combined sex-specific quartiles of total adiponectin with mortality
Administration of TZDs is known to be associated with increased adiponectin levels (18), and in our sample there was higher TZD use among subjects in the fourth quartile of adiponectin, raising concern that TZD use might be a confounder. However, TZD use was not associated with increased mortality (crude HR = 0.8 [95% CI: 0.5–1.3]; adjusted HR = 1.0 [0.6–1.7]); thus TZD use is unlikely to confound our results. We also adjusted for hypertension medication class (β-blocker, calcium channel blocker, diuretic, angiotensin-converting enzyme inhibitor, and angiotensin receptor blocker), and the results were essentially the same. Similarly, there was concern that age might confound the association between adiponectin and mortality since increasing age was associated with increased mortality (crude HR = 1.05 [1.0–1.1]; adjusted HR = 1.05 [1.0–1.1]). However, age did not differ across the quartiles of adiponectin (P = 0.31) and thus is unlikely to be a confounder. Further evidence that TZD use and age are unlikely to act as confounders is the association between higher adiponectin levels and increased hazard of death consistent when stratified by TZD use or age.
Sex was a potential confounder of our results since female sex was associated with higher levels of adiponectin (P < 0.001) and associated with a decreased hazard of death in crude and adjusted analyses (crude HR = 0.7 [95% CI: 0.4–1.0]; adjusted HR = 0.5 [0.3–0.9]). However, stratification by sex did not change our findings.
Because adiponectin increases with weight loss and weight loss is associated with increased mortality in the elderly population, if weight loss occurred in our sample, it could potentially bias our results, creating a stronger association between adiponectin and mortality. In a secondary analysis of n = 446 subjects, ∼10% of the subjects lost weight (n = 41), 10% gained weight (n = 46), and 80% (n = 359) did not have a change in their weight. The association between adiponectin and mortality in this smaller sample was similar to the larger analytic sample: subjects in the fourth adiponectin quartile had an increased hazard of death compared with those in the first quartile (fully adjusted HR = 3.4 [95% CI: 1.4–8.3]). When change in weight was added to the fully adjusted model, the relationship between higher adiponectin and increased mortality was not attenuated (HR = 3.6 [1.4–9.0]) (Supplementary Table 10).
Finally, a sensitivity analysis was performed excluding those subjects who died within 1 year of adiponectin sampling to minimize potential confounding by terminal illness that could cause weight loss and increases in adiponectin. Similar to the previous analyses, those with the highest adiponectin had an increased hazard of death compared with those with the lowest adiponectin (adjusted HR = 4.1 [95% CI: 1.8–9.6]).
CONCLUSIONS
Higher total and HMW adiponectin were associated with increased all-cause mortality in a cohort of elderly, predominantly minority people with type 2 diabetes. This association was not diminished after adjusting for demographic factors, BMI, diabetes control parameters, cardiovascular risk factors, medication usage, and weight loss. Our findings are consistent with most (10–14) but not all (9) of the current literature about the relationship between adiponectin and all-cause mortality in nondiabetic populations.
Adipose tissue is increasingly understood to be an important endocrine organ, secreting adipokines that are important to energy homeostasis. Adiponectin is the most abundant adipokine and has important influences on insulin sensitivity, via the 5′-AMP–activated protein kinase pathway, resulting in improved glucose utilization and fatty acid oxidation and inhibition of serine kinases that antagonize insulin signaling (19,20). Recent evidence also suggests that adiponectin may also mediate insulin sensitivity through a sphingolipid pathway (21). Adiponectin also has antiatherogenic and anti-inflammatory functions via inhibition of the nuclear factor-κB pathway, which results in lower CRP, lower interleukin-8, and downregulation of vascular adhesion molecule expression on endothelial cells (22). Finally, there is evidence to suggest that adiponectin may have a clean-up function through promotion of clearance by macrophages of early apoptotic cells (22). Because of these protective effects against insulin resistance and possibly against atherosclerosis, we hypothesized that higher adiponectin levels would be associated with reduced mortality. Our findings are to the contrary.
Adiponectin exists in plasma in low molecular weight trimers, medium molecular weight hexamers, and HMW multimers, and some authors have suggested that HMW adiponectin, rather than total adiponectin, is the active form (23). Some studies have demonstrated that HMW adiponectin or the ratio of HMW adiponectin to total adiponectin correlates most closely with insulin sensitivity, metabolic abnormalities, and risk of developing type 2 diabetes (24,25), whereas more recent data have provided evidence that total adiponectin, HMW adiponectin, and the HMW-to-total adiponectin ratio all had similar utility for the identification of insulin sensitivity and diabetes risk (26,27). We found similar results for total and HMW adiponectin and chose to present results for total adiponectin.
It is well established that adiponectin levels inversely correlated with adiposity and higher adiponectin levels predict a lower risk of developing diabetes (8). Observational studies have demonstrated that adiponectin is inversely correlated with total cholesterol, LDL, triglycerides, and blood pressure, and it is positively associated with HDL (28,29). We confirm these findings in people with type 2 diabetes by demonstrating that high levels of adiponectin were associated with a more favorable CV risk and lipid profile. In line with these observations, Pischon et al. (30) demonstrated in the Health Professionals Follow-up Study that high adiponectin levels were associated with lower risk of MI in men, but subsequent studies have not agreed. Some authors have shown a lack of association between adiponectin level and prevalent or incident CHD in the general population (28), in patients with pre-existing CHD (28), and in subjects with type 2 diabetes (31). Perhaps surprisingly based on the unclear association of adiponectin and CHD, several studies have consistently demonstrated that high adiponectin levels are associated with increased risk of CHD and all-cause mortality in subjects with prevalent CHD (10), chronic kidney disease (11), chronic heart failure (12), healthy community dwellers (13), and in the elderly (14), whereas some authors have shown no association (9).
To our knowledge, there are few data investigating the role of adiponectin specifically in people with type 2 diabetes. In a subset of 745 diabetic men in the Health Professionals Follow-up Study, the authors found an inverse relationship between adiponectin and incident CHD in a multivariable model adjusting for age, physical activity, family history of MI, hypertension, high cholesterol, aspirin use, tobacco use, fasting status, and duration of diabetes, but this association was attenuated after adjusting for HDL levels (32). In another study, Krzyzanowska et al. (31) found no significant correlation between adiponectin and any clinical parameters (age, BMI, blood pressure, diabetes duration, glomerular filtration rate, albuminuria, triglycerides, LDL, HDL, or CRP) in a cross-sectional analysis of 147 subjects with type 2 diabetes. In a longitudinal analysis of the same subjects, the authors found no association between adiponectin and a composite end point of MI, stroke, and all-cause mortality, which may be explained by the relatively small sample size (n = 147) and short duration of follow-up (median of 19.3 months). In contrast with the previous literature, our current study with more participants and longer follow-up demonstrates that there is in fact a strong association between adiponectin and all-cause mortality in a cohort of patients with type 2 diabetes even after adjusting for demographics, clinical parameters, and potential confounders.
We hypothesized that higher adiponectin would be associated with lower all-cause mortality because it is associated with more favorable cardiovascular and metabolic risk profiles and decreased risk of developing diabetes. On the contrary, our results, and the results of other investigators, demonstrate that higher levels of adiponectin predict an increased risk of all-cause mortality while related to an apparently better metabolic and cardiovascular risk profile. Potential explanations for this paradox that have been suggested include reverse causality (33) or adiponectin resistance (34). In addition, it is possible that there might be a separate phenotype of those people who develop diabetes despite a high adiponectin who have increased mortality as a result of a particularly severe derangement in insulin action. Those excluded from our analytic sample because they did not have adiponectin measured were more likely to have died. Thus, survival bias should be considered a potential explanation for our findings. In addition, our results are only generalizable to elderly survivors with diabetes.
We acknowledge that there are several limitations to our study. We have no direct measure of renal function, and high levels of adiponectin in people with chronic kidney disease is associated with increased mortality (11). We did not have data on cause-specific mortality, and all-cause mortality is heterogeneous in underlying pathology. Being able to analyze relationships of adiponectin specifically with cardiovascular mortality may have helped us to understand the mechanisms at play. We lacked measures of central adiposity such as waist-to-hip ratio; in our sample adiponectin was not related to BMI, and a measure of central adiposity may have been better correlated with adiponectin. We only have one measurement of adiponectin and are thus unable to make any comments on change in adiponectin level and mortality. Finally, there were more deaths in the sample missing adiponectin measurements, which could be a source of bias. It is possible that the association we report is restricted to survivors and that an inverse association could be found in participants missing data. However, it is important to point out that our results are consistent with some other reports that include people with and without diabetes.
In conclusion, our study finds that higher adiponectin is associated with increased mortality in a cohort of elderly people with type 2 diabetes. Further studies should confirm this association and should specifically investigate cause-specific mortality.
Acknowledgments
This work is supported by National Institute on Minority Health and Health Disparities grant P60 MD 000206 (J.A.L., W.P., S.S., J.T.), Alzheimer’s Association grant IIRG-05-15053 (J.A.L.), the Fidelity Foundation (J.A.L.), and Cooperative Agreement 95-C-90998 from the Centers for Medicare and Medicaid Services (R.W., S.S., J.T., W.P., J.A.L.).
No potential conflicts of interest relevant to this article were reported.
J.R.S. helped with the conception of the project, conducted statistical analyses and interpreted findings, and had primary responsibility for writing the manuscript. W.P. was involved in data collection and supervision of the manuscript. J.T. provided statistical expertise, assisted in obtaining funding, and had primary responsibility for data management. R.W. was involved in data collection and provided expertise as an endocrinologist in the interpretation of results. S.S. was involved in obtaining funding, data collection, and interpretation of the findings. J.A.L. was responsible for project conception, statistical analyses, interpretation of results, and manuscript writing with J.R.S. J.A.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 34th Annual Meeting of the Society of General Medicine, Phoenix, Arizona, 4–7 May 2011.
Footnotes
Clinical trial reg. no. NCT00271739, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2215/-/DC1.
A slide set summarizing this article is available online.
References
- 1.Centers for Disease Control and Prevention National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008 [Google Scholar]
- 2.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginsberg HN, Elam MB, Lovato LC, et al. ACCORD Study Group Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116:1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care 2003;26:2442–2450 [DOI] [PubMed] [Google Scholar]
- 8.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2009;302:179–188 [DOI] [PubMed] [Google Scholar]
- 9.Maiolino G, Cesari M, Sticchi D, et al. Plasma adiponectin for prediction of cardiovascular events and mortality in high-risk patients. J Clin Endocrinol Metab 2008;93:3333–3340 [DOI] [PubMed] [Google Scholar]
- 10.Dekker JM, Funahashi T, Nijpels G, et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab 2008;93:1489–1496 [DOI] [PubMed] [Google Scholar]
- 11.Menon V, Li L, Wang X, et al. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2599–2606 [DOI] [PubMed] [Google Scholar]
- 12.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation 2005;112:1756–1762 [DOI] [PubMed] [Google Scholar]
- 13.Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol 2007;165:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poehls J, Wassel CL, Harris TB, et al. Health ABC Study Association of adiponectin with mortality in older adults: the Health, Aging, and Body Composition Study. Diabetologia 2009;52:591–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shea S, Starren J, Weinstock RS, et al. Columbia University’s Informatics for Diabetes Education and Telemedicine (IDEATel) Project: rationale and design. J Am Med Inform Assoc 2002;9:49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc 2006;13:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starren J, Hripcsak G, Sengupta S, et al. Columbia University’s Informatics for Diabetes Education and Telemedicine (IDEATel) project: technical implementation. J Am Med Inform Assoc 2002;9:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajaj M, Suraamornkul S, Piper P, et al. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab 2004;89:200–206 [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–1295 [DOI] [PubMed] [Google Scholar]
- 20.Lancaster GI, Febbraio MA. Adiponectin sphings into action. Nat Med 2011;17:37–38 [DOI] [PubMed] [Google Scholar]
- 21.Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 2011;17:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouchi N, Walsh K. A novel role for adiponectin in the regulation of inflammation. Arterioscler Thromb Vasc Biol 2008;28:1219–1221 [DOI] [PubMed] [Google Scholar]
- 23.Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab 2007;9:282–289 [DOI] [PubMed] [Google Scholar]
- 24.Hara K, Horikoshi M, Yamauchi T, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 2006;29:1357–1362 [DOI] [PubMed] [Google Scholar]
- 25.Nakashima R, Kamei N, Yamane K, Nakanishi S, Nakashima A, Kohno N. Decreased total and high molecular weight adiponectin are independent risk factors for the development of type 2 diabetes in Japanese-Americans. J Clin Endocrinol Metab 2006;91:3873–3877 [DOI] [PubMed] [Google Scholar]
- 26.Zhu N, Pankow JS, Ballantyne CM, et al. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. J Clin Endocrinol Metab 2010;95:5097–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeda-Valdes P, Cuevas-Ramos D, Mehta R, et al. Total and high molecular weight adiponectin have similar utility for the identification of insulin resistance. Cardiovasc Diabetol 2010;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sattar N, Wannamethee G, Sarwar N, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation 2006;114:623–629 [DOI] [PubMed] [Google Scholar]
- 29.Ryo M, Nakamura T, Kihara S, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J 2004;68:975–981 [DOI] [PubMed] [Google Scholar]
- 30.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004;291:1730–1737 [DOI] [PubMed] [Google Scholar]
- 31.Krzyzanowska K, Aso Y, Mittermayer F, Inukai T, Brix J, Schernthaner G. High-molecular-weight adiponectin does not predict cardiovascular events in patients with type 2 diabetes. Transl Res 2009;153:199–203 [DOI] [PubMed] [Google Scholar]
- 32.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes 2005;54:534–539 [DOI] [PubMed] [Google Scholar]
- 33.Sattar N, Nelson SM. Adiponectin, diabetes, and coronary heart disease in older persons: unraveling the paradox. J Clin Endocrinol Metab 2008;93:3299–3301 [DOI] [PubMed] [Google Scholar]
- 34.Teoh H, Strauss MH, Szmitko PE, Verma S. Adiponectin and myocardial infarction: A paradox or a paradigm? Eur Heart J 2006;27:2266–2268 [DOI] [PubMed] [Google Scholar]